Abstract
Aim:
Previous studies demonstrated l-carnitine decreasing doxorubicin-induced cardiotoxicity. Our objectives were to study carnitine levels and cardiac functions in children treated with doxorubicin and the effect of short-term l-carnitine supplements.
Materials and Methods:
Serial carnitine levels and cardiac functions were obtained in children with newly diagnosed solid malignancies before doxorubicin, after cumulative doses of ≥150 mg/m2 and ≥300 mg/m2, respectively. Oral l-carnitine 100 mg/kg/day for 3 days were given to the children treated with doxorubicin at cumulative doses of ≥150 mg/m2 and ≥300 mg/m2. Carnitine levels and cardiac functions were also obtained in those children before and after short-term oral l-carnitine at each cumulative dose of doxorubicin.
Results:
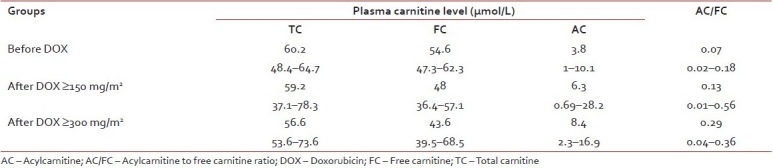
Five children (3 females), median age of 9.1 years (range 1.5–13 years) with newly diagnosed solid malignancies were enrolled in the study. Free carnitine (FC) tended to decrease while acyl-carnitine (AC) increased making AC/FC ratio increased after cumulative dose of ≥150 and ≥300 mg/m2 but the statistics was not significant. Left ventricular (LV) systolic function was not significantly changed. Interestingly, LV global function (LV myocardial performance index) was significantly increased after 150 mg/m2 (median 0.39, 0.27–0.51) and 300 mg/2 (median 0.46, 0.27–0.50) when compared to baseline (median 0.28, 0.14–0.48) (P=0.05). Carnitine levels and cardiac functions were not significantly changed after oral l-carnitine supplement at cumulative dose of ≥150 mg/m2 (n=6) and ≥300 mg/m2 (n=9).
Conclusions:
Carnitine levels tended to decrease after doxorubicin treatment. LV global dysfunction was documented early after doxorubicin. However, short-term l-carnitine supplement did not improve cardiac function.
Keywords: Anthracycline, cardiac function, cardiac toxicity, children
INTRODUCTION
Chemotherapy regimens based on doxorubicin, which is anthracycline antibiotics, are well established in the treatment against a variety of solid malignancies in children.[1] However, the optimal clinical usefulness of doxorubicin is usually limited secondary to the development dose-dependent and irreversible cardiotoxicity.[2] Several studies have shown that doxorubicin induced cardiomyopathy through several mechanisms including generation of free radicals and lipid peroxidation of cardiac membranes,[3] cardiac calcium overload,[4] formation of doxorubicin-iron complex,[5] and inhibition of beta-oxidation of long chain fatty acids with the consequent depletion of cardiac ATP.[6]
However, the exact mechanism for doxorubicin-induced cardiomyopathy remains unclear. Recently, it has been suggested that doxorubicin may exert at least a part of its cardiotoxicity by inhibition of long-chain fatty acid oxidation in the heart.[7] There have been extensive clinical and experimental researches to overcome doxorubicin-induced cardiotoxicity. One of the studied drugs is l-carnitine, which is an essential cofactor for mitochondrial transport and oxidation of long-chain fatty acids. Previous studies have demonstrated that l-carnitine could protect the myocardium against doxorubicin-induced cardiotoxicity.[6,8,9] The objectives of this study were to evaluate serial plasma carnitine levels and cardiac functions in patients treated with doxorubicin at the different cumulative doses and to study the effect of short-term l-carnitine supplement on cardiac function.
MATERIALS AND METHODS
Part one of the study was a prospectively longitudinal cohort study of children aged less than 15 years with newly diagnosed solid malignancies including rhabdomyosarcoma, Ewing's sarcoma, osteosarcoma, fibrosarcoma, and hemangiopericytoma who were planning to receive doxorubicin for their chemotherapy regimens at single center of the University Hospital between March 2008 and April 2009. Carnitine levels and echocardiography were performed in these children before doxorubicin treatment, after cumulative dose of doxorubicin ≥150 and 300 mg/m2, respectively.
Part two of the study was a cross-sectional study of children aged less than 15 years with solid malignancies as described above who received doxorubicin ≥150 and 300 mg/m2. These children received l-carnitine orally at 100 mg/kg/day for three consecutive days. Carnitine levels and echocardiography were performed in these children before and after l-carnitine supplementation.
Exclusion criteria were primary/secondary carnitine deficiency and inborn error of metabolism, cardiovascular diseases, liver diseases, renal diseases, malabsorption or chronic diarrhea, immunocompromised patients, and valproic acid therapy. The study was conducted following an approval by Institutional Review Board and written informed consents given by parents of the patients.
Plasma carnitine measurement
Five milliliter of heparinized blood was collected from each patient at the times described above in those two parts of the study. Plasma specimens obtained by centrifugation at 3000 g for 10 min at room temperature were kept frozen at –80°C until analysis. Plasma total carnitine (TC) and free carnitine (FC) levels were determined by using spectrophotometric method previously described by Alhomida et al.,[10] with some modifications.[11,12] Acylcarnitine (AC) level was calculated as the difference between TC and FC levels. All carnitine levels were expressed as median and rage. The criteria for diagnosis of carnitine deficiency are FC<20 μmol/L (absolute deficit) or AC/FC ratio >0.4 (relative deficit).
Echocardiography
A 2D, M-mode, color, Doppler echocardiography was performed in all patients at the times described above by single pediatric cardiologist (A.K.) blinded to the clinical data. Systolic function was shown as left ventricular (LV) ejection fraction (EF) and fractional shortening (FS) calculated by M-mode of the left ventricle in systole and diastole. Myocardial performance index (MPI) of the left ventricle was calculated by Doppler of the mitral valve inflow and the LV outflow as described.[13] The normal values for LVEF and LVFS are ≥55% and ≥25%, respectively.
The normal range for LV MPI is 0.33±0.05.[14] In this study, the authors used LV MPI ≥0.40 as an cut-off for LV global dysfunction.
Statistical analysis
All continuous data were expressed as median and range and were compared using Mann-Whitney U test in two independent samples and Kruskal-Wallis test for several independent samples. All data were analyzed by a statistical analysis system (SPSS: Statistical Package for the Social Science version 16.0). P value≤0.05 was considered statistically significant.
RESULTS
Part I of the study
Five children (3 females), median age of 9.1 years (range 1.5–13 years) with newly diagnosed solid malignancies were enrolled in the study. Their demographic data were shown in Table 1.
Table 1.
Patients′ characteristics

Serum TC, FC, AC, and AC/FC ratio were not significantly changed before and after doxorubicin ≥150 and ≥300 mg/m2. However, there was a trend that FC decreased while AC increased making AC/FC ratio to be increased while TC was unchanged [Table 2].
Table 2.
Serial plasma carnitine levels before and after doxorubicin treatment (median, range)
LV systolic function represented by LVEF and LVFS was not significantly changed before and after doxorubicin at cumulative dose of ≥150 and ≥300 mg/m2. One patient had LV systolic dysfunction (EF 54% and FS 21%) after doxorubicin at cumulative dose of ≥300 mg/m2. Interestingly, this patient was discontinued doxorubicin but his LVEF was still worsening; he died due to severe sepsis, septic shock, and severe LV dysfunction 3 months later.
LV global function represented by LV MPI was significantly increased after doxorubicin at cumulative dose of ≥150 (median 0.39, range 0.27–0.51) and ≥300 mg/m2 (median 0.46, range 0.27–0.50) when compared to before doxorubicin (median 0.28, range 0.14–0.48), P=0.05. Moreover, 2/5 and 3/5 patients at cumulative dose of ≥150 and ≥300 mg/m2, respectively, had LV MPI ≥0.40, which indicated LV global dysfunction. However, LV MPI after doxorubicin at cumulative dose of ≥150 mg/m2 was not significant different when compared to LV MPI after doxorubicin at cumulative dose of ≥300 mg/m2 [Figure 1].
Figure 1.
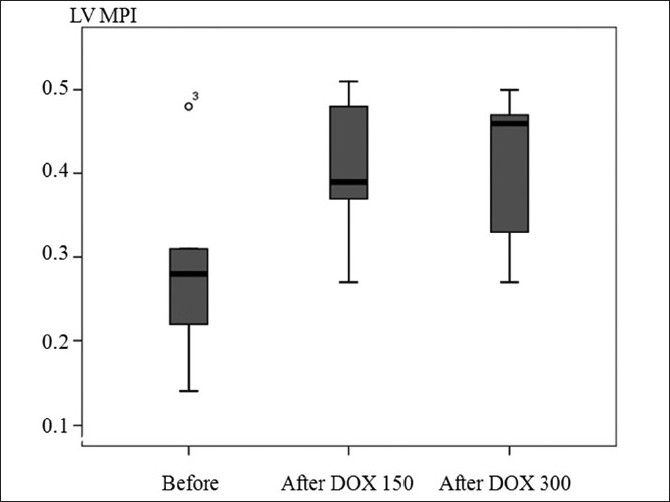
Boxplot of left ventricular myocardial performance index (MPI) before doxorubicin (DOX) treatment, after doxorubicin treatment at cumulative dose >150 and >300 mg/m2
Part II of the study
Six children (3 females), median age of 9.6 years (range 1.5–14 years) having cumulative dose of doxorubicin ≥150 mg/m2 and 9 children (5 females), median age of 9.0 years (range 1.5–13 years) having cumulative dose of doxorubicin ≥300 mg/m2 were enrolled in the study. After oral l-carnitine at 100/kg/day for three consecutive days in children having cumulative dose of doxorubicin ≥150 mg/m2, median TC, FC, AC, and AC/FC levels were not significantly increased [Table 3], whereas median LVEF, 60.5% (52–65%); LVFS 32.5% (30–34%); and LV MPI 0.49 (0.27–0.56) were also not significantly changed when compared to those after l-carnitine supplement for 3 days; LVEF 64.5% (50–72%); LVFS 34% (32–42%), and LV MPI 0.53 (0.32–0.66), respectively.
Table 3.
Plasma carnitine levels before and after short-term oral l-carnitine supplement for 3 days (median, range)
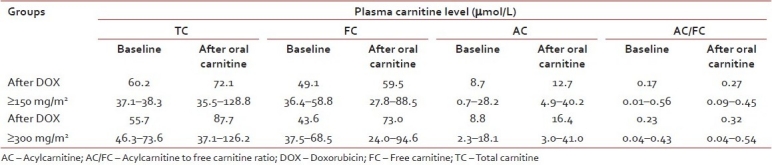
After oral l-carnitine at 100/kg/day for three consecutive days in children having cumulative dose of doxorubicin ≥300 mg/m2, TC and FC were significantly increased (P=0.005) [Table 3]; however, median LVEF 61% (54–68%); LVFS 33% (28–38%); and LV MPI 0.46 (0.18–0.63) were not significantly changed when compared to those after l-carnitine supplement for three consecutive days; LVEF 64% (44–74%); LVFS 35% (21–43%); and LV MPI 0.37 (0.28–0.55), respectively.
DISCUSSIONS
Doxorubicin can lead to cardiotoxicity which can be acute occurring during and within few days after administration including chest pain due to pericarditis, palpitation due to arrhythmias, and acute left ventricle failure due to myocarditis.[15] All these are reversible with appropriate treatments. The incidence of acute cardiotoxicity is approximately 11%.[15] The incidence of chronic doxorubicin cardiotoxicity is approximately 1.7%, which leads to doxorubicin cardiomyopathy primarily related to the accumulative dose of doxorubicin.[16] The present study demonstrated that systolic function assessed by LVEF and LVFS was not significantly changed after the cumulative dose ≥150 and ≥300 mg/m2. Interestingly, one case developed LV systolic dysfunction (LV EF=54%) at cumulative dose ≥300 mg/m2. As a guideline for discontinuing doxorubicin, the suggested values of LVEF and LVFS are below 55% and 29%, respectively.[17] Although doxorubicin was discontinued in this patient, he died 3 months later due to severe sepsis and more progressive on his LV dysfunction.
In contrast, LV MPI which represented LV global function (combined systolic and diastolic myocardial function)[13] was significantly increased after doxorubicin treatment even after the cumulative dose ≥150 mg/m2. Moreover, 2/5 and 3/5 patients at cumulative dose ≥150 and ≥300 mg/m2, respectively, had LV MPI ≥0.40 which indicated LV global dysfunction. This may indicate that even at the low cumulative dose of doxorubicin, the LV global dysfunction had already settled. Senju et al.[18] reported in 23 adult patients treated with doxorubicin and demonstrated that a change in the Tei-index or LV MPI was more sensitive indicator of early cardiotoxicity induced by doxorubicin than LVEF. Our findings may indicate early myocardial damage induced by lower cumulative dose of doxorubicin than that required to cause obvious cardiotoxicity represented by a significant decrease in LVEF or LVFS. The authors considered that LV MPI may be a good indicator of doxorubicin-induced cardiotoxicity and should be included in routine echocardiography for follow up in these patients.
Yaris et al.,[19,20] reported that there was a trend toward decreasing serum carnitine levels with increasing the cumulative doses of doxorubicin and found no significant relationship between carnitine levels and nutritional status of patients either at diagnosis or during treatment. From these results, it is implied that inadequate intake of carnitine or its precursors could not be solely responsible for decrease in carnitine levels. In contrast, Yoon et al.,[21] studied in rats and reported that doxorubicin caused significant elevations of plasma FC, AC, and TC levels with relatively higher increase in AC than FC, resulting in increased AC/FC ratio. Recent study in rat demonstrated that doxorubicin therapy resulted in dose-dependent decrease in FC and increase in AC causing increased AC/FC ratio in both serum and rat heart tissues, leading to the conclusion that myocardial carnitine level may be a mechanism of development of doxorubicin cardiotoxicity.[7] Carnitine level in patients with solid tumor after doxorubicin treatment in the present study had a trend of decreased FC, increased AC, and increased AC/FC ratio as previously reported in animal models [Table 2], although the statistics was not significantly different, probably due to the small number of the patients. According to the criteria for diagnosis of absolute carnitine deficiency (FC<20 μmol/L) or for relative carnitine deficiency (AC/FC ratio >0.4), only one patient met the criteria for the diagnosis of relative carnitine deficiency. At the cumulative dose of doxorubicin ≥150 mg/m2, this patient had normal FC (50.1 μmol/L) but had high AC/FC (0.56). This patient had normal LV systolic function but impaired LV global dysfunction (LIMP=0.56). Interestingly, at the cumulative dose of doxorubicin ≥300 mg/m2, this patient did not have carnitine deficiency (FC 57.8 μmol/L and AC/FC 0.04) and his LV global dysfunction returned to normal (LIMP=0.33).
Various drugs have been tried to prevent doxorubicin-induced cardiotoxicity including l-carnitine.[22] In the present study, the authors evaluated the short-term effect of l-carnitine by supplying 100 mg/kg/day of oral l-carnitine for 3 days and found that no significant changes of serum carnitine levels and cardiac functions. Cardiac function, either systolic function, assessed by LVEF, LVFS, or global function, assessed by LV MPI, were not significantly changed after short-term supplement of l-carnitine. This may be explained by two reasons: first, there was no carnitine deficiency before treatment and second, the sample size was small. Given the essential role of l-carnitine in transporting long fatty acids from the cytoplasmic compartment into mitochondria for beta-oxidation to produce energy,[23] inhibition of this vital pathway in the heart as a result of carnitine deficiency has been shown to impair myocardial function.[24] Moreover, decreased myocardial level of FC is thought to be part of the mechanism involved in the progression of doxorubicin-induced heart failure and cardiomyopathy.[24] The present study demonstrated that there was a trend toward decreasing plasma carnitine levels after increasing cumulative doses of doxorubicin. So, the authors recommended to check carnitine level in children treated with doxorubicin especially when the cardiac function is impaired. In those selected cases, a supplement of l-carnitine may be helpful in the improvement of cardiac function.
CONCLUSIONS
LV systolic function was not found at lower cumulative doses of doxorubicin, but LV global dysfunction was documented earlier after doxorubicin therapy. This should indicate the awareness and proper management in these patients when LV global dysfunction developed. Although carnitine level tended to decrease after doxorubicin treatment, short-term l-carnitine supplement for 3 days did not improve cardiac function. A longer period of carnitine supplement or other modalities of treatment needed to be further investigated.
Acknowledgments
This work was supported by grants (2552-4) from Mahidol University to DW. DW is recipient of the Research Career Development Award, Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
Footnotes
Source of Support: Mahidol University (2552-4)
Conflict of Interest: None declared.
REFERENCES
- 1.Carter SK. Adriamycin: A review. J Natl Cancer Inst. 1975;55:1265–74. doi: 10.1093/jnci/55.6.1265. [DOI] [PubMed] [Google Scholar]
- 2.Kantrowitz NE, Bristow MR. Cardiotoxicity of antitumor agents. Prog Cardiovasc Dis. 1984;27:195–200. doi: 10.1016/0033-0620(84)90004-5. [DOI] [PubMed] [Google Scholar]
- 3.al-Shabanah O, Mansour M, el-Kashef H, al-Bekairi A. Captopril ameliorates myocardial and hematological toxicities induced by adriamycin. Biochem Mol Biol Int. 1998;45:419–27. doi: 10.1080/15216549800202802. [DOI] [PubMed] [Google Scholar]
- 4.Huang XM, Zhu WH, Kang ML. Study on the effect of doxorubicin on expressions of genes encoding myocardial sarcoplasmic reticulum Ca2+ transport proteins and the effect of taurine on myocardial protection in rabbits. J Zhejiang Univ Sci. 2003;4:114–20. doi: 10.1631/jzus.2003.0114. [DOI] [PubMed] [Google Scholar]
- 5.Myers C. The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol. 1998;25:10–4. [PubMed] [Google Scholar]
- 6.Sayed-Ahmed MM, Shaarawy S, Shouman SA, Osman AM. Reversal of doxorubicin-induced cardiac metabolic damage by L-carnitine. Pharmacol Res. 1999;39:289–95. doi: 10.1006/phrs.1998.0438. [DOI] [PubMed] [Google Scholar]
- 7.Sayed-Ahmed MM. Increased serum and cardiac acyl-carnitine/free carnitine ratio during development of doxorubicin-induced cardiotoxicity. Saudi Pharm J. 2007;15:120–6. [Google Scholar]
- 8.Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A. Cardioprotection against the toxic effects of anthracyclines given to children with cancer: A systematic review. Health Technol Assess. 2007;11:1–84. doi: 10.3310/hta11270. [DOI] [PubMed] [Google Scholar]
- 9.Sayed-Ahmed MM, Shouman SA, Rezk BM, Khalifa MH, Osman AM, El-Merzabani MM. Propionyl-L-carnitine as potential protective agent against adriamycin-induced impairment of fatty acid beta-oxidation in isolated heart mitochondria. Pharmacol Res. 2000;41:143–50. doi: 10.1006/phrs.1999.0583. [DOI] [PubMed] [Google Scholar]
- 10.Alhomida AS, Duhaiman AS, al-Jafari AA, Junaid MA. Determination of L-carnitine, acylcarnitine and total carnitine levels in plasma and tissues of camel (Camelus dromedarius) Comp Biochem Physiol B Biochem Mol Biol. 1995;111:441–5. doi: 10.1016/0305-0491(95)00014-y. [DOI] [PubMed] [Google Scholar]
- 11.Pastoris O, Dossena M, Foppa P, Catapano M, Arbustini E, Bellini O, et al. Effect of L carnitine on myocardial metabolism: Results of a balanced, placebo-controlled, double-blind study in patients undergoing open heart surgery. Pharmacol Res. 1998;37:115–22. doi: 10.1006/phrs.1997.0281. [DOI] [PubMed] [Google Scholar]
- 12.Nemoto S, Yasuhara K, Nakamura K, Miyoshi Y, Sakai A. Plasma carnitine concentrations in patients undergoing open heart surgery. Ann Thorac Cardiovasc Surg. 2004;10:19–22. [PubMed] [Google Scholar]
- 13.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function-a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–66. [PubMed] [Google Scholar]
- 14.Eto G, Ishii M, Tei C, Tsutsumi T, Akagi T, Kato H. Assessment of global left ventricular function in normal children and in children with dilated cardiomyopathy. J Am Soc Echocardiogr. 1999;12:1058–64. doi: 10.1016/s0894-7317(99)70102-1. [DOI] [PubMed] [Google Scholar]
- 15.Singal PK, Deally CM, Weinberg LE. Subcellular effects of adriamycin in the heart: A concise review. J Mol Cell Cardiol. 1987;19:817–28. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- 16.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–7. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 17.Okada Y, Horikawa K, Sano M. Echocardiographic evaluation of cardiotoxicity induced by anthracycline therapy. Gan To Kagaku Ryoho. 1997;24:585–9. [PubMed] [Google Scholar]
- 18.Senju N, Ikeda S, Koga S, Miyahara Y, Tsukasaki K, Tomonaga M, et al. The echocardiographic Tei-index reflects early myocardial damage induced by anthracyclines in patients with hematological malignancies. Heart Vessels. 2007;22:393–7. doi: 10.1007/s00380-007-0985-x. [DOI] [PubMed] [Google Scholar]
- 19.Yaris N, Akyuz C, Coskun T, Buyukpamukcu M. Serum carnitine levels of pediatric cancer patients. Pediatr Hematol Oncol. 2002;19:1–8. doi: 10.1080/088800102753356130. [DOI] [PubMed] [Google Scholar]
- 20.Yaris N, Ceviz N, Coskun T, Akytuz C, Buyukpamukcu M. Serum carnitine levels during the doxorubicin therapy.Its role in cardiotoxicity. J Exp Clin Cancer Res. 2002;21:165–70. [PubMed] [Google Scholar]
- 21.Yoon HR, Hong YM, Boriack RL, Bennett MJ. Effect of L-carnitine supplementation on cardiac carnitine palmitoyltransferase activities and plasma carnitine concentrations in adriamycin-treated rats. Pediatr Res. 2003;53:788–92. doi: 10.1203/01.PDR.0000057988.62605.13. [DOI] [PubMed] [Google Scholar]
- 22.Andrieu-Abadie N, Jaffrezou JP, Hatem S, Laurent G, Levade T, Mercadier JJ. L-carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: Role of inhibition of ceramide generation. FASEB J. 1999;13:1501–10. doi: 10.1096/fasebj.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 23.Goa KL, Brogden RN. L-Carnitine. A preliminary review of its pharmacokinetics, and its therapeutic use in ischaemic cardiac disease and primary and secondary carnitine deficiencies in relationship to its role in fatty acid metabolism. Drugs. 1987;34:1–24. doi: 10.2165/00003495-198734010-00001. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki N, Lee JD, Shimizu H, Ueda T. Long-term L-carnitine treatment prolongs the survival in rats with adriamycin-induced heart failure. J Card Fail. 1996;2:293–9. doi: 10.1016/s1071-9164(96)80016-9. [DOI] [PubMed] [Google Scholar]


