Abstract
Background
Smoking behavior in industrialized nations has changed dramatically over the second half of the 20th century, with diverging patterns in male and female smoking rates.
Objective
We examined whether male-to-female incidence of MS changed concomitantly with smoking, as would be expected if smoking truly increased MS risk.
Methods
We identified relevant studies reporting male and female age-specific incidence of MS throughout the world using within-country birth cohorts as units of observation. We then correlated the male to female ratio of MS incidence in each birth cohort with the corresponding male to female ratios in smoking behavior obtained from national statistics. We also examined in depth the within-country trends of smoking and MS in Canada and Denmark, two populations in which statistics on MS are readily available.
Results
We show that, on the natural log scale, the gender ratio of MS is correlated with the gender ratio of smoking (r = 0.16 (95% CI: 0.06, 0.26; p = 0.002)). Additionally, we estimated an overall incidence rate ratio of 1.50 (95% CI: 1.17, 2.01) of MS for ever smokers as compared with never smokers. The trend in the gender ratio of smoking, however, is driven by a decline in smoking among men, rather than by an increase in women as observed for MS incidence.
Conclusion
Our results are consistent with the hypothesis that smoking increases the risk of MS and explains in part the divergence in MS incidence rates in men and women. Some other factor, however, must account for the increasing MS incidence among women.
Keywords: Multiple Sclerosis, smoking, sex ratio, epidemiology
INTRODUCTION
A marked increase in the female to male sex ratio of multiple sclerosis (MS) incidence has been reported in Canada, Denmark and other countries over the past 50 years. [1, 2] In Denmark, this change in the female to male ratio is driven by an increasing MS incidence in women rather than a decline in men [3], but trends in sex-specific MS incidence in other countries are less reliable. Nevertheless, this change suggests that environmental factors have an important influence on MS incidence and that many cases of MS could be preventable.
Cigarette smoking has been proposed as a risk factor for MS [4, 5] and a harmful effect has been reported repeatedly in prospective epidemiological studies.[4, 6–8] However, as with even the most carefully designed epidemiological study, confounding cannot be excluded as an explanation. In this analysis, we examined for a relationship between female to male sex ratio of smoking and MS in order to ascertain whether the relationship between smoking and MS could be real or is the result of confounding.
During the last century, opposing trends have developed in the smoking behavior in men and women [9–12], resulting in the consistent increase in the female to male ratio in prevalence of smoking over the last few decades in developed countries.[13] (Figure 1) In this analysis, we exploit this ‘natural experiment.’ During the 19th and early 20th centuries, social norms discouraged women from smoking. Later in the 20th century, particularly in industrialized countries, women have become increasingly active in society and the workspace, and smoking among women became common while smoking among men in the same countries declined.[9–12] Further, gender-specific smoking rates vary greatly across countries.[13] Here, we examine whether these variations in smoking between men and women are correlated to changes in rates of MS.
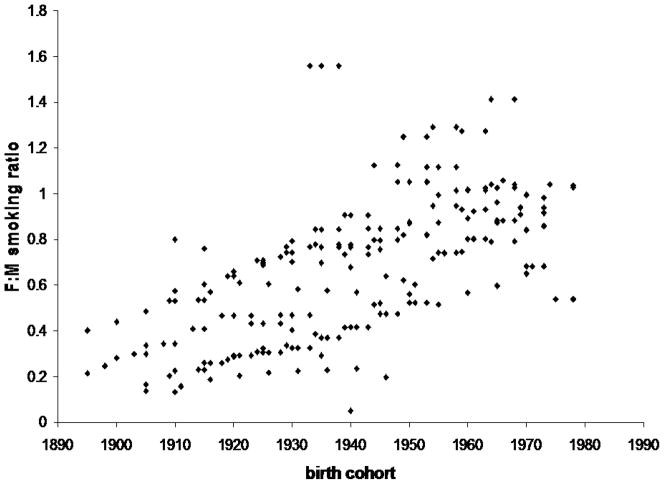
Figure 1.
Historical trend in prevalence of smoking among women relative to that in men.
| Ratio of Female to Male prevalence of smoking from 1890 to 2000
|
Estimated ratio of female to male prevalence of smoking from 1890 to 2000. Figure shows female to male smoking ratios for all the birth cohorts and countries included in this analysis [18]. Source: International Smoking Statistics. A collection of historical data from 30 economically developed countries. [13].
We have previously reported a strong association between the aforementioned trends in male to female smoking and opposite trends in male to female ratios of Parkinson’s Disease (PD). [14] In that work, we also validated our analytic approach by conducting a similar analysis for lung cancer, a disease whose association with smoking is clearly established.[15–17]
METHODS and MATERIALS
Cross-country birth cohort analysis
The methods used in this analysis closely resemble those used in our previously published work on Parkinson Disease (PD). [14] Briefly, we modeled the sex ratio in disease incidence in the following way:
| (eq 1) |
where sw and sm are the gender-specific prevalences of smoking in that population, and IRRsmk_w and IRRsmk_m are the gender-specific incidence rate ratios associated with smoking, that is, the ratios between the incidence rates of the disease in smokers and those in non-smokers, and is the male to female ratio of disease in never smokers (see online appendix for full derivation). These ratios can also be written as (1+α w) and (1+αm), where α w and αm are the proportional increases in the incidence rate of MS caused by smoking in women and men. For example, if smoking increase MS incidence rate in women by 50%, then α w =0.5, and IRRsmk_w =1.5 = 1+0.5.
Replacing IRRsmk_w and IRRsmk_m with (1+αw) and (1+α m), equation 1 simplifies to:
| (eq 2) |
Further, to jointly estimate α and d0w/d0m we made the assumption that αm = αw = α. If the value of (1+α) is assumed to be constant across time and space and the same for men and women, the female to male ratio of disease incidence ( ) can be written as:
| (eq 3) |
Assuming that is also constant, the equation above simplifies to:
| (eq 4) |
Because the distribution of was closest to a log-normal distribution, analyses were performed transforming the disease incidence rate ratios to the natural log scale.
In the first step of our analysis, we calculated the correlation coefficient between the natural log of the smoking-based predicted sex ratio, , and the natural log of the observed sex ratio of MS. We then estimated the regression equation relating the natural log of the smoking-based predicted sex ratio, , to the natural log of the observed sex ratio of MS.
In a sensitivity analysis, we repeated the correlation calculations above using as weight the total number of cases in each corresponding study. Because the studies included in this manuscript were heterogenous by country, in a further sensitivity analysis, we used a generalized linear model (SAS 9.2 MIXED procedure) to estimate the correlation, using data points from each distinct country as repeated measures. To estimate the correlation coefficient in this model, the regression was fit to standardized predicted and observed ratios (standardized to have mean = 0 and standard deviation = 1), allowing the resulting regression coefficients to be interpreted as correlation coefficients.
Subsequently, we directly estimated the excess risk of MS associated with smoking, α, using the minimum sum of errors squared. [10] Allowing a to vary from −1 to 5 in intervals of 0.1 [as α is the IRR -1, it cannot take on values less than −1] and the female to male ratio of MS among never smokers, , to vary from 0 to 5 in intervals of 0.1 [the IRR cannot take on values less than 0], we calculated the sum of errors squared associated with each pair of α and . This method allowed us to directly estimate the values of α and . The analyses and visualization for the multi-country analyses were done with MATLAB version 7.6.
In this analysis, we included manuscripts that were selected for a published analysis of temporal trends and MS, all of which report age and gender-specific incidence of MS and were identified with a comprehensive search of Medline and Embase of manuscripts published between 1966 and February 2007.[18] Most studies contributed information on more than one birth cohort, so that the total number of data points included in the analyses was 374.
Data on smoking was obtained in a manner analogous to our previous work on PD and smoking [14]. Briefly, for each 5-years birth cohort, the prevalence of smoking was obtained from an appendix of ‘International Smoking Statistics’ [13] which reports, for selected countries, the prevalence of smoking by 5-years age groups during 5-year calendar intervals, from 1956–60 to 1991–95. For example, the prevalence of smoking among men born in 1931–36 was estimated using smoking data obtained in 1956–60 for their smoking behavior at age 25–29 years, data obtained in 1961–65 for their smoking behavior at ages 30–34, and so forth. Because we were only interested in the proportion of ever smokers, we attributed to each birth cohort the highest prevalence of smoking recorded at any age, usually corresponding to 20–24 or 25–29 years of age, except for the 1976 birth cohort, for which only smoking prevalence at ages 15–19 was available.
Within-country analyses, Canada and Denmark
In an additional analysis, we examined in depth the within-country trends of smoking and MS for two populations: Canada and Denmark. We chose to focus on Canada because of the recently published data on female to male ratio in MS incidence, [1] and on Denmark, because of the existence of a nation-wide MS registry since the 1940’s.[3, 19] Data from Denmark were provided by the Danish MS Registry.[19]
In the within country analyses for Canada and Denmark, we relaxed one of our key assumptions, allowing the incidence rate ratio in smokers [expressed as (1+ α) above] to differ with gender. We used equation 2 to model the female to male ratio of MS in these countries.
| (eq 2) |
While in the multi-country analyses discussed above (equation 1) we assumed that the incidence rate ratio of MS associated with smoking is equal in men and in women (this allowed us to jointly estimate α and d0w/dm), equation 2 does not make this assumption and allows the relative risk associated with MS to vary with gender.
For both the Canada and the Denmark datasets, we used equation 2 to calculate the female to male MS ratios that would be expected if smoking increased MS risk. These expected ratios were then compared to those observed by using Pearson’s correlation coefficients and by calculating the change over time in the expected ratio as a percent of the observed change. To calculate the expected ratios, we used the IRR of MS estimated in our multi-country analyses (assumption 1). We also conducted sensitivity analyses assuming a IRR of 2.7 in men and 1.6 in women, based on the only published source that provided sex-specific IRR estimates (assumption 2). The MS female to male ratios among never smokers were assumed to be constant over birth cohorts and were arbitrarily set at the values that resulted in the computed female to male ratios being equal to the observed ratios for the 1931–36 birth cohort in each country. This ratio varied with the assumption of IRR above and was higher in Canada (2.16 and 3.64 under assumptions 1 and 2 respectively) than in Denmark (1.62 and 2.64 under assumptions 1 and 2 respectively), a difference that suggests that factors other than smoking also contribute to determine the female to male ratio, although incomplete inclusion of male cases in the Canadian database, which is not based on a complete national registry, cannot be excluded. Most importantly, assigning different values to this ratio would not affect the correlation between the expected and observed female to male ratios nor the estimation of the percent of the change in this ratio that is attributable to changes in smoking behavior.
RESULTS
Cross-country birth cohort analysis
The diverging smoking trends in men and women by birth cohort (real smoking prevalence data used in analyses presented in this manuscript) are shown in Figure 1.
Based on prior studies, we assumed that smoking increases the risk of MS by 50% in men and women (α = 0.5, IRR = 1.5). We found a correlation of 0.16 (95% CI: 0.06, 0.26; p = 0.002) between the natural log of the smoking based predictor (see equation 1 above), , and the natural log of observed ratio of MS incidence, , which is consistent with a hypothesis that smoking increases MS risk.
As part of a sensitivity analysis, we repeated the correlation analysis using as weight the number of cases on which each of the MS incidence data points is based. In some data points (168 of the 375 datapoints considered), the total number of cases was missing. For these datapoints, we used as the weight the median of the number of cases of all the other datapoints. The correlation coefficient was 0.30 (95% CI: 0.20, 0.39), p <0.0001) in the weighted analysis. When, in addition to the number of cases, we took into account the number of observations in each country, the correlation changed to 0.30 (95% CI: 0.21, 0.38; p <0.0001).
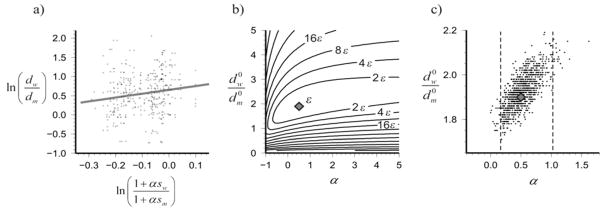
Using simple linear regression, we estimated the following regression equation to describe the relationship between the actual and predicated rates of MS (Figure 2a):
Figure 2.
Comparison of the rates of MS to those predicted from smoking rates: a) Correlations of the logarithms of dw/dm ratios and their estimators, ln (1 + αsw)/(1 + αsm), with α = 0.5 (dots). Line shows the linear dependence . b) Optimization for and α. The diamond at the center represents the best-fit point with , α = 0.5 (corresponding to IRR = 1. 5), and squared data misfit ε. Contours indicate the data misfits for other combinations of model parameters. c) Results of 1000 bootstrap resamplings with replacement of the original dataset. The diamond represents the best-fit estimate as in plot b). 95% of bootstrapped (α, ) values fall within the range of α = 0.17 to 1.01 (dashed lines).
In a sensitivity analysis, accounting for country of study as multiple observations, we estimated the following regression equation to describe the relationship between the actual and predicated rates of MS:
In estimating the excess risk of MS, α, directly from our data by minimizing the sum of sum of errors squared, we found that smoking was associated with a 50% increased risk of MS (α = 0.5, IRR = 1.50) in men and women. As discussed above, this analysis was done under the assumption that the increase in risk is equal for men and women. Figure 2b shows the minimum sum of errors squared (center diamond), with the contours representing each subsequent two-fold increase in the sum of errors squared. Figure 2c shows the results of a bootstrapping procedure; the estimation in Figure 2b. was repeated 1000 times by sampling with replacement from the original dataset. From minimizing the sum of errors squared we conclude that the optimum α is 0.5 and from bootstrapping procedure, we estimate the 95% confidence interval around α as (0.17, 1.01), translating into an incidence rate ratio (1+α) of 1.5 (95% CI: 1.17, 2.01).
Within-country analyses, Canada and Denmark
The female to male ratios in smoking prevalence in Canada and Denmark are shown in Table 1.
Table 1.
Percent smoking in women, men, and their ratio in Canada and Denmark by birth cohort.
| Birth Cohort | % ever smokers | ||
|---|---|---|---|
| Canada | Female | Male | F:M Ratio |
| 1931–35 | 42.50* | 82.60 | 0.51 |
| 1936–40 | 42.20 | 81.50 | 0.52 |
| 1941–45 | 42.60 | 62.40 | 0.68 |
| 1946–50 | 42.50 | 57.20 | 0.74 |
| 1951–55 | 41.60 | 54.10 | 0.77 |
| 1956–60 | 46.40 | 50.70 | 0.92 |
| 1961–65 | 41.60 | 40.70 | 1.02 |
| 1966–70 | 35.20 | 35.80 | 0.98 |
| 1971–75 | 30.10 | 31.60 | 0.95 |
| 1976–80 | 20.90** | 19.60** | 1.07 |
| Denmark | |||
| 1931–35 | 64.20 | 81.00 | 0.79 |
| 1936–40 | 57.90 | 75.50 | 0.77 |
| 1941–45 | 56.50 | 72.70 | 0.78 |
| 1946–50 | 55.60 | 69.90 | 0.80 |
| 1951–55 | 56.20 | 64.60 | 0.87 |
| 1956–60 | 54.70 | 55.00 | 0.99 |
| 1961–65 | 50.20 | 49.30 | 1.02 |
| 1966–70 | 45.30 | 47.10 | 0.96 |
| 1971–75 | 41.80 | 42.00 | 1.00 |
| 1976–80 | 25.80** | 24.10** | 1.07 |
Unless noted, prevalence of smoking was for ages 20–25 or 25–29.
Prevalence of smoking at age 30–34
Prevalence of smoking at age 15–19
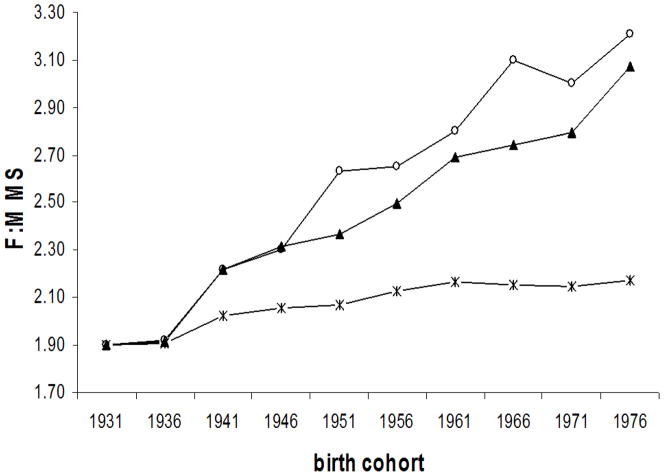
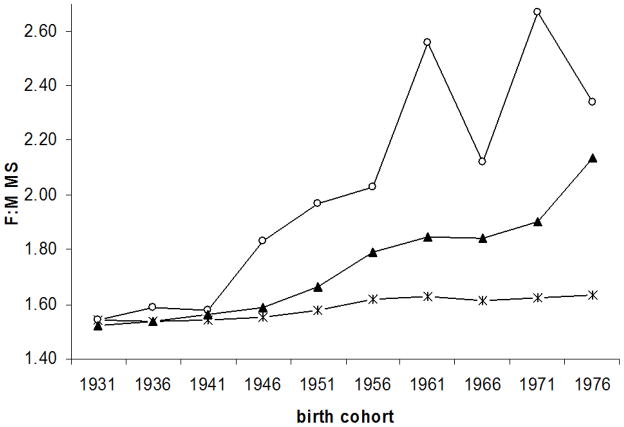
The Pearson correlation coefficients between the female to male ratio in smoking prevalence and the corresponding ratio in MS incidence was 0.94 (p <0.0001) in Canada, and 0.90 (p = 0.0004) in Denmark. The female to male ratio in MS incidence expected under the hypothesis of a causal effect of smoking increased from 1.90 to 2.17 in Canada, and from 1.54 to 1.63 in Denmark (figure 3). The percent increase in the observed female to male ratio of MS potentially explained by smoking was 21% (1.58% per 5-year expected over 7.66% observed) in Canada, and 14% in Denmark (0.67% over 4.81%).
Figure 3.
Observed (
 ) and expected [under assumption 11: (
) and expected [under assumption 11: (
 ) and under assumption 22(▴) female to male MS incidence by birth cohort.
) and under assumption 22(▴) female to male MS incidence by birth cohort.
| A. Canada
|
|
|
| B. Denmark
|
Assumptions:
1: In both males and females, the IRR of MS in smokers compared to non-smokers =1.4
2: In females, the IRR of MS in smokers compared to non-smokers =1.6 In males, the IRR of MS in smokers compared to non-smokers = 2.7 [24]
A) Canada:
Under assumption 1 above, rate of change in observed MS: 7.66% per 5 year period; Rate of change in expected MS: 1.58% per 5 year period; Percent of F:M MS ratio potentially explained by smoking: 20.6%
Under assumption 2 above, rate of change in observed MS: 7.66% per 5 year period; Rate of change in expected MS: 6.86% per 5 year period; Percent of F:M MS ratio potentially explained by smoking: 89.6%
B) Denmark:
Under assumption 1 above, rate of change in observed MS: 4.8 % per 5 year period; Rate of change in expected MS: 0.67% per 5 year period; Percent of F:M MS ratio potentially explained by smoking by birth cohort: 14.0%
Under assumption 2 above, rate of change in observed MS: 4.8 % per 5 year period; Rate of change in expected MS: 4.5% per 5 year period; Percent of F:M MS ratio potentially explained by smoking by birth cohort: 94%
These results were very sensitive to the strength of the association between smoking and MS among men. In particular, the % of the female to male change in MS risk potentially explained by smoking increases monotonically with increasing values of the IRR associated with smoking in men -- if the IRR associated with smoking was assumed to be 2.7 and 1.6 in men and women respectively, smoking would potentially explain as much as 90 % (6.86% per 5-year expected over 7.66% observed) of the increase in the female to male ratio of MS in Canada and 94% of the increase Denmark (4.5% per 5-year expected over 4.81% observed).
DISCUSSION
In this study, we show a significant association between the ratios of smoking prevalence in women and men in different countries and birth cohorts and the corresponding ratios in MS incidence. Based on these data, we estimate that smoking is associated with a 40% average increase in risk of MS.
Our previous publication on smoking and Parkinson’s disease trends applies this approach to PD and also presented a validation of this approach by showing that smoking, when modeled in this way, is strongly predictive of lung cancer, a disease for which the causal association with smoking is widely accepted.
A positive association between smoking and Multiple Sclerosis has been found in all of the prospective studies that have addressed this question. In the Oxford Family Planning Association Study [6] and the Royal College of General Practitioners’ Oral Contraception Study [7], women who smoked over 15 cigarettes per day were, respectively, at an 80% and a 40% increased risk of MS compared to never smokers. In the Nurses Health Study, and the Nurses Health Study II, the RR for women smoking 25 pack years or more compared with never smokers was 1.7 (95% CI, 1.2–2.4; p<0.01).[4] In a nested case-control study within the General Practice Research Database, smokers had a 30% increased risk of MS compared to never smokers (95% CI, 1.0–1.7). Our study, although ecological in nature, offers a unique and complementary approach to supplement these longitudinal studies: by examining trends in the female to male ratios of MS and smoking we are able to address the possible confounding issues that may have been present in the longitudinal analysis. The problem of confounding is addressed because we are examining the sex RATIO: if one assumes that whatever confounding factor affects men and women to a similar extent on a multiplicative scale, it is eliminated by dividing female by male rates of MS and of smoking. Thus, taken in the context of prospective epidemiologic studies, this work provides an important addition to the evidence in favor of a harmful effect of smoking on risk of MS.
Possible biological explanations of how smoking may raise the risk of MS include possible neurotoxic [20] effects of the constituents of cigarette smoke, as well as its’ immunomodulatory effects [21], as suggested by the increased risk of other autoimmune diseases, (rheumatoid arthritis, lupus, Graves’ disease and cirrhosis) [22] among smokers.
The assumptions inherent in our method are discussed in detail in our manuscript on PD and smoking trends.[14] Briefly, two important assumptions are required for the validity of this approach: 1) that the probability of being recruited into each study included in the analyses is independent from gender, so that the ratios of MS incidence in men and women in these investigations approximate the same ratios in the populations from which the cases were drawn; and 2) that the gender ratio of MS incidence in these groups will not systematically deviate from the ratio among all individuals belonging to the same birth cohort in the same country.
Additionally, in our in-depth analysis of Canadian and Danish nationwide MS registries and the corresponding national statistics on smoking behavior, we found that the female to male ratio in smoking prevalence increased in parallel and is strongly correlated with the female to male ratio in MS incidence in both countries. The stronger correlation in within-country analyses as compared to between-country analyses suggests that factors other than smoking contribute to the international variations in the gender ratio in MS frequency.
If smoking increased MS risk, as consistently suggested by the results of multiple longitudinal studies [5], a substantial proportion of the increase in the female to male ratio of MS could be explained by differential changes over time in the smoking behaviors of men and women. Although the available data do not allow detailed analyses based on duration and intensity of smoking, the smoking statistics used here provide a reasonable approximation of the average prevalence of smoking in early adult life for each birth cohort.
It remains possible that other factors associated with both smoking and MS explain both the results of longitudinal studies and the ecological correlations reported here. For example, over the period of time covered in this analysis, there may have been a reduction in exposure to UV light, which is the primary source of vitamin D, because of increasing awareness of its possible relation with skin cancer. If such a decrease has affected women more than men, and if high vitamin D levels, as suggested in previous studies, decrease MS risk, an increase in the female to male ratio in MS could be expected.[5] Other changes that have occurred among women over the period of interest could also contribute, including increasing participation in the work force, increased use of oral contraceptives, changes in sexual behavior, lower parity and increased age at first birth, although there is no clear association between any of these factors and MS risk in longitudinal studies. [5, 23]
In summary, in this investigation based on the analysis international trends in smoking behavior and MS incidence, we found a significant correlation between changing smoking patterns in men and women and the increasing female to male ratio in MS incidence. This result is consistent with the hypothesis that smoking not only increases MS risk [4, 5], but is also one of the factors driving the divergence between the female and male incidence of MS. Changes in smoking behavior, however, cannot explain the recent trend of increasing MS incidence in women.
Acknowledgments
Grants and Financial Support
Dr. Ascherio is the recipient of grants from the U.S. National Institutes of Health / National Institute of Neurological Diseases and Stroke for the investigation of Multiple Sclerosis. Natalia Palacios, at the inception of this project was supported by the Training Program in Environmental Epidemiology funded under grant no. T32 ES07069 and is currently supported under NIH Career Development K01 Award 1K01ES019183-01.
The Danish Multiple Sclerosis Society finances the Danish Multiple Sclerosis Registry.
Thank – you’s
The authors would like to thank Leslie Unger for administrative support and Eilis O’Reilly for statistical support. We would also like to thank the anonymous reviewers of this manuscript for their insightful comments.
Footnotes
HUMAN SUBJECTS
No human subjects were involved in this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orton SM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5(11):932–6. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 2.Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention. Semin Neurol. 2008;28(1):17–28. doi: 10.1055/s-2007-1019126. [DOI] [PubMed] [Google Scholar]
- 3.Koch-Henriksen N. The Danish Multiple Sclerosis Registry: a 50-year follow-up. Mult Scler. 1999;5(4):293–6. doi: 10.1177/135245859900500418. [DOI] [PubMed] [Google Scholar]
- 4.Hernán MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol. 2001;154(1):69–74. doi: 10.1093/aje/154.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61(6):504–13. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 6.Villard-Mackintosh L, Vessey MP. Oral contraceptives and reproductive factors in multiple sclerosis incidence. Contraception. 1993;47:161–168. doi: 10.1016/0010-7824(93)90088-o. [DOI] [PubMed] [Google Scholar]
- 7.Thorogood M, Hannaford PC. The influence of oral contraceptives on the risk of mulitple sclerosis. Br J Obstet Gynaecol. 1998;105:1296–1299. doi: 10.1111/j.1471-0528.1998.tb10008.x. [DOI] [PubMed] [Google Scholar]
- 8.Hernan MA, et al. Cigarette smoking and the progression of multiple sclerosis. Brain. 2005;128(Pt 6):1461–5. doi: 10.1093/brain/awh471. [DOI] [PubMed] [Google Scholar]
- 9.Birkett NJ. Trends in smoking by birth cohort for births between 1940 and 1975: a reconstructed cohort analysis of the 1990 Ontario Health Survey. Prev Med. 1997;26(4):534–41. doi: 10.1006/pmed.1997.0169. [DOI] [PubMed] [Google Scholar]
- 10.Osler M, et al. Trends in smoking prevalence in Danish adults, 1964–1994. The influence of gender, age, and education. Scand J Soc Med. 1998;26(4):293–8. doi: 10.1177/14034948980260041101. [DOI] [PubMed] [Google Scholar]
- 11.Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: the influence of gender and education. Am J Public Health. 1996;86(2):231–6. doi: 10.2105/ajph.86.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronneberg A, Lund KE, Hafstad A. Lifetime smoking habits among Norwegian men and women born between 1890 and 1974. Int J Epidemiol. 1994;23(2):267–76. doi: 10.1093/ije/23.2.267. [DOI] [PubMed] [Google Scholar]
- 13.Forey B, Lee P. Estimation of sex-specific smoking statistics by standardized age groups and time periods. In: Forey B, et al., editors. International Smoking Statistics. A collection of historical data from 30 economically developed countries. Wolfson Institute of Preventative Medicine and Oxford University Press; London and Oxford: 2002. pp. 1–201. [Google Scholar]
- 14.Morozova N, O’Reilly EJ, Ascherio A. Variations in gender ratios support the connection between smoking and Parkinson’s disease. Mov Disord. 2008;23(10):1414–9. doi: 10.1002/mds.22045. [DOI] [PubMed] [Google Scholar]
- 15.Doll R, et al. Smoking and Dementia in male British doctors: prospective study. BMJ. 2000;320:1097–1102. doi: 10.1136/bmj.320.7242.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. Jama. 2005;294(12):1505–10. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BE. Tobacco and lung cancer. Prim Care. 1998;25(2):279–91. doi: 10.1016/s0095-4543(05)70064-6. [DOI] [PubMed] [Google Scholar]
- 18.Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: A systematic review. Neurology. 2008;71(2):129–135. doi: 10.1212/01.wnl.0000316802.35974.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch-Henriksen N, et al. The Danish Multiple Sclerosis Registry. History, data collection and validity. Dan Med Bull. 2001;48(2):91–4. [PubMed] [Google Scholar]
- 20.Smith ADM, Duckett S, Waters AH. Neuropathological changes in chronic cyanide intoxication. Nature. 1963;200:179–81. doi: 10.1038/200179a0. [DOI] [PubMed] [Google Scholar]
- 21.Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83(1–2):148–56. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- 22.Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15(11):737–45. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 23.Hernán MA, et al. Oral contraceptives and the incidence of multiple sclerosis. Neurology. 2000;55(6):848–854. doi: 10.1212/wnl.55.6.848. [DOI] [PubMed] [Google Scholar]
- 24.Riise T, Nortvedt MW, Ascherio A. Smoking is a risk factor for multiple sclerosis. Neurology. 2003;61(8):1122–4. doi: 10.1212/01.wnl.0000081305.66687.d2. [DOI] [PubMed] [Google Scholar]