Abstract
This study assessed the role of aryl hydrocarbon receptor (AHR) affinity, and cytochrome P4501A (CYP1A) protein and activity in polyaromatic hydrocarbon (PAH)-induced oxidative stress. In the 1–100 nM concentration range benzo[a]pyrene (BaP) but not benzo[e]pyrene (BeP) competitively displaced 2 nM [3H]2, 3, 7, 8-tetrachloro-dibenzo-p-dioxin from rainbow trout AHR2α. Based on appearance of fluorescent aromatic compounds in bile over 3, 7, 14, 28 or 50 days of feeding 3 μg of BaP or BeP/g fish/day, rainbow trout liver readily excreted these polyaromatic hydrocarbons (PAHs) and their metabolites at near steady state rates. CYP1A proteins catalyzed more than 98% of ethoxyresorufin-O-deethylase (EROD) activity in rainbow trout hepatic microsomes. EROD activity of hepatic microsomes initially increased and then decreased to control activities after 50 days of feeding both PAHs. Immunohistochemistry of liver confirmed CYP1A protein increased in fish fed both PAHs after 3 days and remained elevated for up to 28 days. Neither BaP nor BeP increased hepatic DNA adduct concentrations at any time up to 50 days of feeding these PAHs. Comet assays of blood cells demonstrated marked DNA damage after 14 days of feeding both PAHs that was not significant after 50 days. There was a strong positive correlation between hepatic EROD activity and DNA damage in blood cells over time for both PAHs. Neither CYP1A protein nor 3-nitrotyrosine (a biomarker for oxidative stress) immunostaining in trunk kidney were significantly altered by BaP or BeP after 3, 7, 14, or 28 days. There was no clear association between AHR2α affinity and BaP and BeP-induced oxidative stress.
Keywords: cytochrome P4501A activity, oxidative stress, benzo[a]pyrene, benzo[e]pyrene, aryl hydrocarbon receptor, biliary excretion, fish
INTRODUCTION
Environmental polycyclic aromatic hydrocarbon (PAH) contamination persists as a public health and environmental quality concern (van Metre et al, 2000; Zhang and Tao, 2009). Natural PAH sources include volcanic activity and wildfires. The current major environmental PAH source is incomplete combustion of carbonaceous materials by humans for energy and other industrial processes. Petroleum losses during extraction, transportation, and refining are another significant source of PAH contamination. Regardless of source, environmental PAH contamination exits as complex mixtures.
The toxicity of PAHs is a continuing subject of intense investigation (Incardona et al, 2006; Nebert et al, 2004; Park et al., 2009). Metabolic activation of PAHs to reactive intermediates and aryl hydrocarbon receptor (AHR)-dependent alterations in gene expression are prominent modes of toxic action. Xue and Warshawsky (2005) describe three principal metabolic pathways that yield reactive PAH intermediates. (1) Electrophilic diol-epoxides result from sequential PAH oxidation by cytochrome P-450 (CYP) enzymes, hydrolysis of the resulting arene oxides by microsomal epoxide hydrolase, and a second CYP-catalyzed oxidation. (2) One electron oxidation of PAHs by CYP peroxidase yields the radical anion. (3) PAH-o-quinones result from sequential actions of CYP1A1, epoxide hydrolase, dihydrodiol dehydrogenases, and tautomerization between the ketol and a catechol. Reduction of the catechol produces an o-semiquinone anion radical which undergoes further reduction to the o-quinone. The first two pathways yield electrophiles that may covalently bind DNA and lead to cancer. The PAH-o-quinone pathway releases reactive oxygen species (ROS) that can produce oxidative stress. Planar xenobiotics including many PAHs bind cytosolic AHR with varying affinity, induce translocation of a complex to the nucleus, and activate ligand-dependent signaling which alters gene expression (Oesch-Barlomowicz and Oesch, 2009). An endogenous pathway involving cAMP appears to activate cytosolic AHR for nuclear translocation and mediate gene expression for normal physiological events. Induction of CYP1A mRNA and protein is perhaps the best indicator of AHR-dependent gene activation by planar xenobiotics. Persistent AHR activation by such xenobiotics perhaps mediates various toxicological events in tissues including liver, vascular and immune systems. Application of morpholino knockdown technology blocks elevation of CYP1A protein after PAH treatment of zebrafish (Incardona et al.) Despite this, benz [a] anthracene produces cardiac defects in zebrafish embryos in an AHR2-dependent. This suggests interactions with genes other than those coding for CYP1As underlie this toxicity.
The primary objective of the work herein is to compare and contrast biomarkers for oxidative stress and AHR activation (CYP1A protein and enzymatic activity) after subchronic benzo [a] pyrene (BaP) and benzo [e] pyrene (BeP) treatments of rainbow trout. Purification and reconstitution of CYP isoforms from hepatic microsomes of β-naphthoflavone treated rainbow trout demonstrates CYP1A proteins catalyze more than 98% of ethoxyresorufin-O-deethylase (EROD) activity (Williams and Buhler, 1983). BaP and BeP are both five-ring, unsubstituted PAHs with very similar physical and chemical properties (Jonsson et al., 2007). Both isomers are highly lipophilic with nearly equivalent molecular areas and volumes. However, BaP is a several hundred-fold more potent AHR agonist than BeP (Machala et al, 2001).
METHODS
Receptor binding
Competition of BaP and BeP with [3H]2,3,7,8-tetrachlorodibenzo-p-dioxin ([3H]TCDD) for binding to a trout AHR2α was measured by velocity sedimentation in sucrose gradients in a vertical tube rotor (Tsui and Okey 1981) as described earlier (Billiard et al. 2002; Karchner et al. 1999). DMSO (Sigma-Aldrich, Milwaukee, WI) solutions that contained 10 μM of BaP and BeP were prepared for the AHR2α binding assay. Rainbow trout AHR2α (Abnet et al. 1999) was expressed by in vitro transcription and translation, diluted 1:1 in MEDMG buffer, and incubated with 2nM [3H]TCDD in DMSO, BaP or BeP (1, 10 or 100 nM final concentration) overnight on ice. The incubations were then layered on sucrose gradients, centrifuged, and fractionated as described (Karcher et al. 1999). UPL (unprogrammed TNT lysate) was used as a control for non-specific binding.
Animal Treatments
Juvenile (10–15g at the beginning of treatments) rainbow trout (Oncorhynchus mykiss) of the Shasta strain were held in flowing fresh water (10 °C, pH 7). Fish were fed a commercial diet (Trout diet #4, Ranger Inc. Angleton, TX) at 2% of their body weight per day. Diets contained 160 parts per million (ppm) of BaP or BeP or no detectable PAHs. This yielded 0 PAH (undetectable), 3 μg BeP or BaP/g fish/day, a PAH concentration near the middle of the range for stomach contents of PAH mixtures in salmonids at a contaminated site (Bravo et al, 2011) There were 90 fish in each treament group. Methods for diet preparation and PAH analysis, and other details on rearing conditions were published previously (Bravo et al., 2011). Fish were euthanized in 250 mg tricane methane sulphonate (MS-222) (Sigma-Aldrich, St Louis, MO)/L water after 3, 7, 14, 28 or 50 days of dietary treatments for biomaker analyses. Blood was collected by severing the caudal vein and filling a capillary tube. The liver, gallbladder and trunk kidney were excised with scissors and transferred to ice-cold buffer or formalin fixed immediately. Not all biomarkers were examined at all times points (detailed below).
Biomarkers of exposure
Three biomarkers for PAH exposure were measured with composite samples or individual fish fed either 3 μg of BeP or BaP/g fish/ day or control diet. Fluorescent aromatic compounds [FACs] in gallbladder bile were measured by high pressure liquid chromatography with fluorescene detection (Krahn et al., 1993). BaP and phenanthrene served as external standards. EROD activity in hepatic microsomes (n=3, with livers from 4 fish composited per sample) was determined at all time-points. Rainbow trout hepatic microsomes were prepared and stored until used as described by Curtis et al. (1995). EROD activities of these preparations were determined by a modified kinetic assay (Eggens and Galgani, 1992). Protein was measured by the Lowry et al. (1951) method. CYP1A immunohistochemistry in liver and trunk kidney tissue (n=3–5 individual fish per treatment) was conducted after 3, 7, 14 and 28 days of PAH treatments; tissues were paraffin embedded (Husoy et al., 1994). Immunohistochemical staining employed an indirect peroxidase method (Myers et al., 1996), Mab 1-12-3 primary antibody, and a biotinylated goat antimouse IgG secondary antibody and the chromagen was 3, 3- diaminobenzidine tetrahydrochloride (Hyyti et al., 2001). Relative staining compared PAH-fed and control diet fed fish liver and trunk kidney on the following scales for intensity; 0=absent, 1=minimal, 2=minimal-mild, 3=mild, 4=mild-moderate, 5=moderate, 6=moderate-intense, and 7=intense; and distribution where 1=focal, 3=multifocal, and 5=diffuse.
Biomarkers of Toxicity
Three biomarkers of PAH toxicity were measured: one each in three different tissues (liver, blood and trunk kidney). DNA adduct were measured in liver since rainbow trout fed BaP developed hepatocarcinoma (Hendricks et al., 1985). The co met assay conducted with whole blood assessed systemic oxidative stress (Mitchelmore and Chipman, 1998). Protein nitration was evaluated in trunk kidney since this damage was detected in human kidney undergoing oxidative stress (MacMillan-Crow et al., 1996).
DNA adducts were measured in liver since rainbow trout fed BaP developed hepatocarcinoma (Hendricks et al., 1985). The comet assay conducted with whole blood assessed systemic oxidative stress (Mitchelmore and Chipman, 1998). Protein nitration was evaluated in trunk kidney since this damage was detected in human kidney undergoing oxidative stress (MacMillan-Crow et al., 1996). DNA adducts in liver tissue (n=3 fish per composite x 3 composites per treatment) were measured all time points. DNA adducts in liver tissue of rainbow trout fed BaP, BeP and control diet were quantified using the 32P-postlabeling protocol as described in detail in Reichert and French (1994). Briefly, DNA extracted from livers was isolated, purified, and hydrolyzed to deoxyribonucleotide-3-monophosphates. Adducted 3-monophosphates were enzymatically labeled with [y32P] ATP (MP Biomedicals Inc, Irvine, CA) and separated by multidimensional thin-layer chromatography. Adduct levels were quantified using storage phosphor imaging technology and Image Quant software (Molecular Dynamics, Sunnyvale, CA).
The comet assay assessed DNA damage in whole blood (n=4 individual fish per treatment) after 14, 28 and 50 days of feeding BaP, BeP and control diet. Analysis of DNA strand breaks with the comet assay followed the method described by Mastaloudis et al. (2004). Whole blood (3 μl) was diluted with 1 ml of calcium and magnesium free phosphate buffer saline (PBS). This resulted in approximately 30,000 blood cells per sample. This cell suspension (10 μl) was added to 80 μl of 0.5% low melting point agarose (LMPA) at 35°C. Most of this agarose mixture (75 μl) was spread onto a slide and a cover glass was applied. The slides were placed in a horizontal gel electrophoresis tank side by side nearest to the anode. Electrophoresis was carried out at 40 volts and 400 milliamperes for 20 min. DNA in the samples was stained with 60 μL of ethidium bromide (20 μg· ml−1) for 5 min. Samples were processed on the same day of collection. Slides were analyzed within 3–4 h of processing. The presence of comets was determined using a Nikon Eclipse sE40 fluorescent microscope (Lake Forrest, CA). Cells were analyzed for DNA tail presence providing a score with Image Analysis software III (Perceptive Instruments Version 3.0, Suffolk, UK). Fifty randomly selected cells per slide were quantified. Results were expressed as means ± standard error in terms of the percentage of DNA that migrated into the comet tail region (tail DNA percentage) (Anderson et al., 1994: Michelmore and Chipman, 1998).
3-Nitrotyrosine immunohistochemistry (3-NT) assessed protein oxidation in trunk kidney tissue (n=4 individual fish per treatment) after 3, 7, 14, and 28 days of PAH feeding. Trunk kidney samples were collected and processed to paraffin, sectioned at 4 μm and placed on Microprobe plus (Fisher Scientific: Pittsburgh, PA) charged slides. Slides were washed and blotted in Tris buffered saline with Tween, pH 7.6 (TBST) (Dako, Denmark), until good capillary flow was obtained. Endogenous peroxides were blocked with 3% H2O2 in TBST for 10 min followed by washing and blotting TBST. Serum free protein (Dako, Denmark) was applied for 10 min followed by blotting and application of the rabbit polyclonal anti 3- NT antibody diluted at 1:500 in antibody diluent for 30 min. Universal negative rabbit control (Dako, Denmark), a immunoglobulin fraction of serum from non-immunized rabbits, was used as a negative reagent control in place of the primary antibody. Positive tissue controls were sections from the site of injection of muscle from rainbow trout treated with bacterial kidney disease (BKD) that were treated with the primary antibody. The inflammation that occurred along the needle tract resulted in positive staining. The negative tissue controls were sections not injected with BKD that were treated with the primary antibody. Following washing and blotting in TBST Dako Envision, anti-rabbit horseradish peroxidase (HRP) (Dako, Denmark) was applied for 30 min. Antibody binding was detected with the chromagen NovaRED ™ (Vector Laboratories Burlingame, CA) and hematoxylin counter stain was applied to each sample for 5 min on an autoimmunostainer (Dako, Denmark) to insure consistent staining times. Replicate slides per sample were counterstained with Harris’s hematoxylin and eosin (Luna 1968) for histopathological assessment and to visualize tissue structures and cells expressing 3-NT in parallel sections. Relative intensities were determined subjectively by comparing the staining of trunk kidney sections to that of similarly treated control-diet fed fish and BaP or BeP-fed fish by light microscopy. Staining was scored as previously described for CYP1A immunohistochemistry.
Statistics
Statistical analyses of the biomarker responses were conducted with Number Cruncher Statistical Software (NCSS: Kaysville, UT) and StatView (Kari, NC). Differences in FACs-BaP and FACs-PHN in bile, EROD activity, comet tail DNA percentage, F2- isoprostanes, and immunohistochemistry (CYP1A and 3-NT) between BaP and BeP-fed and control groups were assessed with one-way ANOVA using tank mean as the experimental unit. Percentage data from comet assays were arcsin transformed which assured normality. Simple linear regression was conducted on EROD activity and CYP1A immunostaining versus transformed comet assay results. In addition, statistical differences between all possible pair-wise comparisons of a particular treatment were assessed with Tukey-Kramer and Kruskal-Wallis Z multiple comparison procedures. Statistically significant difference between pair-wise comparisons was established using an alpha value of 0.05.
RESULTS
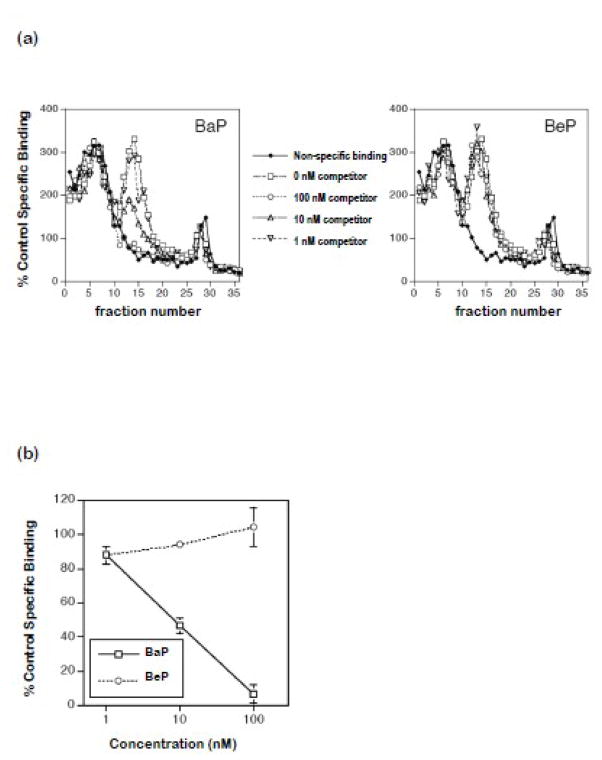
Sedimentation profiles of [3H]TCDD specific binding from sucrose gradients verified BaP but not BeP displaced binding in fractions characteristic of AHR2α (Fig. 1a). The AHR2α peak of specific binding was detected in fractions 10–20. BaP but not BeP displaced [3H]TCDD from rainbow trout AHR2α as expressed by specific binding (Fig. 1b).
Figure 1.
Competitive binding of BaP and BeP to rainbow trout AHR2α.
Trout AhR2α was synthesized by in vitro transcription and translation and incubated (18 h at 4°C) with [3H]TCDD (2 nM) and increasing concentrations of competitor, followed by analysis by velocity sedimentation as described in Materials and Methods. (a) Representative binding curves showing [3H]TCDD binding to AhR2α in the absence or presence of increasing concentrations of BaP (left panel) or BeP (right panel) or to unprogrammed lysate (UPL; a measure of non-specific binding). (b) Specific binding of [3H]TCDD in the presence of increasing concentrations of competitor, expressed as percent control specific binding (binding in the absence of competitor). Error bars indicate the range of values, from two independent binding assays.
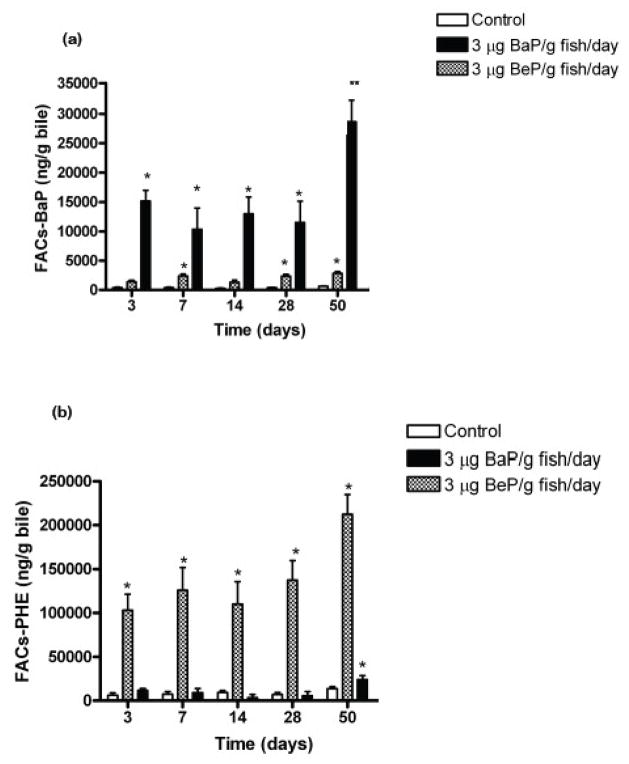
Rainbow trout consumed all ration, exhibited similar weight gain, and appeared healthy in control, BaP, and BeP-fed fish throughout the 50 day dietary treatments (data not shown). Biliary FACs confirmed consistent PAH exposures between 3 and 50 days of dietary treatments for fish fed BaP (Fig. 2a) and BeP (Fig. 2b). FACs in gallbladder bile in fish fed BaP for 50 days was significantly higher than at earlier times.
Figure 2.
Mean concentrations of fluorescent aromatic compounds (FACs) (±SE) measured at benzo(a)pyrene wavelengths 380/430(BaP-FACs) (a) and phenanthrene wavelengths (PHE-FACs) (b) in bile of juvenile rainbow trout fed either 3 μg BaP/g fish/day, 3 μg BeP/g fish/day or control diet for 50 days. Measurements (n=4 for each treatment) indicated with asterisk (*) are significantly different than control (ANOVA, p<0.05). The double asterisk (**) indicates significant difference from other times in this treatment group.
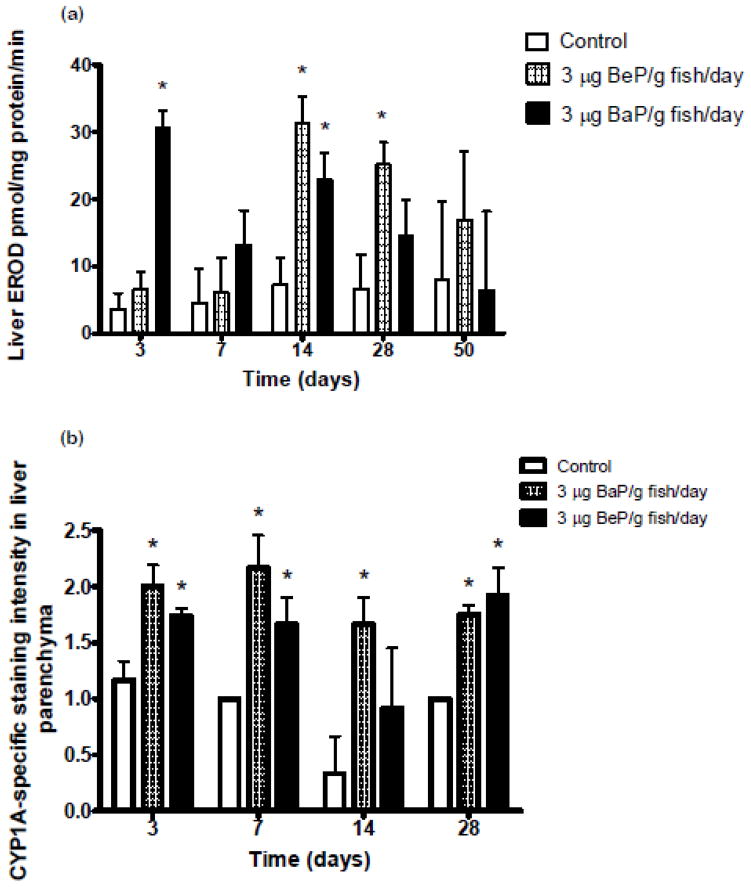
Hepatic microsomal EROD activity increased significantly after 3 and 14 days in BaP-fed fish (Fig. 3a). This activity increased after 14 and 28 days in BeP-fed fish (Fig. 3a). There were no significant differences in EROD activity in hepatic microsomes from fish fed control, BaP, or BeP diets at 50 days. Immunohistochemistry detected increased staining for CYP1A in livers of BaP-fed fish after 3, 7, 14 and 28 days of treatments (Fig 3b). CYP1A staining was significantly increased after 3, 7 and 28 days in BeP-fed fish. There were not statistically significant differences in CYP1A staining in trunk kidney of fish fed control, BaP, or BeP diets after 3, 7, 14, and 28 days (data not shown).
Figure 3.
(a) Changes in ethoxyresorufin-O-deethylase (EROD) activity at 3, 7, 14, 28 and 50 days in liver microsomes of juvenile rainbow trout fed either 3 μg BaP/g fish/day, 3 μg BeP/g fish/day or control diet for 50 days. Measurements (n=3) indicated with asterisk (*) are significantly different than control (ANOVA, p<0.05). (b) Staining intensity (mean ±SE of CYP1A in liver of juvenile rainbow trout at 3, 7, 14 and 28 days fed either 3 μg BaP/g/fish, 3 μg BeP/g/fish or control diet. Measurements (n=3–5) indicated with asterisk (*) are significantly different than control (ANOVA, p<0.05).
There were no statistical differences in hepatic DNA adduct concentrations between controls and PAH treatment groups. DNA adduct concentrations of liver ranged from 1–30 nmol adduct/mol DNA in fish fed control diet, BaP and BeP (data not shown).
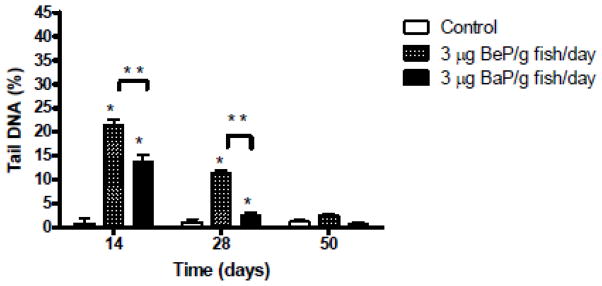
Damage to blood cell DNA increased in fish fed BaP and BeP after 14 and 28 days compared to controls (Fig. 4). DNA damage was more extensive after BeP than BaP exposure at those times. There was no significant elevation in DNA damage compared to controls after 50 days of feeding either PAH.
Figure 4.
Results of the single cell electrophoresis (comet assay) expressed in terms of the percentage of DNA in the comet tail measured in blood cells of juvenile rainbow trout fed either 3 μg BaP/g fish/day, 3 μg BeP/g fish/day or control diet for 14, 28 or 50 days. Results are expressed as mean ±SE for n=4 samples. Measurements indicated with asterisk (*) are significantly different than control (ANOVA, p<0.05).
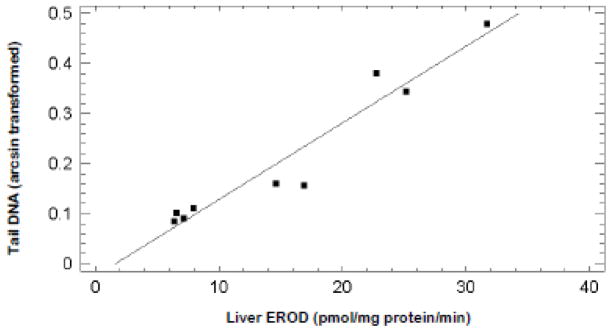
Visual inspection of the data suggested an association between hepatic microsomal EROD activity and whole blood cell tail DNA percentage. Data for both responses were available for fish after 14, 28, and 50 days of feeding control, BaP, and BeP diets. Simple linear regression of EROD activity (independent variable) (Fig. 3) and arcsin transformed tail DNA percentage (dependent variable) (Fig. 4) revealed a strong positive correlation (Fig. 5). The r2 value was 0.93. There was no significant correlation between hepatic CYP1A stain intensity (independent variable) (Fig. 3) and arcsin transformed tail DNA percent (dependent variable) (Fig. 4) for fish after 14 or 28 days of control, BaP or BeP feeding (r2=0.007).
Figure 5.
Simple linear regression of tail DNA from comet assays on whole blood (dependent variable) versus hepatic microsomal EROD activity (independent variable) from rainbow trout fed BaP or BeP for 14, 28, or 50 days. The coefficient of determination (r2) was 0.93 with p=0.00003.
There were no statistically significant differences in 3-NT staining intensity in trunk kidney between control, BaP, and BeP-fed rainbow trout after 3, 7, 14 or 28 days of treatments. Staining intensity was in the mild range for all treatments (data not shown). Histopathological examination of parallel sections revealed no treatment-dependent tissue damage (data not shown).
DISCUSSION
Environmental contamination with complex PAH mixtures persists due to past and continuing releases and long half-lives of these agents complexed in soil and sediment (Van Metre et al., 2000; Zhang and Tao, 2009). The work reported herein compared and contrasted biomarkers for oxidative stress, and hepatic CYP1A protein and EROD activity over 50 days of feeding rainbow trout BaP and BeP. These PAHs substantially differed in AHR affinity (detailed below). In vivo responses of hepatic CYP1A mRNA and EROD activity in mice (Shimada et al, 2003) and activation of an AHR reporter construct in rat hepatoma cells (Machala et al., 2001) indicated substantially higher AHR affinity for BaP than BeP. Displacement of [3H]TCDD from rainbow trout AHR2α by BaP but not BeP (Fig. 1) was consistent with those earlier reports. Despite negligible affinity of BeP for rainbow trout AHR2α it significantly increased hepatic CYP1A protein and activity (detailed below). If on assumed instantaneous absorption and uniform distribution of 3 μg BaP or BeP/kg a tissue concentration of 10 μmol/kg resulted. Curtis et al (1990) intraperitoneally injected rainbow trout with 10 μmol [3H] BaP/kg. After 24 hr liver contained 15–20 μmol total [3H] BaP equivalents/kg, about 5% of which was extracted by hexane and probably represented parent compound. Both these scenarios suggested the PAH contcentrations used in the AHR2α affinity assay were relevant to in vivo doses for this study.
Measurements of PAH metabolites in biological fluids were previously demonstrated as biomarkers of exposures. Bouchard et al. (2009) assessed human PAH exposures by mass spectrometric analysis of urine for PAH metabolites. Krahn et al. (1992) reported HPLC detection of FACs strongly correlated with sums of PAH metabolites quantitated by mass spectrometric analysis in bile of fish collected after an oil spill. As expected, bile from BaP-fed fish fluoresced at wavelengths characteristic of that PAH (Fig. 2a), while bile from BeP-fed fish fluoresced more strongly at phenanthrene wavelengths (Fig. 2b). Most important, analysis of FACs verified treatment groups received the appropriate diets throughout the 50 day time course. It also demonstrated near uniform dosing of across the time course. Significant elevation of FACs in bile of fish fed BaP for 50 days compared to earlier time points perhaps reflected increased hepatic excretory activity. Biliary excretion appeared rate limiting for hepatic elimination of BaP in temperature change experiments with rainbow trout (Curtis et al., 1990). A number of efflux transporters, including three ATP-binding cassette proteins, were identified as key mediators of biliary excretion (Kusuhara and Sugiyama, 2010). Increased activity or content of efflux transporters at the hepatocyte apical surface (bile canalicalus) was a potential explanation for stimulated biliary excretion of PAH metabolites but such proteins were not assessed in this study.
Constitutive CYP1A protein content and EROD activity of rainbow trout hepatic microsomes were quite low (Curtis et al, 1995; Gilroy et al, 1993). Fabacher (1982) reported anesthesia of brook trout with MS-222decreased hepatic microsomal BaP hydroxylase about 25%. Constitutive activity in the current work (Fig. 3a) averaged across all time points was about one-third lower than for hepatic microsomes from rainbow trout killed by a blow to the head (Gilroy et al., 1993). The potential for relatively mild CYP1A inhibition by MS-222 to influence interpretation of responses to PAHs was deemed acceptable in light of animal care and use considerations. Hepatic microsomal EROD activity increased rapidly in BaP-fed fish (about 7-fold higher than controls after 3 days), then trended towards control activity throughout the remaining 50 day treatment period (Fig. 3a). Transient elevations of hepatic microsomal CYP1A protein and EROD activity were previously reported in indole-3-carbinol-fed rainbow trout (Takahaski et al., 1995). During continuous feeding of rainbow trout with indole-3-carbinol over 21 days EROD activity peaked after 3 days (about 95 pmol/mg protein/min), dropped to 10 pmol/mg protein/min after 7 days., and increased to 25 pmol/mg protein/min after 14 days. The underlying mechanism was not resolved. Inactivation of nuclear factor 1 (NF1) by ROS interaction with cysteine residue 427 down regulated the CYP1A1 gene (reviewed by Zanger et al., 2004). Increased hepatic microsomal EROD activity in BeP-fed fish after 14 and 28 days of treatment was unanticipated (Fig. 3a). Two mutually nonexclusive explanations were plausible. First, PAH-o-quinones were reported as ligands for the AHR (Park et al., 2009). The 14 day time delay between initiation of BeP feeding and elevation of EROD activity perhaps reflected the period required for hepatic accumulation of a BeP-derived o-quinone concentration required for induction. Second, a hepatic cytosolic 4S protein was proposed as an alternative signaling pathway for CYP1A induction (Bhat et al., 1997). Sterling et al. (1994) reported BeP activated a CYP1A1 gene construct, induced EROD activity and was a ligand for the 4S protein in mouse hepatoma cells. The 4S protein was detected in fish liver cystosol (Barton and Marletta, 1988). Assessment of BeP as a ligand for this 4S protein or confirmation that protein was a regulator of CYP1A was beyond the scope of this study.
The immunohistochemistry procedure confirmed that CYP1A protein increased in livers of BaP-and BeP-fed, compared to control diet-fed rainbow trout (Fig. 3b). There was not a strong association between CYP1A-specific staining and EROD activity over the 28 days of the time course for which both biomarkers were assessed, however. This was particularly obvious for BeP-fed fish where CYP1A-specific staining increased before EROD activity. BaP metabolism by rat hepatic microsomes was demonstrated to be competitively inhibited by primary BaP metabolites (Keller and Jefcoate, 1984). Monooxygenation of the primary metabolite (7, 8,-dihydrodio) was 5 to 10 –fold more sensitive to this inhibition than BaP itself. EROD activities of recombinant human CYP1A1, CYP1A2 and CYP1B1 were directly inhibited by multiple PAHs including BaP (Shimada et al., 2008). Absence of a close association of EROD activity of hepatic microsomes and immunoreactive CYP1A in liver parenchyma (Fig. 3) was best explained by inhibition of enzymatic activity by BaP, BeP and their metabolites.
The presence of nucleated fish red blood cells greatly facilitated application of the comet assay in blood as a biomarker of systemic oxidative stress. The substantially increased percentage of tail DNA percentage after 14 days in both BaP and BeP-fed fish compared to controls (Fig. 4) suggested the PAH-o-quinone pathway (Xue and Warshawsky, 2005) was important in toxicity of both agents. Significantly higher DNA damage in blood cells of fish fed BeP compared to BaP after both 14 and 28 days of treatments indicated either more of the former PAH was metabolized to the o-quinone or the BeP-o-quinones released more ROS than BaP-o-quinones. The decline in percentage of tail DNA percentage from 14 to 50 days of treatments in both BaP and BeP-fed fish was remarkable. Induction of antioxidant enzymes, perhaps via the Keap1-Nrf2-antioxidant response element pathway (reviewed by Kensler et al., 2007) was likely important in this recovery. Several unpublished results from the Curtis laboratory supported this speculation. In the rainbow trout fed complex a PAH mixture hepatic microsomal catalase activity increased about 5-fold after 14 and 42 days of treatment. Glutathione peroxidase activity increased slightly less than 2-fold after 42 days in these fish. Microarray analysis or hepatic mRNA from fish fed a similar PAH mixture for 50 days detected a 2.4-fold elevation of glutathione peroxidase type 2.
A remarkably strong correlation between tail DNA percentage in blood cells and hepatic microsomal EROD activity (Fig. 5) suggested a prominent role for CYP1A activity in PAH-induced oxidative stress. Zanger et al. (2004) reviewed the role of the CYP system in generation of ROS. One principle conclusion was an importance of inefficient coupling of NADPH consumption (usually less than 50%) to substrate oxidation with consequential generation of ROS. A second key point was implication of ROS in repression of cyp1a1 gene transcription. This downregulation was probably mediated through oxidation of NFI as described above. For the times that both CYP1A immunohistochemistry and comet assay data were available, there was no correlation between these parameters. Diminuation of catalytically active hepatic CYP1A appeared a key event in recovery from toxicity during subchronic PAH treatments (Fig. 5). At 24 hr after intraperitoneal injection with [3H]BaP plasma of intact rainbow contained about 90% of total [3H]BaP equivalents as polar metabolites (Curtis et al, 1990). In rat livers perfused with 4 μM BaP about 4 nmol BaP phenols/g liver/h effluxed into perfusate (Zhong et al., 1994). Therefore, efflux of hepatic BaP and BeP metabolites into blood was a probable source of ROS that damaged blood cell DNA. Inhibition of hepatic CYP1A activity was likely a key mechanism of reduction in ROS generated by PAH metabolism.
Trunk kidney was assessed for oxidative stress to serve as a comparison to systemic (whole blood) and hepatic biomarkers. Bravo et al. (2011) detected transient but significantly increased 3-NT stain intensity coincident with increased CYP1A staining in trunk kidney of rainbow trout fed a PAH mixture. This was interpreted as evidence of initial oxidative stress followed by adaptation as discussed above. 3-NT presence indicated the cellular production of highly reactive nitrogen species such as peroxynitrate that nitrate tyrosine residues in proteins resulting in protein oxidation (Mac Millan-Crow et al., 1996). There was no elevation of 3-NT stain intensity, CYP1A stain intensity, or histological damage in trunk kidney in rainbow trout fed BaP or BeP doses employed herein for up to 28 days (data not shown). Lack of protein nitration suggested absence of marked oxidative stress in this tissue despite significant elevation of DNA damage in blood cells. No elevation of CYP1A protein and low tissue PAH bioactivation perhaps explained this. This further supported a key role of CYP1A in oxidative stress induced by PAHs.
In summary, the results for CYP1A activity (Fig. 3a) and comet assays of blood cells (Fig. 4) clearly demonstrated recovery during subchronic PAH treatments. A strong correlation between two of these parameters (Fig. 5) suggested a key role for inhibition of CYP1A activity in this adaptation. There was no clear association between AHR2α affinity and BaP and BeP-induced oxidative stress.
Highlights.
There was no direct association between aryl hydrocarbon receptor affinity and potency of polyaromatic hydrocarbons to produce oxidative stress.
There was a strong correlation between cytochrome P4501A activity and systemic oxidative stress as measured with the comet assay.
There was no correlation between cytochrome P4501A protein detected by immunohistochemistry and oxidative stress as measured with the comet assay.
Acknowledgments
Funding Sources Statement
The Oregon Agricultural Experiment Station, Northwest Fisheries Science Center, and RO1ES006272 from the National Institute of Health supported this work.
Jim Meador and Lyndal Johnson provided valuable technical advice.
Footnotes
Conflict of Interest Statement
No conflicts of interest exist for the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abnet CC, Tanguay RL, Hahn ME, Heideman W, Peterson RE. Two forms of aryl hydrocarbon receptor type 2 in rainbow trout (Oncorhynchus mykiss): Evidence for differential expression and enhancer specificity. J Biol Chem. 1999;274:15159–15166. doi: 10.1074/jbc.274.21.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Yu TW, Phillips B, Schmezer P. The effects of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the COMET assay. Mutat Res. 1994;307:261–271. doi: 10.1016/0027-5107(94)90300-x. [DOI] [PubMed] [Google Scholar]
- Arkoosh M, Clemons E, Myers M, Casillas E. Suppression of B-cell mediated immunity in juvenile Chinook salmon (Oncorhynchus tshawytsha) after exposure to either a polycyclic aromatic hydrocarbon or to polychlorinated biphenyls. Immunopharm and Immunotox. 1994;16:293–314. doi: 10.3109/08923979409007096. [DOI] [PubMed] [Google Scholar]
- Arkoosh M, Clemons E, Huffman P, Kagley A. Increased susceptibility of Juvenile Chinook salmon to Vibriosis after Exposure to Chlorinated and Aromatic Compounds Found in Contaminated Urban Estuaries. J Aquat Anim Hlth. 2001;13:257–268. [Google Scholar]
- Barton HA, Marletta MA. Kinetic and immunochemical studies of a receptor-like protein that binds aromatic hydrocarbons. J Biol Chem. 1988;263:5825–5832. [PubMed] [Google Scholar]
- Bhat R, Bresnick E. Glycine N-methyltransferase is an example of functional diversity: role as a polycyclic aromatic hydrocarbon-binding receptor. J Biol Chem. 1997;272:21221–21226. doi: 10.1074/jbc.272.34.21221. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Hahn ME, Franks DG, Peterson RE, Bols NC, Hodson PV. Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs) Comp Biochem and Physiol. 2002;133B:55–68. doi: 10.1016/s1096-4959(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Normandin L, Gagnon F, Viau C, Durrias P, Gaudreau E, Temblay C. Repeated measures of validated and novel biomarkers of exposure to polycychic aromatic hydrocarbons in individuals living hear an aluminum plant in Quebec, Canada. J Toxicol Environ Hlth Part A. 2009;72:1534–1549. doi: 10.1080/15287390903129481. [DOI] [PubMed] [Google Scholar]
- Bravo CF, Curtis LR, Myers MS, Meador JP, Johnson LL, Buzitis J, Collier TK, Morrow JD, Laetz CA, Loge FJ, Arkoosh MR. Biomarker responses and disease susceptibility in juvenile rainbow trout (Oncorhynchus mykiss) fed a high molecular weight PAH mixture. Environ Toxicol Chem. 2011;30:1–11. doi: 10.1002/etc.439. [DOI] [PubMed] [Google Scholar]
- Carlson EA, Li Y, Zelikoff JT. Exposure of Japanese medaka oryzias latipes to benzo[a]pyrene suppresses immune function and host resistance against bacterial challenge. Aquat Toxicol. 2002;56:289–301. doi: 10.1016/s0166-445x(01)00223-5. [DOI] [PubMed] [Google Scholar]
- Curtis LR, Frederickson LK, Carpenter HM. Biliary excretion appears rate limiting for hepatic elimination of benzo(a)pyrene by temperature acclimated rainbow trout. Fund Appl Toxicol. 1990;13:495–503. doi: 10.1016/0272-0590(90)90028-i. [DOI] [PubMed] [Google Scholar]
- Curtis LR, Zhang Q, El-Zahr C, Carpenter NM, Miranda CL, Buhler DR, Selivonchick DP, Arbogast DN, Hendricks JD. Temperature-modulated incidence of aflatoxin B1-initiated liver cancer in rainbow trout. Fund Appl Toxicol. 1995;25:146–153. doi: 10.1006/faat.1995.1048. [DOI] [PubMed] [Google Scholar]
- Eggens M, Galgani F. Ethoxyresorufin-O-deethylase (EROD) activity in flatfish: Fast determination with a fluorescent plate-reader. Mar Environ Res. 1992;3:213–221. [Google Scholar]
- Fabacher DL. Hepatic microsomes form freshwater fish-II. Reduction of benzo(a)pyrene metabolism by the fish anesthetics quinaldine sulfate and tricaine. Comp Biochem Phylsiol. 1982;73C:285–288. doi: 10.1016/0306-4492(82)90122-8. [DOI] [PubMed] [Google Scholar]
- Gilroy DJ, Carpenter HM, Siddens LK, Curtis LR. Chronic dieldrin epxosure increases hepatic disposition and biliary excretion of [−14C] dieldrin in rainbow trout. Fundam Appl Toxicol. 1993;20:295–301. doi: 10.1006/faat.1993.1039. [DOI] [PubMed] [Google Scholar]
- Hendricks JD, Meyers TR, Shelton DW, Casteel JL, Bailey GS. Hepatocarcinogenicity of benzo(a)pyrene to rainbow trout by dietary exposure and intraperitoneal injection. J Nat Cancer Inst. 1985;74:839–851. [PubMed] [Google Scholar]
- Hyyti O, Nyman M, Willis ML, Raunio H, Pelkoen O. Distribution of cytochrome P450 (CYP1A) in the tissues of Baltic ringed and gray seals. Mar Environ Res. 2001;51:465–485. doi: 10.1016/s0141-1136(00)00258-0. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Persson Y, Frankki S, van Bavel B, Lundstedt S, Haglund P, Tysklind M. Degradation of polycyclic aromatic hydrocarbons (PAHs) in contaminated soils by Fenton’s reagent: A multivariate evaluation of the importance of spoil characteristics and PAH properties. J Haz Mat. 2007;149:86–96. doi: 10.1016/j.jhazmat.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Powell WH, Hahn ME. Identification and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleost Fundulus heteroclitus. Evidence for a novel subfamily of ligand-binding basic helix-loop-helix Per-ARNT-Sim (bHLH-PAS) factors. J Biol Chem. 1999;274:33814–33824. doi: 10.1074/jbc.274.47.33814. [DOI] [PubMed] [Google Scholar]
- Keller GM, Jefcoate CR. Benzo(a)pyrene activation to 7, 8-dihydrodiol 9, 10-oxide by rat liver microsomes. J Biol Chem. 1984;259:13770–13776. [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi W, Biswal S. Cell survival responses to environmental stress via the Keap1-Nrf2-ARE pathway. Ann Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Krahn MM, Burrows DG, Vlitalo GM, Wigren CA, Collier TK, Chan SL, Varanasi U. Mass spectrometric analysis for aromatic compounds in bile of fish sampled after the Exxon Valdez oil spill. Environ Sci Technol. 1992;26:116–126. [Google Scholar]
- Krahn M, Ylitalo G, Buzitis J, Chan S, Varanasi U. Rapid high-performance liquid chromatography methods that screen for aromatic compounds in environmental samples. J Chromatogr. 1993;642:15–32. [Google Scholar]
- Kusuhara H, Sugiyama Y. Pharmacokinetic modeling of the hepatobiliary transport mediated by cooperation of uptake and efflux transporters. Drug Metab Rev. 2010;42:539–550. doi: 10.3109/03602530903491824. [DOI] [PubMed] [Google Scholar]
- Lowry O, Rosenbrough N, Farr A, Randall R. Protein Measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luna C. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3. McGraw Hill; New York: 1968. pp. 12–47. [Google Scholar]
- Machala M, Vondráček J, Bláha L, Ciganek M, Neča J. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutat Res. 2001;497:49–62. doi: 10.1016/s1383-5718(01)00240-6. [DOI] [PubMed] [Google Scholar]
- Mac Millan-Crow L, Crow J, Kerby J, Beckman J, Thompson J. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastaloudis A, Tian-Wei Y, O’Donnell RP, Frei B, Dashwood RH, Traber MG. Endurance exercise results in DNA damage as detected by the comet assay. Free Rad Bio Med. 2004;36:966–975. doi: 10.1016/j.freeradbiomed.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Mitchelmore C, Chipman J. Detection of DNA strand breaks in brown trout (salmo trutta) hepatocytes and blood cells using the single cell electrophoresis (comet) assay. Aquat Toxicol. 1998;41:161–182. [Google Scholar]
- Myers MS, Willis ML, Mette A, Anders G, Collier TK. Immunohistochemical localization of CYP1A in multiple types of contaminant- associated hepatic lesions in English sole (Pleuronectes vetulus) Mar Environ Res. 1996;39:283–288. [Google Scholar]
- Nebert DW, Dalton TP, Oken AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of CYP1A enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:2384723850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Oesch-Bartloomowicz B, Oesch F. Role of cAMP in mediating AHR signaling. Biochem Pharmacol. 2009;77:627641. doi: 10.1016/j.bcp.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Park JH, Mangal D, Frey AJ, Harvey RG, Blair IA, Penning RM. Aryl hydrocarbon receptor facilitates DNA strand breaks and 8-oxo-2′-deoxyguanosine formation by the aldo-keto reductase product benzo[a]pyrene-7-8-dione. J Biol Chem. 2009;284:29725–29734. doi: 10.1074/jbc.M109.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert W, French B. NOAA Technical Memorandum NMFS-NWFSC-14. 1994. The 32P-Postlabeling Protocols for Assaying Levels of Hydrophobic DNA Adducts in Fish. [Google Scholar]
- Shimada T, Nurayama N, Tanaka K, Takenaka S, Imia Y, Hopkins NE, Foroozesh MK, Alworth WL, Yamazaki H, Guengerich RP, Komori M. Interaction of polycyclic aromatic hydrocarbons with humancytochrome P450 1B1 in inhibiting catalytic activity. Chem Res Toxicol. 2008;21:2313–2323. doi: 10.1021/tx8002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Sugie A, Yamada T, Kawazoe H, Hashimoto M, Azuma E, Nakajima T, Inoue K, Oda Y. Does-response studies on the induction of liver cytrochromes P4501A1 and 1B1 by polycyclic aromatic hydrocarbons in arylhydrocarbon-responsive C57BL/6J mice. Xenobiotica. 2003;33:957–971. doi: 10.1080/0049825031000140896. [DOI] [PubMed] [Google Scholar]
- Sterling K, Raha A, Bresnick E. Induction of CYP1A-1 gene expression in mouse hepatoma cells by benzo(e)pyrene, a ligand of the 4s polycyclic hydrocarbon binding protein. Toxicol Appl Pharmacol. 1994;128:18–24. doi: 10.1006/taap.1994.1175. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Dashwood RH, Bjeldanes LF, Bailey GS, Williams DE. Regulation of hepatic cytochrome P4501A by indole-3-carbinol. Transient induction with continuous feeding in rainbow trout. Food Chem Toxicol. 1995;33:111–120. doi: 10.1016/0278-6915(94)00117-7. [DOI] [PubMed] [Google Scholar]
- Tsui HW, Okey AB. Rapid vertical tube rotor gradient assay for binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to the Ah receptor. Can J Physiol Pharmacol. 1981;59:927–931. doi: 10.1139/y81-143. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ, Furlong ET. Urban sprawl leaves its PAH signature. Environ Sci Technol. 2000;34:4064–4070. [Google Scholar]
- Williams DE, Buhler DR. Comparative properties of purified cytochrome P-448 from β-napthoflavone treated rats and rainbow trout. Comp Biochem Physiol. 1983;75C:25–32. doi: 10.1016/0742-8413(83)90006-3. [DOI] [PubMed] [Google Scholar]
- Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Zangar R, Davydov R, Seema V. Mechanism that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol. 2004;199:316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tao S. Global atmospheric emission inventory of poycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos Environ. 200;43:812–819. [Google Scholar]
- Zhong Z, Goto M, Hijioka T, Oide H, Kauffman FC, Thurman RG. Role of Kupffer cells in storage and metabolism of benzo[a]pyrene in the liver. Drug Metab Dispo. 1994;22:680–687. [PubMed] [Google Scholar]