Abstract
Background:
Lipids are the major cell membrane components essential for various biological functions including cell growth and division for the maintenance of cell integrity of normal and malignant tissues. The changes in lipid profile have long been associated with cancer. Hypocholesterolemia has been observed in patients with cancers of various organs. Therefore, a causative relationship might exist between plasma lipid levels and oral cancer patients.
Aim:
The objective of this study was to investigate the alterations and clinical significance of plasma lipid profiles in untreated head and neck cancer patients.
Materials and Methods:
A total of 30 subjects (25 oral cancer patients and 5 controls) were included. Fasting blood lipid profile including cholesterol (C), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL) and very low density lipoprotein (VLDL) were evaluated using spectrophotometric kits, with CHOD PAP technique. The values were then statistically analyzed using analysis of variance (ANOVA) and post hoc methods.
Results:
The levels of total lipids, cholesterol and HDL were significantly lower in oral cancer patients as compared to controls, but LDL and VLDL values were not significant.
Conclusion:
An inverse relationship was found between the lipid levels and the occurrence of oral cancer. Hence, the lower plasma lipid status may be a useful indicator to detect the initial changes seen in neoplastic process.
Keywords: CHOD PAP, hypocholesterolemia, inverse relationship, lipids, oral cancer
INTRODUCTION
In recent years, emphasis has been placed on detecting molecular markers from body fluids such as saliva, urine and others, for detecting, predicting prognosis, and monitoring the oral cancer progression. The idea of screening and following patients with malignancy by blood based tests is appealing from several points of view including its ease, economic advantage, non invasiveness, and possibility of repeated sampling.[1] Lipoproteins are clusters of proteins and lipids, all tangled up together to carry lipids in our blood.
Lipids are high energy yielding molecules and include fats and oils, waxes, phospholipids, steroids and some other related compounds. Fats and oils are made from two kinds of molecules: one glycerol and three fatty acids joined by dehydration synthesis, known as triglycerides (TGs), which are the major form of energy storage. For transport in plasma, TGs and cholesterol are packaged into lipoproteins, which are then taken up and degraded by cells to fulfill the demands for cellular functions. Lipids are major cell membrane components essential for various biological functions including cell growth and division of normal and malignant tissues, maintenance of the structural and functional integrity of all biological membranes, activity of membrane-bound enzymes and stabilization of DNA helix.[2] There are two main categories of lipoproteins: high density lipoprotein (HDL) being associated with carrying “cholesterol” out of the blood system and low density lipoprotein (LDL) which transports 75% of plasma cholesterol.[3] Cell receptors metabolize circulating LDL and clear nearly 80% of it from the body, while the rest of it is associated with deposition of “cholesterol” on the walls of arteries.
The relation of lipid levels in serum and coronary disease is well established. Rose et al[4] reported an inverse association between blood cholesterol level and the risk of cancer and provided a base for further epidemiological research. Since then, conflicting hypotheses have been put forward by many workers. Several authors propose that hypocholesterolemia is a predisposing factor for cancer development.[5] It is believed that tobacco carcinogens induce generation of free radicals and reactive oxygen species responsible for the high rate of oxidation/peroxidation of polyunsaturated fatty acids which affects the cell membrane and are thus involved in carcinogenesis.Because of the lipid peroxidation, there is a greater utilization of lipids for new membrane biogenesis.[2] In oral cancer development, tobacco plays an etiologic role. Hence, the aim of the study was to compare the serum lipid profiles of different histological grades of oral squamous cell carcinoma with that of clinically normal controls and also amongst the grades of carcinoma.
MATERIALS AND METHODS
A total of 30 male patients in the age range of 22-64 years, with a mean age of 50.66 years, were selected [Table 1]; 25 patients were histopathologically confirmed Oral Squamous cell Carcinoma, diagnosed at the Gujarat Cancer and Research Institute, Ahmedabad, and 5 controls with no systemic disease were selected randomly. The patients comprised 10 subjects each of well-differentiated (WD) carcinoma and moderately differentiated (MD) carcinoma and 5 patients of poorly differentiated (PD) carcinoma. Two milliliters of fasting blood was collected from each patient in red color-coded vacutainers. The blood was allowed to clot and was then centrifuged to separate the serum for lipid analysis. Lipid analysis was done on a chemical analyzer (Erba chem 5x analyzer) based on spectrophotometric principle. The analysis was made by an enzymatic photometric test using a wavelength of 546 nm and an optical path of 1 cm and is known as “CHOD PAP”. The serum lipid profile in the form of total cholesterol, HDL, LDL, very low density lipoprotein (VLDL) and TGs was analyzed on the same day of the withdrawal of blood. Serum cholesterol was estimated by mixing 0.01 ml serum sample with 1 ml of working reagent. This mixture was incubated at 37°C for 5 minutes, and the absorbance of the assay mixture was measured after 60 minutes by a spectrophotometer at 546 nm, against distilled water as a blank. Similarly, different working reagents for all lipids were used for their estimation. All data were expressed as mean±standard deviation. Analysis of variance (ANOVA) and post hoc tests were performed for comparison between patient groups. A value of P<0.05 was considered significant.
Table 1.
Mean age and standard deviation for the oral cancer patients and controls
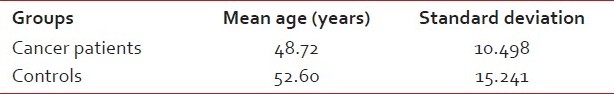
RESULTS
In the present study, all the patients were of low socioeconomic status and were having a poor nutritional status and had various grades of squamous cell carcinoma lesions. Of them 80 and 70% patients of WD and MD carcinoma, respectively, were in the age range of 46-65 years, while 60% patients of the PD carcinoma were in the range of 20-45 years. 40% patients were having lesion on buccal mucosa, 44% on tongue and the rest 16% at the other sites of the oral cavity (including floor of the mouth and palate). Of these, 68% of the patients had the habit of tobacco chewing and 32% had tobacco smoking habit [Table 2]. The mean, standard deviation and P value of all the lipid values in the oral cancer patients and controls were calculated and compared [Table 3 and Figure 1]. There was a significantly lower level of mean serum cholesterol (196.26±25.3 mg/dl), HDL (50.62±4.08 mg/dl), TGs (154.31±20.6 mg/dl) and total lipids (546.18±64.6 mg/dl) found in all groups of oral cancer patients as compared to the controls (cholesterol 231.0±33.05 mg/dl, HDL 59.94±3.08 mg/dl, TGs 249.80±51.9 mg/dl, total lipids 688.78±52.14 mg/dl), and their P values suggest that the cholesterol was significant, while the HDL, TGs and total lipids were highly significant in oral cancer patient compared to controls (P<0.05 is significant). While comparing all the lipid levels between three different groups of oral cancer patients, there was no significant difference found, but MD carcinoma group of oral cancer patients showed somewhat higher values of total lipids as compared to other two groups, and this was not significant.
Table 2.
The demographic data of squamous cell carcinoma patients
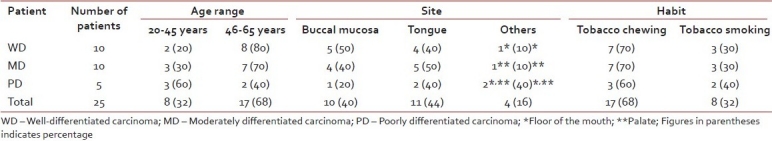
Table 3.
Mean±standard deviation of all lipids (mg/dl) in oral cancer patients and control groups, with their statistical significance expressed as P value, when compared to control group
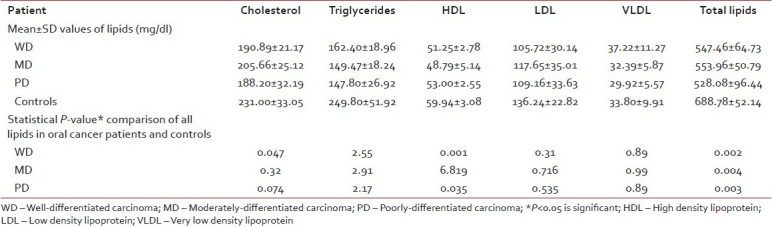
Figure 1.
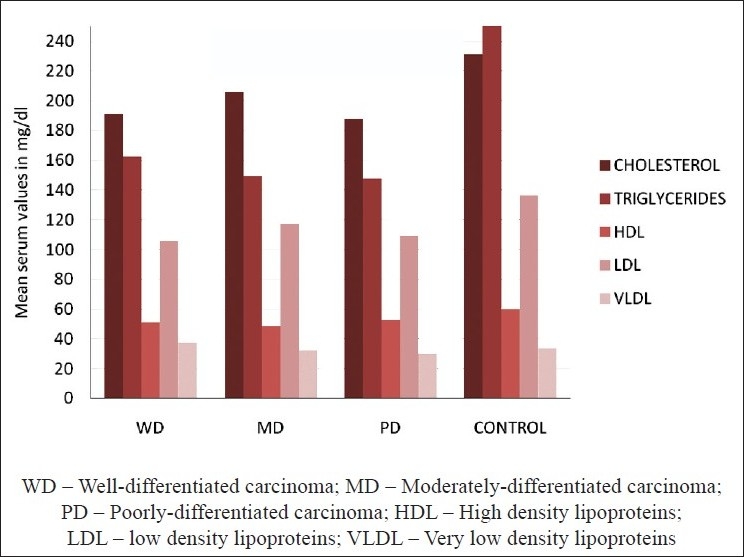
Mean serum lipid profile levels in oral cancer patients and controls
DISCUSSION
There are three main competing hypotheses to explain the inverse association between cholesterol concentrations and the incidence of cancer. Firstly, lower cholesterol values, even before the manifestation or detection of cancer, may be a result of the cancer process. Secondly, lower cholesterol values may precede the development of cancer but the association with cancer is secondary, i.e. cholesterol serves as a marker for some other causal set of variables. Thirdly, lower cholesterol values may precede the development of cancer and may be causally associated with the occurrence of some forms of cancer.[6]
In the present study, a significant decrease in plasma total cholesterol, TGs and HDL was observed in oral cancer patients as compared to the controls. Plasma LDL and VLDL levels did not reveal any significant difference among the two groups. The reduced levels of serum HDL in patients with tumors may be a consequence of the disease, probably mediated by the greater utilization of cholesterol for new membrane biogenesis and by the accumulation of esterified cholesterol in tumoral tissues. The role of LDL and TGs in the change of overall pattern of total cholesterol is less clear. The blood cholesterol undergoes early and significant change in malignant diseases, but there was no significant relation to the histological type of cancer.[1] Halton et al.[7] showed that patients with widespread disease had lower concentration of cholesterol and HDL than patients with localized tumors. Rose et al.[4] first reported the inverse relation between blood cholesterol level and the risk of cancer. It appears to be strongest in the first 2 years of occurrence following the base line cholesterol measurement and this phenomenon has been termed as “preclinical cancer effect”.[8,9] In the oral malignancy, serum cholesterol undergoes early and significant changes. Low levels of cholesterol in the proliferating tissue and in the blood compartment could be due to the rapidly dividing cells in the oral malignancy.[3] Therefore, the change in lipid levels may have a diagnostic role in the early diagnosis of oral cancer. Winn[10] demonstrated the protective role of nutritional factors in cancer, but all the patients in our study were of low socioeconomic status and poorly nourished, which enhances the risk of development of cancer.
CONCLUSION
To conclude, it appears that the lower serum lipid status may be considered as a useful indicator for initial changes occurring in the neoplastic cells. Also, the mean serum lipid profile levels between histological grading of the oral cancer had no statistical significance. The present findings are drawn by a smaller sample size but the findings strongly warrant an in-depth study on a larger sample size of oral cancer patients. As early detection is also called secondary prevention, the programs for cancer control are based on the premise that the earlier cancer is diagnosed, the better the outcomes in terms of increased survival and reduced mortality.[1]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lohe VK, Degwekar SS, Bhowate RR, Kadu RP, Dangore SB. Evaluation of correlation of serum lipid profile in patients with oral cancer and precancer and its association with tobacco abuse. J Oral Pathol Med. 2010;39:141–8. doi: 10.1111/j.1600-0714.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- 2.Patel PS, Shah MH, Jha FP, Raval GN, Rawal RM, Patel MM, et al. Alterations in plasma lipid profile patterns in head and neck cancer and oral precancerous conditions. Indian J Cancer. 2004;41:25–31. [PubMed] [Google Scholar]
- 3.Mehrotra R, Pandya S, Chaudhary AK, Singh HP, Jaiswal RK, Singh M, et al. Lipid profile in oral submucous fibrosis. Lipids Health Dis. 2009;29:1–7. doi: 10.1186/1476-511X-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose G, Shipley MJ. Plasma lipids and Mortality: a source of error. Lancet. 1980;1:523–6. doi: 10.1016/s0140-6736(80)92775-0. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos CG, Blatsios B, Avgerinos A. Serum Lipids and Lipoprotein Disorders in Cancer Patients. Cancer. 1987;60:3065–70. doi: 10.1002/1097-0142(19871215)60:12<3065::aid-cncr2820601234>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Kark JD, Smith AH, Hames CG. Serum retinol and the inverse relationship between serum cholesterol and cancer. Br Med J (Clin Res Ed) 1982;284:152–4. doi: 10.1136/bmj.284.6310.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halton JM, Nazir DJ, McQueen MJ, Barr RD. Blood Lipid Profiles in Children with Acute Lymphoblastic Leukemia. Cancer. 1998;83:379–84. [PubMed] [Google Scholar]
- 8.Wald NJ, Thompson SG, Law MR, Densem JV, Bailey A. Serum cholesterol and subsequent risk of cancer: results from the BUPA study. Br J Cancer. 1989;59:936–8. doi: 10.1038/bjc.1989.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kritchevsky SB, Wilcosky TC, Morris DL, Truong KN, Tyroler HA. Changes in Plasma Lipid and Lipoprotein Cholesterol and Weight prior to the Diagnosis of Cancer. Cancer Res. 1991;51:3198–203. [PubMed] [Google Scholar]
- 10.Winn DM. Diet and nutrition in the etiology of oral cancer. Am J Clin Nutr. 1995;61:437s–45s. doi: 10.1093/ajcn/61.2.437S. [DOI] [PubMed] [Google Scholar]


