Abstract
The number of young cancer survivors is increasing owing to advances in cancer therapeutics, but many face infertility as a result of their treatment. Technologies that already exist for cancer patients concerned about their future fertility include sperm banking for men and hormonal intervention followed by in vitro fertilization and embryo cryopreservation for women. However, logistical barriers to timely patient referral and coordination of care between specialties can limit patient access to all the available options. Moreover, there are few alternatives for young women and girls who cannot delay their cancer treatment, or who are unable to undergo hormonal intervention. The Oncofertility Consortium is a network of researchers, physicians and scholars who are advancing fertility preservation options for young cancer patients. Research into the societal, ethical, and legal implications is also an important part of the work performed by the Oncofertility Consortium, which is providing new perspectives on patient decision making about how to access these emerging reproductive technologies. Experts in the fields of oncology, reproductive medicine, the social sciences, law, education, and the humanities are working together to develop next generation reproductive interventions and promote communication between scholars, clinicians, patients, and the public to ensure that young cancer patients are equipped with the most appropriate information and options for having a family in the future.
Introduction
Late on a Friday afternoon, the Oncofertility Consortium national helpline (Fertline) received a call from a pediatric oncologist with an urgent request. His patient was a girl of high-school age who had just learned that the treatment for her cancer, which needed to start on Monday morning, would likely cause infertility or sterility. She told her doctor that she did not want to go ahead with the life-preserving treatment unless her fertility could be spared.
Every year, 1.4 million people are diagnosed with cancer in the USA. Most people associate cancer with old age, but 10% of those diagnosed are <45 years of age.[1] Owing to more-aggressive and advanced treatment, an increasing number of patients are surviving their disease. Unfortunately, these cancer treatments can also cause infertility, sterility, or early menopause.[2] Providers and patients alike have tolerated these adverse side effects with the notion that surviving the disease was the only goal, and any unintended consequences of treatment should be endured. The desire to address post-cancer infertility has come to the fore in the past decade, as patients have begun to express their belief that the ability to reproduce should be an imperative and not an afterthought.
Lance Armstrong, the world-famous cyclist, was 25 years of age when he was diagnosed with metastatic cancer. Unlike most cancer patients, however, Armstrong was advised by his oncologist to have his sperm frozen. After he survived the disease, three of his children were born using his banked sperm. By contrast, a woman named Lindsay Nohr Beck was diagnosed with cancer at the age of 21, but was not informed of any fertility-sparing options by her health-care providers. Instead, she delayed her cancer treatment and found a physician who was willing to perform hormonal stimulation and bank her eggs. Beck went on to found Fertile Hope, an organization that provides reproductive information and support to cancer patients and survivors.
Despite the intense interest of cancer patients and their families in preserving fertility, there are gaps in our understanding of the underlying biology, clinical techniques, and patient and provider awareness,[3] particularly for young women and girls diagnosed with cancer. The primary barrier to fertility preservation in men is knowledge about a fertility threat at the time of diagnosis. By contrast, young women and girls who face a similarly devastating cancer diagnosis have no easy method to preserve their fertility. Unlike sperm, the female germ cell (oocyte or egg) must be retrieved surgically, is available in limited numbers, and will be at varying degrees of maturity depending on the time of the menstrual cycle.[4] To offer the hope of future fertility to young women and girls with cancer, knowledge of ovarian function is needed to establish methods that support follicle growth and oocyte development in vitro.
Oncofertility is a new concept that describes an integrated network of clinical resources that focus on developing methods to spare or restore reproductive function in patients diagnosed with cancer. Solving this problem requires progress at both the bench and bedside in concert with an awareness of the societal, ethical, and legal issues that arise as a result of the introduction of new reproductive interventions.[5–7] There are three reasons why meeting the fertility needs of young men, women, and children who face a cancer diagnosis and fertility- threatening treatment has been such a challenge: the science and supporting technology are not mature, solving the problem requires an interdisciplinary approach by research and clinical communities, and the work requires an unorthodox funding mechanism that supports diverse groups of investigators and scholars. The Oncofertility Consortium was founded to address the questions that exist at the intersection of oncology, reproductive medicine, and the public. By integrating the bench (basic and social research sciences), bedside (clinicians and clinical researchers), and community (the humanities, law, and education) in an over arching program, the Oncofertility Consortium represents a new way to approach a previously intractable problem (Figure 1). This review will discuss the need for fertility preservation in cancer patients and describe how the Oncofertility Consortium has become a nationwide resource for addressing the fertility needs of young people who have been diagnosed with cancer.
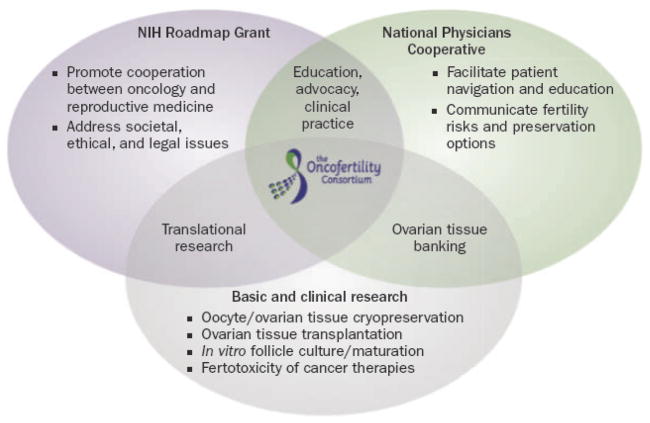
Figure 1.
Structure of the Oncofertility Consortium. Three major gaps have prevented the fertility needs of young men, women, and children who face a cancer diagnosis and fertility-threatening treatment from being met. First, the science and supporting technology are not mature (scientific gap). Second, solving the problem requires an interdisciplinary approach by research and clinical communities (structural gap). Third, the work requires an unorthodox funding mechanism that supports diverse groups of investigators and scholars (tactical gap). The NIH Roadmap Grant, the National Physicians Cooperative, and basic and clinical research intersect and work together to ensure that advances in the laboratory are translated efficiently and safely to the bedside.
The concept of oncofertility
The term ‘oncofertility’ was coined in 2006, although the history of oncofertility dates to 1971 when the US president at the time, Richard Nixon, signed the National Cancer Act. This act marked the start of the ‘war on cancer’ and allowed the necessary funding for a new National Cancer Institute (NCI), which would focus on the diagnosis and treatment of the disease, as well as its etiology. The resources provided by the NCI have been responsible for earlier diagnosis and more-aggressive and better treatment of cancer, which have resulted in a prodigious increase in the number of cancer survivors.
At the time when the NCI was formed, reproductive scientists were developing new technologies to assist infertile women. The birth of Louise Brown in 1978—the first baby born using in vitro fertilization (IVF)—preceded three decades of steady advances in reproductive interventions. Today, IVF using eggs matured in vivo with hormonal treatments and freezing (cryopreserving) the embryos for implantation at a later date is routine.[8] Oocyte cryopreservation and vitrification (an ultrarapid freezing process that prevents ice crystal formation) technologies have greatly improved, and the number of babies born from the fertilization of mature eggs that were once frozen has now surpassed 1,000.[9–11] The reported clinical pregnancy rate per IVF cycle using once-vitrified oocytes is between 35% and 65%,[12–14] which is comparable to rates achieved using standard IVF with fresh eggs as reported by American, Canadian, and European registries.[15–18] Despite the number of live births, however, oocyte cryopreservation technology remains inefficient and is still considered investigational. Ideally, advances in cancer care and reproductive technologies should have converged and provided fertility options to young cancer patients years ago. Conceptually, this task seems logical and inevitable, yet its execution has been complicated by the challenge of combining the separate specialties of oncology and reproductive medicine.
The gaps in fertility preservation
Prior to the creation of the Oncofertility Consortium, three main gaps posed substantial obstacles to solving the problem of fertility preservation in young cancer patients: tactical, structural, and scientific.
Funding a multidisciplinary project
The first gap facing the Oncofertility Consortium was tactical. Developing fertility interventions for cancer patients has not been a research priority for the biotechnology and pharmaceutical industries. These industries focus on creating life-saving medicines, but view the ancillary effects of these treatments on reproductive potential as a quality of life concern that only affects a small number of people. The classification of this research area was also problematic—is it a cancer project or a reproductive science project? New analytical tools and methodological approaches are required that integrate intellectual discovery, basic science breakthroughs, and new medical practice between two special ties—oncology and reproductive medicine—that have traditionally been distant. Fortunately, the NIH addressed this issue with the creation of the roadmap funding initiative, which was established specifically for solving multidisciplinary problems at the inter section of the NIH structure. The roadmap grants were somewhat controversial, as they were considered detrimental to individual investigator initiated projects.[19,20] However, r01-funded science is often carried out in isolation, and the fragmentation of new ideas can slow their implementation into everyday medicine. Furthermore, r01 projects do not usually solve large-scale multi factorial scientific and medical problems or processes. The roadmap initiative allows teams to work on problems that have not been tackled previously, and turns ideas into tangible solutions.[21] Another component of the roadmap initiative was to involve multiple NIH institutes and program officers from interdisciplinary units. The Oncofertility Consortium has nine program officers involved in its roadmap grant, each bringing their own culture and rules.
Under the NIH roadmap grant mechanism, the fields of oncology, pediatrics, reproductive science and medicine, biomechanics, materials science, mathematics, social science, bioethics, religion, policy research, law, and cognitive and learning science have been brought together into a single discipline—oncofertility. Researchers and clinicians are accepting this new specialty as a mechanism that can address a number of questions, including: how to optimally store and recover gonadal tissue; how to promote primate-derived immature follicle growth and oocyte maturation in a three-dimensional environment; predicting how new cancer drugs will affect fertility; describing the ethical and legal concerns surrounding the use of advanced reproductive technologies in cancer patients; assessing the cost/benefit analysis of fertility preservation; and how these new technologies will operate in the health-care market place. Other issues include recognizing how families facing a child’s cancer diagnosis make the decision to participate in an ovarian cryopreservation program; identifying what role health-care practitioners and religious counselors have in patient decision-making with regard to fertility preservation; outlining how women with cancer share their concerns about infertility with their physicians, and how their decisions impact on their lives and relationships after cancer; and determining how sex, race or ethnicity, socioeconomic background, and family status influence these decisions. Work that attempts to answer some of these questions would be too unorthodox to be funded under the traditional r01 peer-review process, but all of these questions need to be asked if the basic and translational work is to move at an effective pace and with timely transition from the bench to the bedside.
It is hoped that the Oncofertility Consortium will become a template for solving other large, complex, and intractable problems. By creating an infrastructure and toolbox for clinicians, rapid change has occurred based on existing and emerging technologies, a willing partnership has been created between unorthodox groups of scholars and practitioners, and an urgent unmet need of patients is being met. The transformative nature of the NIH roadmap grant—in terms of the numbers of lives that will be touched—is incalculable. This multidisciplinary approach should become the standard for NIH-funded science, and would increase both the rate and the quality of research. Moreover, the translation of basic science into technology that can be used in the clinic would become the norm rather than the exception. This approach would move NIH-funded science in a new direction, from only seeking the publication of scientific papers to seeking solutions.[22] Team-based science at the intersection of the NIH institutes is the best way to stretch research funding further and progress faster. Other national funding programs, such as the Medical research Councils in the UK and Australia, and the Canadian Institutes of Health research, should be encouraged by the success of the Oncofertility Consortium to take a similar approach to funding this type of inter disciplinary program. Pitfalls have been encountered that should be addressed as other funding agencies, countries, and groups begin to offer fertility management options for cancer patients. Many fertility preservation technologies are still in the research stage, with the discoveries made today becoming available in the clinic in 5 to 10 years. Offering a balance between hope and hype is vital to the integrity of any new program. The desire to have a child is an overwhelming human emotion, and it must be balanced against what options are available and the primary objective, which is to survive cancer.
The National Physicians Cooperative
The second gap to overcome was structural; a national clinical collaboration was needed to navigate patients between the fields of oncology and reproductive medicine. Creating the National Physicians Cooperative (NPC) addressed this problem. The NPC consists of 50 different sites across the USA—each committed to the guidelines and initiatives of the cooperative—that provide access to tissue repositories, a national biomaterials core, and teams of interdisciplinary specialists, such as ethicists, economists, law and education scholars, social and behavioral scientists, patient advocates, basic reproductive scientists, and clinical investigators.[23] These specialists share a common vision for creating, cultivating, and applying the best technology and methods to confront the issues surrounding the impending infertility or sterility experienced by many cancer patients as a result of therapy.
The purpose of the NPC is threefold. First, it provides a system through which patients can find reliable information about their individual fertility needs and creates a personalized management plan that accounts for the time available between diagnosis and treatment, as well as the long-term wishes of patients and family members.[24] The Oncofertility Consortium has created a wealth of resources and guidelines for the NPC community, including documents that outline treatment options and the risks and benefits of fertility options, shared informed consent documents, and consensus group decisions on ovarian tissue acquisition and storage. Second, the NPC provides optimized protocols for patient access to a number of mature and investigational fertility preservation technologies, as well as information about non-biological parenting options. Finally, basic research discoveries have expanded our knowledge of the fertility threat posed by specific treatments and have led to the development of more robust fertility preservation options and medical interventions that protect the gonads from the lethal effects of certain cancer treatments.[25] Through this national cooperative, human ovarian tissue is being acquired primarily for the investigation of biological questions regarding ovarian function and maturation, as well as for cryopreservation and possible future use by patient donors. Participating fertility clinics have adjusted their practices and procedures to meet the specific needs of cancer patients, which often cannot be addressed by the standard of care. All of this work coincides with the development of a team of health-care providers who enable the patient to make an informed decision about fertility interventions while ensuring the best outcome from cancer treatment.[26]
By developing national centers distributed across the USA (Figure 2), the program ensures that rapid referral occurs as close to the patient’s home as possible, options for fertility preservation take into account the patient’s individual cancer diagnosis and its planned treatment, the most mature technologies are offered as the first-line fertility preservation options, non biological or non interventional strategies are discussed, and any experimental options are performed according to institutional review board specifications. Importantly, both the American Society for reproductive Medicine (ASRM) and ASCO support the discussion of fertility options with young cancer patients.[27,28] The structure of the NPC is now being adopted around the world; a global network will further increase the capacity of clinicians to act on behalf of cancer patients using best practices developed by the practitioner community, for the practitioner community.
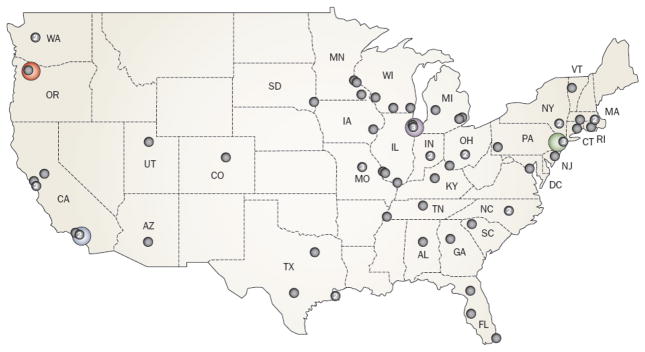
Figure 2.
The National Physicians Cooperative. The National Physicians Cooperative is a federation of fertility programs that work together to share practice plans and ensure that patients receive the most authoritative and timely care. There are four core centers in the USA where research is being conducted (denoted in color): Northwestern University, Chicago, IL; University of Pennsylvania, Philadelphia, PA; Oregon National Primate Research Center, Beaverton, OR; University of California, San Diego, CA. An additional 68 allied centers are located across the USA (denoted by black dots; numbers indicate more than one center in the same location). A national fertility helpline (Fertline) is operated at Northwestern University on behalf of the entire network.
The scientific gap
The final gap was scientific. Sperm banking, although difficult for pre-pubertal boys, is an easy option for pubertal boys and men if the patient is appropriately counseled and followed-up after a cancer diagnosis. By contrast, obtaining and storing mature eggs from young women is interventional, time-consuming, and has a lower efficiency. Some young women do not have time to postpone cancer treatment, and if they wish to preserve their fertility, an ovary must be retrieved surgically. Moreover, once the ovary is removed, it is unclear how it should be stored until the patient is ready to start a family. Understanding how ovarian follicles and the enclosed oocyte mature, is essential to allow female cancer patients to one day use the ovarian tissue they bank.
Fertility preservation options
Options for men and boys
Most male cancer patients can bank sperm.[29,30] Despite this, few men provide sperm for cryopreservation before undergoing treatment. This discrepancy may reflect a lack of patient or provider awareness, or it may be related to an inability to locate a competent andrology laboratory prior to initiation of cancer treatment. An assessment of pediatric oncologists in the USA revealed that although 80% of survey respondents acknowledged that male fertility threats are a major concern for them and 86% agreed that all post-pubertal male cancer patients should be offered a fertility consultation, only 46% had referred male cancer patients to a fertility specialist prior to cancer treatment >50% of the time.[31] The discrepancy between awareness of the issue and referral to a fertility specialist is the biggest hurdle to preserving fertility in male cancer patients. Consequently, effective education programs and awareness campaigns are two ways the Oncofertility Consortium assists men who are facing a fertility threat.
The nonprofit advocacy community has been exceedingly helpful in assisting men preserve their fertility. Both Fertile Hope and the Lance Armstrong Foundation promote greater awareness of the fertility threat of cancer treatments and help patients pursue sperm banking at participating centers or with in-home semen banking.[32] These groups also provide financial support for the laboratory work and have a 1-year storage commitment with participating patients. However, there are some men and boys (particularly pre-pubertal boys) who cannot provide a semen sample. For these patients, fertility preservation efforts have focused on cryopreservation of testicular tissue. After the removal and storage of testicular tissue, a survivor could pursue one of several transplant methods to obtain mature spermatozoa for fertilization in vitro.[33] In testicular grafting, thawed immature testicular tissue fragments are transplanted ectopically. Alternatively, spermatogonial stem cells can be isolated from the tissue and infused into the seminiferous tubules to initiate spermatogenesis. The ability to mature spermatocytes from stem cells to spermatids in vitro would offer pediatric cancer patients an important fertility-sparing option; however, hurdles remain in bringing in vitro maturation of sperm to the clinical setting.[34]
Options for women and girls
The central problem identified by the Oncofertility Consortium was the paucity of biological fertility preservation options for young women. Currently, fertility preservation for men and women revolves around the common themes of gamete storage and future utilization; however, the options available to young women present unique limitations, particularly for young cancer patients. One fertility preservation technique for this group of patients involves hormonal stimulation and collection of mature oocytes, followed by IVF and then cryopreservation of the resulting embryos for use at a later date.[35,36] Embryo banking prior to cancer treatment is the most mature technology, and whenever practical, this method should be the first option for fertility management. However, embryo banking raises a variety of concerns that must be factored into the decision-making process. First, the procedure requires 2–5 weeks to complete and may delay cancer treatment. Second, an available sperm donor is required, and changes in the status of the sperm donor, such as in cases of divorce, can introduce various legal implications with regard to the ownership of the resulting embryos.[10] Third, hormonal stimulation is not an option for pre-pubertal girls, and although it can be performed on teenagers, concerns surrounding embryo creation have limited its utility in this group of patients. Finally, some young women may not wish to create or store embryos for ethical, religious, or personal reasons.[11]
Advances in cryopreservation through vitrification of mature, unfertilized oocytes expand hormonal intervention options to a greater number of female cancer patients.[14,16,37,38] In vitro maturation of immature follicles followed by oocyte vitrification is available, and has resulted in the birth of four healthy babies.[16] In vitro maturation combined with oocyte vitrification is a viable option when there is limited time between cancer diagnosis and treatment, or when hormonal stimulation for IVF is contraindicated. In a case reported by Rao et al.,[38] a 33-year-old woman presented with stage II breast cancer and was scheduled to begin chemotherapy in 1 week; the time frame from initial fertility consultation to retrieval of immature germinal vesicle-stage oocytes was only 2 days. Even with techniques such as vitrification, many mature oocytes must be frozen in order to have a reasonable chance of a later successful pregnancy.[39] whether pursuing embryo or oocyte cryopreservation, however, patients with limited uterine capacity may require retrieval of a greater number of oocytes for a statistically realistic chance of pregnancy. Moreover, patients with uterine defects, or those who have undergone a hysterectomy or have severe uterine damage owing to chemotherapy or radiation, will require the use of a gestational carrier regardless of whether oocytes or embryos are stored. This option must be offered to the patient along with details of its limitations. Because many young women wish to have a child that is biologically related to their future husband or partner, opting to bank unfertilized eggs as well as embryos created with donor sperm may provide the best combination for both success and peace of mind.
Ovarian tissue banking
Ovarian tissue banking is an option for patients whose cancer is aggressive and for whom a delay in treatment for hormonal stimulation is not possible, patients whose cancer is hormone-responsive, patients who do not wish to undergo hormonal intervention, and adolescents or Pre-pubertal girls.[40] Ovarian tissue is retrieved in an outpatient laparoscopy procedure. Strips of tissue from the outer cortex of the ovary, which contains mostly small, immature follicles, are isolated, frozen, and stored for future use. Ovarian tissue banking is available worldwide, but the major hurdle faced by the research community is what to do with the tissue once it has thawed. Two investigational options exist: ovarian tissue transplant[41–48] and in vitro follicle maturation,[49–58] with the former already achieving live human births. Both methods have theoretical and practical advantages and limitations, and it is very important that a patient who opts for tissue banking is aware of these issues.
Ovarian tissue transplantation
Major advances in human ovarian tissue transplantation have occurred in the past 3 years, initially with women who experienced premature ovarian failure but who were otherwise healthy,[41–47] and in women made sterile by cancer treatment. To date, all women with premature ovarian failure who underwent tissue transplant have had their endocrine function restored, though few have subsequently delivered healthy babies.[44–47] In cancer patients, approximately 30 autotransplantations have resulted in the birth of 12 children.[41–43] Despite this advance, success rates are still low; in one case report, a young breast cancer patient underwent ovarian tissue cryopreservation and subsequent transplantation of the tissue 6 years later.[59] After eight cycles of IVF, six with high-dose gonadotropin, a total of 20 oocytes were produced, of which two were fertilized. One normal embryo was transferred, but no pregnancy resulted. When it does work, the advantage of ovarian tissue transplant technology is the restoration of both endocrine and fertility function. In women with premature ovarian failure, undergoing ovarian tissue transplantation has led to spontaneous pregnancies without assisted reproductive technologies. The disadvantage of transplantation is that the ovarian tissue is leukocyte-rich and, in some types of cancer, there is a risk of reintroducing aggressive cancer cells. Ovarian tissue transplants also have a finite lifespan, which correlates to the amount of ovarian tissue inserted.[60] Additional work is needed, therefore, to improve transplant success rates, use methods that minimize the risk of cancer recurrence owing to the transplanted tissue, and maximize the likelihood of having a biological child.
In vitro follicle maturation
An alternative to tissue transplantation is in vitro maturation of ovarian follicles (Figure 3). Follicle growth and maturation is a complex process that requires communication between the oocyte and its surrounding somatic cells. Females are born with a finite number of primordial ovarian follicles (approximately 1 million follicles are present in the ovaries at birth) that contain a small, dormant oocyte and some somatic cells. This immature follicle pool represents the source of a female’s reproductive potential, the so-called ovarian reserve. These follicles remain in a dormant stage until a female reaches sexual maturity, at which point a number of follicles are selected with each menstrual cycle to begin the developmental process that leads to hormone sensitivity and production, and the ultimate release of a single, mature egg during ovulation. The scientific hurdle is to grow immature follicles and support the development of an egg in vitro that may be fertilized.
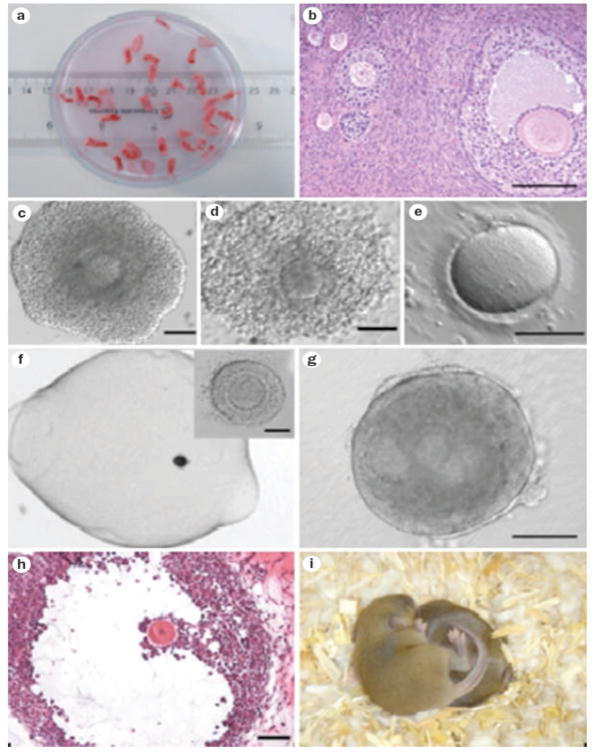
Figure 3.
In vitro follicle maturation. a | Before cancer treatment, the ovary is removed and the outer cortex is dissected into small pieces that can be cryopreserved easily and may be transplanted at a later date. b | The tissue pieces contain a variety of follicle stages depending on the patient age and fertility status prior to cancer diagnosis. c–e | Immature cumulus oocyte complexes can be aspirated from the tissue and matured in vitro; eggs in metaphase II can then be cryopreserved for use at a later date. f | Immature human follicles can be isolated and matured in vitro in a three-dimensional biomaterial matrix (~140 μm). g | After 15 days in culture, morphological hallmarks of a developing follicle can be observed, including the antrum and the asymmetrically located oocyte (~400 μm). h | After 30 days, cultured human follicles reach the antral stage (~1 mm). i | The birth of viable and fertile offspring have been achieved using oocytes from mouse follicles cultured in vitro.
In the past, impediments to in vitro maturation of ovarian follicles stemmed from the limitations of two-dimensional culture systems. Specifically, these systems cannot maintain the follicle architecture and interactions between the oocyte and somatic cells that are critical for follicle growth and oocyte maturation. Breakthroughs in the fields of materials science and reproductive biology have allowed the development of a three-dimensional culture system based on the biomaterial alginate, which mimics the natural ovarian structure in the late stages of follicle maturation. The system has been tested using follicles from mice,[7,52,56] rhesus monkeys,[57] and humans.[54,55,61] with this in vitro culture system, normal growth is fostered by adding soluble factors (insulin, trans ferrin, selenium, and fetuin) that regulate growth, and by maintaining necessary somatic–germ cell interactions in a physically supported environment. Follicles grow normally in the alginate system, and fully grown germinal vesicle-intact oocytes have been collected from mouse or primate follicles after 8 or 30 days in culture, respectively. Metaphase II-arrested mouse eggs have been produced by in vitro follicle maturation and have been fertilized in vitro. The resulting embryos have been transferred into foster mothers, with live births achieved from 20% of the transferred embryos.[56]
Studies of human follicles from an ovary removed laparoscopically before cancer treatment and grown in the alginate culture system, are underway to evaluate the efficacy of this technique for female cancer survivors. Currently, it is possible to grow healthy human follicles for 30 days in vitro.[55] The terminal meiotic maturation of the oocyte, fertilization, and transfer to a human recipient await development. The advantage of this system is that follicle growth is carried out entirely in vitro, providing a mature egg that can be fertilized. The embryo, which contains no somatic cells from the donor, can be transferred to the patient or to a gestational carrier without the risk of reintroducing cancer cells to the patient who has just survived her disease. However, this method does not restore the endocrine function of the patient, who will need endocrine management for the pregnancy and the duration of her life owing to premature ovarian failure secondary to cancer treatment.
Both ovarian tissue transplantation and in vitro follicle maturation remain highly experimental. However, removing an ovary is straightforward and may be the only chance for a patient to preserve her fertility before undergoing a potentially sterilizing treatment for a condition such as aggressive cancer. The length of time between cancer treatment and having a child will depend on the course of treatment. Immediately after cancer treatment, patients should be advised to take hormonal contraceptives to prevent pregnancy during recovery and to prevent symptoms associated with menopausal hormone levels, such as hot flashes, bone loss, and vaginal dryness. Ensuring the patient is well informed about the immediate steps she can take, as well as the limitations and expectations of the reproductive interventional methods, is essential to a successful outcome.
Educating patients and providers
One limitation to fertility preservation in young women with cancer is not related to science and technology, but rather to the beliefs and actions of the provider regarding fertility interventions. A disparity exists in provider attitudes about provision of fertility options to men versus women. In a survey of pediatric oncologists,[31] most respondents acknowledged that fertility threats to female patients are a major concern for them (83%) and agreed that all pubertal female cancer patients should be offered fertility consultation (73%). However, only 12% reported that they refer female cancer patients to a fertility specialist prior to cancer treatment >50% of the time, compared with 46% who refer male cancer patients.[31] The barriers to referrals for women include a lack of knowledge about fertility management options or referral centers, the concern that fertility preservation procedures take too much time, a bias towards cancer patients who are married, the notion that banking embryos is not appropriate for a cancer patient who might not survive her disease, and concerns about the ability of the patient who has survived the disease to utilize the banked embryos.[31] Note that all of these issues are based on provider bias and not on informed decision-making by the patient. Ensuring that providers are aware of the available options, as well as their limitations and new opportunities is, therefore, an important part of the work undertaken by the Oncofertility Consortium.
Finally, not all women will seek fertility options or opt for interventional methods, even when fully advised of the fertility risks. In a prospective trial involving 215 women of reproductive age with breast cancer, 63% were unwilling to undergo fertility preservation using hormonal stimulation owing to concerns about safety, the high cost of the procedure, and the required delay in cancer treatment to undergo stimulation.[62] All patients who choose not to pursue fertility preservation before treatment are counseled about the fertility risks and the range of post-treatment biological and nonbiological reproductive options that are available to them, including adoption.
Educating the public
Educating the next generation of scholars to participate in the multidisciplinary field of oncofertility is a fundamental mission of the Oncofertility Consortium program. However, education is not only important for training new scientists and clinicians, it is critically important to the development of educated patients and an educated public who can search for information and are more aware of their own health and welfare. The need to communicate the importance and relevance of scientific and medical research to the public has never been greater. Critical issues, including global warming, genetic testing, and stem cell research, affect us all on both personal and political levels. Yet studies show that the American public, although interested in science, maintains a low level of scientific literacy not only with regard to scientific facts, but also with regard to a clear understanding of the process by which scientific theories are tested, validated, and developed into tomorrow’s medical breakthroughs.[63] Furthermore, patients find it hard to make informed decisions if they are unfamiliar with mathematical principles, such as percentages and risk calculations, which are commonly used to describe scientific and medical research findings. Advances in reproductive science and medicine must, therefore, be communicated in a clear way.
To address the disparity between those who are fortunate enough to be informed of their fertility-sparing options and the remaining young cancer patient cohort, the Oncofertility Consortium program provides information that is authoritative and current. As an example, a patient-facing website has been developed by an interdisciplinary team of educators, communication specialists, patient advocates, clinicians, and basic scientists to provide an interactive, online platform to address patient questions.[64] The website features graphics, animations, and videos that describe and explain fertility preservation technologies available for cancer patients, and highlight patient experiences in grappling with these choices.
The Oncofertility Consortium has also developed an educational program that provides opportunities for high-school girls to work at an Oncofertility research site on weekends (Oncofertility Saturday Academy) or during the summer. This program offers hands-on experiences that engage young scholars in cutting-edge basic science research, clinical medicine, and bioethics. Additionally, by making students aware of the real issue of fertility preservation in cancer patients, the program creates a context for discussions of reproductive physiology, contraceptive biology, and sexuality. One-on-one interactions between the students and researchers in diverse fields, as well as the involvement of parents, the university, and the high school, provide the opportunity to build lasting relationships that support students when they are in high school and into the future. Educating high-school students in this way helps them to understand the role of science in society, and formulate their own opinions about research that is often presented as controversial. This trained cohort will eventually feed back into the system, leading to improved scientific literacy and more well-educated health-care consumers, and perhaps also creating the next generation of basic science and clinical researchers.
The humanities and social sciences
An essential facet of the Oncofertility Consortium is its ability to bring together an interdisciplinary pool of experts, including scholars from the humanities and social sciences, to examine the complex issues raised by developments in oncofertility.[65] Among these issues are the ethics of harvesting tissue from children and adolescents, the identification of the legal owner of the tissue, insurance and reimbursement for procedures, the discussion of fertility preservation with young adults, children and their parents, and the role of justice in a society where these procedures may be available only in certain locations and to those who can afford them.[5,8,32,33,67] Additionally, it is necessary to understand the views of various religious communities about both cancer treatment and fertility interventions when navigating individual patients through the decision-making process in fertility preservation. Infertility treatments have often been considered an enhancement rather than a necessary treatment that is covered by health-care insurance, which may impact who is able to pay for these therapies. we advocate for an insurance mandate to ensure that interventions to avoid sterility as a result of their impending cancer treatment are covered by insurers.[67] Finally, among the many cultural and societal issues highlighted by oncofertility is how to address the fact that women are delaying child bearing in many western countries, and who may not have achieved their ideal family size when diagnosed with cancer. Issues relating to feminism and women’s agency should, therefore, be addressed in their own reproductive schedule. Also, both men and women are having second families, and stored gametes inevitably become part of the social dialogue.
Metrics of success
The scientific community will be able to measure the success of the Oncofertility Consortium in several ways. First, conventional metrics such as the number of peer reviewed publications will reflect the quality and pace of the work achieved during the grant’s time frame, and will ensure that interdisciplinary science achieves the traditional goals of basic science and clinical research. Second, a greater understanding about the basic biology of the human follicle will allow the emergence of new reproductive technologies that can be translated into clinical applications. Third, the Oncofertility Consortium will be successful if it provides a means of gamete preservation to women at risk of losing their fertility. Finally, success will be measured by ensuring that new chemotherapeutics are tested for fertotoxicity prior to clinical use, and that efforts are focused on developing drugs that have fertoprotective properties. For example, alkylating agents such as cyclophosphamide, are particularly toxic to the primordial follicles in the ovary and to sperm. New formulations and delivery systems for chemotherapeutic agents are being developed to reduce gonado toxicity while retaining specificity for and efficacy against cancer cells. Furthermore, drugs that target cellular pathways that are not present in the oocyte or sperm cells should be safer to use in young men and women. Limiting damage to the ovary would reduce or eliminate the need for an oncofertility program, which would be a true mark of success. Ultimately, however, success will be measured by the number of young cancer patients who are provided with information at the time of their diagnosis about the potential effects of treatment on their fertility and the options available to preserve their fertility prior to initiating treatment, with the goal of helping these patients achieve successful pregnancies that fulfill their expectations of becoming both cancer survivors and parents. The work being done today is allowing the future fertility needs of disease-free cancer survivors to be met tomorrow.
Looking to the future
Despite the rapid growth of the Oncofertility Consortium, further work is needed to improve this initiative, which can be achieved through a greater understanding of follicle development, learning about the fertility risks of new cancer patients, and exploring the best ways to discuss fertility options at a time of extraordinary stress. The ultimate goal is to find noninterventional or medical ways to eliminate the risk of losing fertility in young people being treated for cancer. The development of chemo therapeutics that are targeted to individual cancers is necessary, in addition to symptom management strategies for cancer patients, finding ways to address sexual dysfunction, maintaining bone health, and meeting the unique psycho social needs of young, sterilized cancer survivors. Moreover, the treatment of other medically severe conditions can pose a fertility threat to young people; thus, it is necessary to be aware of the fertility preservation options for these patients. The financial aspects of fertility preservation must be considered, and participation in the health-care debate on the implementation of newly emerging technologies is essential. Finally, it is necessary to think in a global manner about resource allocation, surviving cancer, and the expectations of family. Much has been accomplished in the short time the Oncofertility Consortium has been funded, but much more remains to be achieved.
Conclusions
By the time the young cancer patient discussed at the beginning of this review was aware of the potential threat to her fertility, she was excluded from all traditional fertility options because her chemotherapy treatment needed to start immediately. However, she was able to undergo unilateral oophorectomy at an Oncofertility Consortium NPC site that offers ovarian tissue cryopreservation. After a short recovery, the family returned home and the patient received chemotherapy the following day. This young woman was provided with the best cancer care and the most advanced fertility preservation option the Oncofertility Consortium could offer, which is truly a measure of success. The clinical management team acted quickly and efficiently to handle this urgent case. The prognosis for the patient is good and the technology that is being developed under the auspices of the Oncofertility Consortium will hasten the ordinary pace of research and help ensure that when this patient is ready to start her family, the Consortium will be ready to help her. Without the collective work of the Oncofertility Consortium and unique mechanism of the NIH roadmap grant, this would not be possible.
Key points.
Advances in cancer diagnostics and therapeutics have increased the number of young cancer survivors; however, some life preserving cancer treatments can pose a threat to a patient’s fertility
The Oncofertility Consortium is a confederation of partners funded by an NIH Roadmap grant that is advancing fertility preservation options for young cancer patients
The NIH Roadmap Grant program provides a unique structure that supports the work of multidisciplinary teams to solve large, intractable problems in health care
The development of a National Physicians Cooperative brings together the specialties of oncology and reproductive medicine to more efficiently guide patients after a cancer diagnosis
Collaborations between biomaterials scientists, reproductive endocrinologists, and clinical researchers have led to breakthroughs that may improve and expand fertility preservation options for women
Cooperation with the social sciences, humanities, law, and education allows a comprehensive means of understanding and fulfilling the needs of patients wishing to preserve fertility after a cancer diagnosis
Acknowledgments
The Oncofertility Consortium is funded by a number of NIH Interdisciplinary Research Consortium grants: NIDCR 8UL1DE019587, NICHD 1RL1HD058293, NICHD 1RL1HD058294, NICHD 5RL1HD058295, NICHD 5RL1HD058296, NIBIB 5PL1EB008542, NCI 1PL1CA133835, NCI 5RL5CA133836, NCI 5TL1CA133837/5RL9CA133838, NCI 1KL1CA133839. The original in vitro follicle maturation studies were funded by a Specialized Cooperative Centers Program in Infertility Research (SCCPIR) NIH/NICHD U54HD041857. The author is grateful for the comments of an interdisciplinary team of readers (listed alphabetically: Lisa Campo Engelstein, Marla Clayman, Francesca Duncan, Shauna Gardino, Marybeth Gerrity, Sarah Rodriguez, Lonnie Shea, Candace Tingen and Min Xu). The author also thanks Stacey Tobin for assistance with language editing.
Footnotes
Review criteria
Information for this Review was compiled by searching the PubMed and MEDLINE databases using the terms “fertility preservation”, “fertility after cancer”, “oncofertility”, and “cancer”. English language articles published from 1971 to 2010 were evaluated. Additional literature discussed in the Review can be found on the Oncofertility Consortium website (http://oncofertility.northwestern.edu), and the Oncofertility Consortium patient information website (http://myoncofertility.org). Some data described was presented at the World Congress on Fertility Preservation, Brussels, Belgium, 10–12th December, 2009.
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn GP, et al. Discussion of fertility preservation with newly diagnosed patients: oncologists views. J Cancer Surviv. 2007;1:146–155. doi: 10.1007/s11764-007-0019-9. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal SK, Chang RJ. Fertility management for women with cancer. Cancer Treat Res. 2007;138:15–27. doi: 10.1007/978-0-387-72293-1_2. [DOI] [PubMed] [Google Scholar]
- 5.Snyder KA. Oncofertility and the social sciences. Cancer Treat Res. 2007;138:137–148. doi: 10.1007/978-0-387-72293-1_10. [DOI] [PubMed] [Google Scholar]
- 6.Dolin G, Roberts D, Rodriguez L, Woodruff T. Medical hope, legal pitfalls: potential legal issues in the emerging field of oncofertility. Santa Clara Law Rev. 2009;49:673–716. doi: 10.1007/978-1-4419-6518-9_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backhus LE, Zoloth L. Today’s research, tomorrows cures: the ethical implications of oncofertlity. Cancer Treat Res. 2007;138:163–179. doi: 10.1007/978-0-387-72293-1_12. [DOI] [PubMed] [Google Scholar]
- 8.Pauli SA, Berga SL, Shang W, Session DR. Current status of the approach to assisted reproduction. Pediatr Clin North Am. 2009;56:467–488. doi: 10.1016/j.pcl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Kelly SM, Buckett WM, Abdul Jalil AK, Tan SL. The cryobiology of assisted reproduction. Minerva Ginecol. 2003;55:389–398. [PubMed] [Google Scholar]
- 10.Chian RC, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16:608–610. doi: 10.1016/s1472-6483(10)60471-3. [DOI] [PubMed] [Google Scholar]
- 11.Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18:769–776. doi: 10.1016/s1472-6483(10)60025-9. [DOI] [PubMed] [Google Scholar]
- 12.Chian RC, et al. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009;91:2391–2398. doi: 10.1016/j.fertnstert.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Antinori M, et al. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod Biomed Online. 2007;14:72–79. doi: 10.1016/s1472-6483(10)60766-3. [DOI] [PubMed] [Google Scholar]
- 14.Cobo A, et al. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89:1657–1664. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 15.Society for Assisted Reproductive Technology; American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2000 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2004;81:1207–1220. doi: 10.1016/j.fertnstert.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Gunby J, Bissonnette F, Librach C, Cowan L. IvF Directors Group of the Canadian Fertility and Andrology Society. Assisted reproductive technologies in Canada: 2005 results from the Canadian Assisted Reproductive Technologies Register. Fertil Steril. 2009;91:1721–1730. doi: 10.1016/j.fertnstert.2008.02.125. [DOI] [PubMed] [Google Scholar]
- 17.Castilla JA, et al. Assisted reproductive technologies in public and private clinics. Reprod Biomed Online. 2009;19:872–878. doi: 10.1016/j.rbmo.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Yli Kuha AN, Gissler M, Luoto R, Hemminki E. Success of infertility treatments in Finland in the period 1992–2005. Eur J Obstet Gynecol Reprod Biol. 2009;144:54–58. doi: 10.1016/j.ejogrb.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Marks AR. Rescuing the NIH before it is too late. J Clin Invest. 2006;116:844. doi: 10.1172/JCI28364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley W, et al. The Clinical Research Forum and Association of American Physicians disagree with criticism of the NIH Roadmap. J Clin Invest. 2006;116:2058–2059. doi: 10.1172/JCI29557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutes of Health. The NIH Common Fund: About the NIH Roadmap. 2009 [online], http://nihroadmap.nih.gov/aboutroadmap.asp.
- 22.Delvecchio R. Panel looks at control of emissions. San Francisco Chronicle; Mar 22, 2007. [Google Scholar]
- 23.Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- 24.Klock SC, Zhang JX, Kazer RR. Fertility preservation of female cancer patients: early clinical experience. Fertil Steril. doi: 10.1016/j.fertnstert.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 25.West ER, et al. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53:289–295. doi: 10.1002/pbc.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clayman ML, Galvin KM, Arntson P. Shared decision making: fertility and pediatric cancers. Cancer Treat Res. 2007;138:149–160. doi: 10.1007/978-0-387-72293-1_11. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, et al. American Society of Clinical Oncoloy recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 28.Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–1628. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal R. Semen banking in patients with cancer: 20 year experience. Int J Androl. 2000;23 (Suppl 2):16–19. doi: 10.1046/j.1365-2605.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 30.Schover LR, Agarwal A, Thomas AJ., Jr Cryopreservation of gametes in young patients with cancer. J Pediatr Hematol Oncol. 1998;20:426–428. doi: 10.1097/00043426-199809000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Kohler TS, et al. Gender disparity in delivery of fertility preservation message to adolescents with cancer. Cancer. doi: 10.1007/s10815-010-9504-6. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fertile Hope. Sperm banking—Sharing Hope program for men. 2009 [online], http://www.fertilehope.org/financialassistance/spermbanking.cfm.
- 33.Schlatt S, Ehmcke J, Jahnukainen K. Testicular stem cells for fertility preservation: preclinical studies on male germ cell transplantation and testicular grafting. Pediatr Blood Cancer. 2009;53:274–280. doi: 10.1002/pbc.22002. [DOI] [PubMed] [Google Scholar]
- 34.Nayernia K, et al. RETRACTION—In vivo derivation of human sperm from embryonic stem cells. Stem Cells Dev. doi: 10.1089/scd.2009.0063. [DOI] [PubMed] [Google Scholar]
- 35.Oktay K, et al. Fertility preservation in breast cancer patients: IvF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18:90–95. doi: 10.1093/humrep/deg045. [DOI] [PubMed] [Google Scholar]
- 36.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 37.Huang JYJ, Buckett WM, Gilbert L, Tan SL, Chian RC. Retrieval of immature oocytes followed by in vitro maturation and vitrification: a case report on a new strategy of fertility preservation in women with borderline ovarian malignancy. Gynecol Oncol. 2007;105:542–544. doi: 10.1016/j.ygyno.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Rao GD, Chian RC, Son WS, Gilbert L, Tan SL. Fertility preservation in women undergoing cancer treatment. Lancet. 2004;363:1829–1830. doi: 10.1016/S0140-6736(04)16320-4. [DOI] [PubMed] [Google Scholar]
- 39.Gosden RG. Prospects for oocyte banking and in vitro maturation. J Natl Cancer Inst Monogr. 2005;34:60–63. doi: 10.1093/jncimonographs/lgi007. [DOI] [PubMed] [Google Scholar]
- 40.Gosden R. Gonadal tissue cryopreservation and transplantation. Reprod Biomed Online. 2002;4 (Suppl 1):64–67. doi: 10.1016/s1472-6483(12)60014-5. [DOI] [PubMed] [Google Scholar]
- 41.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 42.Donnez J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 43.Meirow D, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 44.Silber SJ, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- 45.Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med. 2007;356:1382–1384. doi: 10.1056/NEJMc066574. [DOI] [PubMed] [Google Scholar]
- 46.Silber SJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- 47.Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359:2617–2618. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- 48.Andersen Y, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 49.Cortvrindt R, Smitz J, van Steirteghem AC. In vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepubertal mice in a simplified culture system. Hum Reprod. 1996;11:2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 50.Eppig JJ, O’Brien MJ. Development in vitro of mice oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 52.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the threedimensional culture of granulosa cell oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 53.Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod. 1994;9:527–532. doi: 10.1093/oxfordjournals.humrep.a138539. [DOI] [PubMed] [Google Scholar]
- 54.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two step serum free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 55.Xu M, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue engineered follicles provide live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu M, et al. Encapsulated three dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang JYJ, Tulandi T, Holzer H, Tan SL, Chian RC. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89:567–572. doi: 10.1016/j.fertnstert.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 59.Oktay K, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 60.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 61.Amorim CA, van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human preantral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–99. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- 62.Azim AA, Costantini Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 63.Miller JD, Pardo R, Niwa F. Public Perceptions of Science and Technology: a Comparative Study of the European Union, the United States, Japan, and Canada. BBv Foundation Press; Madrid: 1997. [Google Scholar]
- 64.MyOncofertility.org. A patient education resource provided by the Oncofertility Consortium. 2009 [online], http://www.myoncofertility.org.
- 65.Woodruff TK, Campo Engelstein L, Rodriguez S, Zoloth L, editors. Oncofertility: Perspectives from the Humanities and Social Sciences. Springer; New York: 2010. [Google Scholar]
- 66.Zoloth L, Backhus L, Woodruff TK. Waiting to be born: the ethical implications of the generation “NUBorn” and “NUAge” mice from pre pubertal ovarian tissue. Am J Bioeth. 2008;8:21–29. doi: 10.1080/15265160802248203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campo Engelstein L. Consistency in insurance coverage for iatrogenic conditions resulting from cancer treatment including fertility preservation. J Clin Oncol. 2010;28:1284–1286. doi: 10.1200/JCO.2009.25.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]