Abstract
Objectives. To test the relation between white matter lesions (WML) location and physical performance, in aged patients. Methods. Subjects: 29 patients (17 males), aged >65 (mean age 72.6 ± 5.2), with leukoaraiosis. WML was quantified with a visual scale; Apparent Diffusion Coefficient (ADC) was measured bilaterally in frontal periventricular lesioned white matter and frontal and parieto-occipital normal appearing white matter (NAWM). Motor performance was studied using the Short Physical Performance Battery (SPPB), single leg stand time, finger tapping and grooved pegboard tests (GPT). Results. There were significant correlations between the frontal region visual scale scores and SPPB chair stands (r = −0.379; P = .039) and Grooved Pegboard (r = 0.393; P = .032); frontal NAWM ADC values and SPPB standing balance (r = −0.450; P = .014) and SPPB 4 meter walk (r = −0.379; P = .043). Conclusion. Frontal WML are negatively related to motor performance in patients with leukoaraiosis. DWI results suggest that this may be true even for NAWM.
1. Introduction
Leukoaraiosis was the first term introduced to characterize areas of loss of density of white matter observed on computerized tomography of the brain [1]. Later recognized as hyperintense areas on T2-weighted MR images, these findings, although probably related to vascular risk factors, are common in advanced age and thus usually designated age-related white matter changes (ARWMC). Several clinical features have been related to ARWMC, including cognitive impairment, depressive symptoms, mood disturbance, urinary dysfunction, and motor deficits. Among these, gait disturbance probably deserves further attention on account of related functional impairment [2, 3].
The pathophysiology of motor impairment in old age patients with leukoaraiosis is not fully understood. Although frequently attributed to frontal lobe dysfunction, related to disconnection effects of the lesions on white matter tracts [4], few studies have devoted their attention to the relation between ARWMC localization and motor deficits. Recently, conventional MRI studies have suggested that gait disturbance [5] and postural control [6] could be related not only to frontal but also to parieto-occipital (PO) lesions. Others have found association mainly with frontal lesions, when compared with basal ganglia and infratentorial lesions [7].
Difficulty in locating the cause of motor disturbance in patients with leukoaraiosis could be due to the low sensitivity of conventional imaging techniques to more subtle ischemic changes, occurring even in normal appearing white matter (NAWM). This could explain some mismatch between clinical and imaging data. Average apparent diffusion coefficient (ADC), a measure of water diffusion in tissues, presents higher sensitivity to white matter structural changes. It is increased in chronic ischemic WML, probably due to breaking of the anatomic barrier formed by the myelinated axons and also in surrounding NAWM [8]. It could thus be a useful technique to study vascular damage in leukoaraiosis and also in tissue not visibly damaged in conventional MRI, but potentially altered at a microstructural level, permitting to analyze more accurately the relation between white matter vascular alterations and motor function in patients with leukoaraiosis.
On a previous study, we presented data regarding the association between age-related white matter changes and cognitive function, on a sample of old age, community-dwelling subjects with leukoaraiosis [9]. In the present analysis, we present data concerning the relation between motor function and location of ischemic white matter changes.
2. Materials and Methods
2.1. Subjects
We included 30 subjects, according to the following criteria: age above 65 years; no (or mild) disability assessed by Instrumental Activities of Daily Living scale (IADL); presence of ARWMC on CT-Scan, defined as periventricular or subcortical areas of hypodensity (all criteria needed). We exclude patients who presented with leukoencephalopathy from other identified cause, neurological or general incapacitating chronic disease, Modified Rankin Scale >2, dementia, aphasia, and general contraindications for undergoing MRI. Patients were referred to Egas Moniz Hospital Outpatient Neurology Clinic by general practitioners for reasons not directly related to the presence of ARWMC: cognitive complaints (6), stroke (12), gait disturbance (1), depression (2), incidental CT/MRI findings (4), vertigo (2), and other neurological symptoms (3). Stroke was confirmed, by history taking and revision of imaging findings, in 9 patients. Ictus occurred at least three months before study inclusion, leaving no neurological signs. They were all minor strokes, with the following classification: lacunar (5), cardioembolic (2), large artery disease (1), and other cause (1) strokes, occurring. All patients underwent MRI scanning, according to a standardized protocol. All patients provided informed consent, and the local ethic committee approved the protocol. The same sample was also investigated for cognitive impairment. Data related to neuropsychological variables is discussed elsewhere [9].
2.2. MRI Protocol
2.2.1. Imaging Acquisition Protocol
the head was imaged from foramen magnum to superior convexity with General Electric Sigma CV/i 1.5T equipment. Structural acquisition consisted in localizing sequence Sagital T1(FOV 24 cm, thickness 5 mm, Gap 1.5 mm, Matrix 256 × 224, Nex 2); Proton Density and T2 FSE (ET = 4, TR 4000, TE 102, FOV 22 cm, thickness 4 mm, Gap 0, Matrix 320 × 256, Nex 2 and ET = 16, TR 4000, TE 102, FOV 22 cm, thickness 4 mm, Gap 0, Matrix 320 × 256, Nex 2); T2 Fast FLAIR (TR 10000, TE 130, FOV 24 cm, thickness 4 mm, Gap 0-Double acquisition, Matrix 256 × 192, Nex 2); DWI (TR 10000, TE min, FOV 32 cm, thickness 5 mm, Gap 0, Matrix 128 × 128, Nex 1, B = 0 and B = 1000 s/mm2). Diffusion gradients were applied sequentially along six noncollinear directions.
2.3. Image Processing
Two consecutive slices were selected to study average ADC, one for LWM and parieto-occipital NAWM and another for frontal NAWM, respectively. The size of the ROI and the selected planes were previously decided in order to obtain consistent measures and avoid regions with both LWM and NAWM. The observer was unaware of motor test results and ARWMC rating results.
Average ADC was measured on circular, 58 voxel (standard deviation ≤10%) regions of interest (ROI), directly on ADC maps. They were placed bilaterally in parieto-occipital and frontal NAWM, avoiding areas with white matter lesions. We also measured ADC on lesioned frontal periventricular white matter (LWM), on 30 voxel oval ROI, placed near but at least 4 voxels apart from the tip of the anterior horns. (Figure 1).
Figure 1.
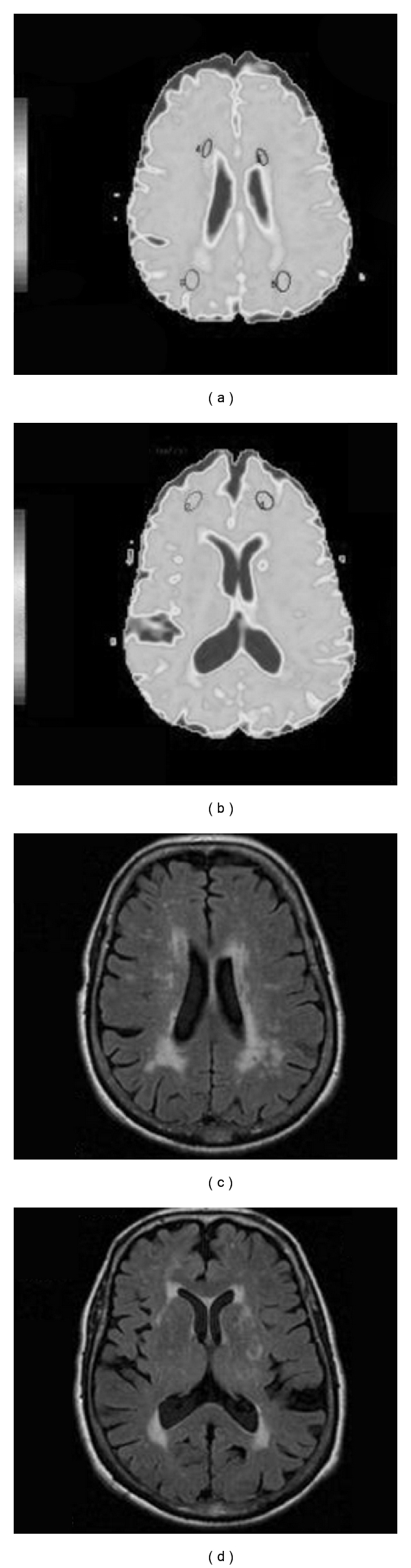
(a) and (b) ADC Map with representation of Regions of interest in Parieto-Occipital Normal Appearing White Matter (NAWM) and lesioned white matter (LWM) (a) and Frontal Normal Appearing White Matter (NAWM) (b). (c) and (d) Corresponding planes in FLAIR sequences.
2.4. White Matter Lesion Load
White matter lesions were scored on FLAIR sequences, bilaterally in frontal, parieto-occipital, temporal, basal ganglia, and infratentorial regions. They were rated retrospectively, by an experienced neuroradiologist (C. J.), blinded to clinical evaluation and DWI results, using a validated visual rating scale [10]. Mean regional scores were used, derived from the average of left and right values.
2.5. Motor Function Assessment
2.5.1. Subjects Underwent the Following Motor Function Tests
Short Physical Performance Battery [11] —
This widely used scale tests lower extremity function, by measures of standing balance (including tandem, semitandem, and side-by-side stands), walking speed (fastest of two times to walk 4 meters), and ability to rise from a chair (time to rise to stand and sit five times). There are five performance scores, ranging from 0 (inability to complete the test) to 4 (highest level of performance). A total score is derived from adding the three partial scores. Based on this score, patients can be divided in three categories: higher mobility (SPPB = 11 or 12), intermediate mobility (SPPB = 9 or 10), or lower mobility (SPPB < 9).
Single Leg Stand Time Best Trial in Seconds —
the subjects were instructed to balance as long as possible on one leg; two trials for each leg were performed, the best time of the four trials being used for analysis.
Finger Tapping from the Unified Parkinson's Disease Rating Scale [12] —
In this section of the Unified Parkinson's Disease Rating Scale the patient is required to tap his/her thumb with index finger in rapid succession. The test is rated as follows: 0 = normal; 1 = mild slowing and/or reduction in amplitude; 2 = moderately impaired; definite and early fatiguing; may have occasional arrests in movement; 3 = severely impaired; frequent hesitation in initiating movements or arrests in ongoing movement; 4 = can barely perform the task. For correlation analysis, right and left scores were averaged.
Grooved Pegboard [13] —
The Grooved Pegboard is a manipulative dexterity test, involving eye-hand coordination capacities. It consists of 25 holes with randomly positioned slots. Pegs with a key along one side must be rotated to match the hole before they can be inserted. The subjects are timed to determine how quickly they can insert grooved pegs into 25 holes in the pegboard, with both hands. For correlation analysis, left and right scores were averaged.
2.6. Statistical Analyses
Differences between ARWMC scores in the various regions and between mean ADC values in different ROI were tested using ANOVA followed by Bonferroni post hoc comparisons analysis.
We performed a data reduction study, using principal component analysis, in account of the high number of motor variables. Eigen values greater than 1 were considered as loading on a factor. Two rotated principal components accounted for 60.07 % of the total variance: Factor 1 (F1) was related mainly to Grooved Pegboard time (r = −0.835), SPPB standing balance (r = 0.764), and SPPB 4 meter walk (r = 0.753); Factor 2 (F2) was related mainly to SPPB chair stands (r = 0.764). Each patient was given a score on these factors. The relation between ADC and Visual Scale scores and motor tests, including F1 and F2, was tested with bivariate correlation analysis (Spearman or Pearson correlation coefficient, as appropriated). P < .05 was considered significant.
3. Results
Of the 30 subjects selected, 1 was excluded from analysis, because technical problems precluded utilization of MRI data. 17 subjects were male. Age ranged from 66 to 85 (mean 72.6 ± standard deviation 5.2).
Motor tests results are shown in Table 1. Regarding SPPB total score, most patients were on the higher (10) and intermediate (17) mobility group, with just two patients in the lower mobility group.
Table 1.
Motor performance results.
| Minimum | Maximum | Mean | Standard deviation | |
|---|---|---|---|---|
| SPPB standing balance | 2 | 4 | 3.87 | 0.43 |
| SPPB 4 meter walk | 3 | 4 | 3.90 | 0.30 |
| SPPB chair stands | 1 | 4 | 2.33 | 0.99 |
| SPPB total score | 7 | 12 | 10.10 | 1.21 |
| Single leg stand time best trial (seconds) | 5 | 60 | 34.87 | 19.83 |
| Finger tap | 0 | 1.50 | 0.75 | 0.54 |
| Grooved pegboard | 80.50 | 417.00 | 126.98 | 60,50 |
SPPB: short physical performance battery.
Visual scale regional scores were significantly different (F = 39.54, P < .0001), with Bonferroni post hoc comparison analysis showing higher scores for frontal and PO regions (P < .0001), with no statistical difference between them. Basal ganglia, temporal, and subtentorial regions were excluded from correlation analysis because there were too many null values. ADC means were significantly higher in LWM (P < .0001). There was no significant difference between PO and frontal means (imaging data comparisons are discussed elsewhere [9]).
We found a global trend to negative correlations between the frontal lobe visual scale scores and ADC and motor performance. Correlations were significant between frontal visual scale scores and Factor 1 (r = −0.468; P = .012). There were significant correlations between the frontal region and SPPB chair stands (r = −0.379; P = .039) and Grooved Pegboard (r = 0.393; P = .032) (Table 2). Frontal NAWM ADC values were significantly correlated to Factor 1 (r = −0.379; P = .043). Significant correlations were found between frontal NAWM and SPPB standing balance (r = −0.450; P = .014) and SPPB 4 meter walk (r = −0.379; P = .043) (Table 2).
Table 2.
Correlations between imaging data and motor performance tests.
| VS: frontal | VS: PO | DWI: LWM | DWI: frontal NAWM | DWI: PO NAWM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Factor 1 | −0.468 | .012* | −0.364 | .052 | −0.364 | .052 | −0.379 | .043* | −0.048 | .804 |
| Factor 2 | −0.176 | .353 | −0.017 | .931 | −0.017 | .931 | 0.212 | .270 | −0.007 | .972 |
| SPPB standing balance | −0.251 | .180 | −0.333 | .078 | −0.333 | .078 | −0.450 | .014* | −0.335 | .076 |
| SPPB 4 meter walk | −0.189 | .316 | −0.108 | .576 | −0.108 | .576 | −0.379 | .043* | −0.230 | .230 |
| SPPB chair stands | −0.379 | .039* | −0.156 | .420 | −0.156 | .420 | 0.269 | .158 | −0.116 | .548 |
| Single leg stand time best trial (seconds) | −0.057 | .767 | −0.152 | .430 | −0.152 | .430 | −0.234 | .221 | −0.072 | .711 |
| Finger Tap | 0.358 | .052 | 0.258 | .176 | 0.258 | .176 | 0.279 | .142 | 0.092 | .636 |
| Grooved Pegboard | 0.393 | .032* | 0.291 | .126 | 0.291 | .126 | 0.274 | .151 | 0.131 | .500 |
Values are as follows: r: spearman coefficient; P: level of significance; *P < .05; SPPB: short physical performance battery; VS: visual scale score; DWI: diffusion-weighted imaging; LWM: lesioned white matter; NAWM: normal appearing white matter; PO: parieto-occipital.
To account for the potential influence of age, correlations between imaging and motor variables were repeated, using partial correlation analysis, controlling for age. Correlations between SPPB and frontal ADC remained significant for NAWM and became significant for LWM. Correlation between frontal visual scale scores and SPPB became less significant, but still showed a trend to significance (P < .1) (Table 3).
Table 3.
Correlations between imaging data and motor performance tests, controlling for age.
| Frontal | PO | LWM | Frontal NAWM | PO NAWM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Factor 1 | −0.107 | .582 | 0.259 | .174 | −0.433 | .021* | −0.382 | .045* | −0.155 | .430 |
| Factor 2 | −0.107 | .582 | 0.259 | .174 | −0.011 | .957 | 0.197 | .316 | −0.027 | .893 |
| SPPB standing balance | −0.083 | .669 | −0.006 | .975 | −0.458 | .014* | −0.465 | .013* | −0.217 | .267 |
| SPPB 4 meter walk | 0.062 | .749 | 0.406 | .029* | −0.113 | .566 | −0.199 | .310 | −0.136 | .491 |
| SPPB chair stands | −0.341 | .070 | −0.199 | .300 | −0.099 | .616 | 0.400 | .035* | −0.114 | .564 |
| Single leg stand time best trial (seconds) | 0.036 | .853 | 0.327 | .083 | −0.148 | .453 | −0.107 | .588 | −0.066 | .740 |
| Finger Tap | 0.271 | .155 | 0.034 | .861 | 0.245 | .209 | 0.129 | .513 | −0.012 | .952 |
| Grooved Pegboard | 0.086 | .656 | 0.255 | .181 | −0.325 | .092 | −0.283 | .144 | 0.001 | .997 |
Values are as follows: r: partial correlation coefficient, controlling for age; P: level o0f significance, *P < .05; SPPB: short physical performance battery; DWI: diffusion-weighted imaging; LWM: lesioned white matter; NAWM: normal appearing white matter; PO: parieto-occipital.
To avoid the potential bias produced by the inclusion of stroke patients in our subject group, we compared imaging and physical performance results between patients with and without a history of stroke (independent samples Student's t-tests). We found no significant differences.
4. Discussion
We have investigated the relation between ischemic changes and motor function in a sample of 29 community-dwelling old age patients, with relatively good motor performance, as shown by high scores on the SPPB (most patients were in the higher/intermediate mobility categories). Significant correlations were found between ischemic white matter changes identified by conventional and DWI imaging techniques and several motor performance variables, including a rotated principal component (F1), related both to lower and upper limb function. These results are in accordance with several studies [14–26] and confirm the influence of white matter changes on old age subjects' motor function. Single variable analysis was in accordance with principal factor analysis, showing significant correlations between imaging data and SPPB items (standing balance, chair stands, walking speed) and Grooved Pegboard, suggesting a relation between white matter damage and gait velocity, balance and upper limb dexterity.
Some findings may additionally contribute to a better understanding of the pathophysiology of motor disturbance caused by leukoaraiosis. Patients with leukoaraiosis and those with frontal lobe lesions (or, in which, basal-ganglia/frontal lobe connections are deranged) show some similarities in gait disturbance. This leads to the concept that gait disturbance in leukoaraiosis could be due to disconnection between the motor and premotor frontal regions and the basal ganglia, related to frontal ARWMC [4]. In the present study we found significant correlation between gait dysfunction and frontal but not PO white matter lesions, although both regions showed similar degrees of white matter damage, thus corroborating the frontal lobe hypothesis.
As far as we are aware, few studies have investigated the relation between white matter lesions in particular regions and motor function, none of them using DWI techniques. Using the same gait measures (SPPB), despite different imaging methods, Benson and coworkers [5] found frontal and parieto-occipital lesions to be related to lower mobility in patients with leukoaraiosis. While the first location showed high sensitivity, despite low specificity, to predict gait impairment, the latter showed high specificity but low sensitivity. Contrary to Benson et al., we did not find any significant correlation between PO ischemic changes and motor function. This is true also for PO NAWM, as shown by the DWI study, which has higher sensitivity for subtle white matter changes. Thus, these results do not support the hypothesis put forward by the authors that PO WML low sensitivity for gait impairment could be due to underestimation of tissue damage caused by microscopically, nonvisible alterations. We should say, however, that our patients generally presented with higher mobility than those reported in that study, and, therefore, they could be in a less advanced stage of the disease. This would suggest that frontal lesions could have a more important role in gait impairment on the initial stages of the disease. Novak and collaborators' study [6] found that PO lesions also correlated significantly with postural control measures. This study used automated measures of gait and posture and assessed a great number of patients, which may have permitted to detect more subtle relations between motor variables and WML.
Results from visual scale scoring and DWI point roughly to the same conclusions, that is, a relation between motor function and frontal lobe ischemic changes. However, the significant correlations found between frontal NAWM and several motor variables suggest that nonvisible white matter damage can have influence on patients' motor ability, as has been already shown for cognitive functions [9, 27]. More recently, a study using magnetic transfer imaging also showed relation between motor dysfunction in old age subjects and microstructural tissue damage, not seen in conventional MRI [28].
Our study has some limitations, one being not using automated methods to quantify WML. We should say, however, that in a recent analysis both automated methods and the visual scale used in this study had comparable power in discriminating between leukoaraiosis patients with and without gait complaints [29]. The large number of motor performance variables and the relatively low level of significance of the correlations found (P < .05) could have made separate variables correlation analysis less reliable. However, we should point that these results were mostly concordant with the results from data reduction analysis. Besides, they were similar in both imaging techniques. We also did not use diffusion tensor imaging (DTI) techniques, which are considered to be more specific than ADC in detecting white matter structural changes, and we did not perform inter- or intraobserver reproducibility tests for image analysis. As main advantages of the present study, we underline the small number of diffusion studies addressing motor disturbances in patients with white matter lesions, ADC measurement in both LWM and NAWM, and use of regional scores to quantify lesion load. In conclusion, our findings suggest that white matter changes are negatively related to balance, gait velocity, and hand dexterity in old age patients with leukoaraiosis and that this could be due mainly to frontal lobe derangement. DWI results suggest that this may be true even for NAWM.
Acknowledgment
This investigation was partially supported by a grant from Bristol-Myers Squibb, Portugal.
References
- 1.Hackinski VC, Potter P, Merskey H. Leuko-araiosis. Archives of Neurology. 1987;44(1):21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 2.Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger’s report: a review. Stroke. 1995;26(7):1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- 3.Baune BT, Schmidt WP, Roesler A, Berger K. Functional consequences of subcortical white matter lesions and mri-defined brain infarct in an elderly general population. Journal of Geriatric Psychiatry and Neurology. 2009;22(4):266–273. doi: 10.1177/0891988709342722. [DOI] [PubMed] [Google Scholar]
- 4.Thompson PD, Marsden CD. Gait disorder of subcortical arteriosclerotic encephalopathy: Binswanger’s disease. Movement Disorders. 1987;2(1):1–8. doi: 10.1002/mds.870020101. [DOI] [PubMed] [Google Scholar]
- 5.Benson RR, Guttmann CRG, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58(1):48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- 6.Novak V, Haertle M, Zhao P, et al. White matter hyperintensities and dynamics of postural control. Magnetic Resonance Imaging. 2009;27(6):752–759. doi: 10.1016/j.mri.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blahak C, Baezner H, Pantoni L, et al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80(6):608–613. doi: 10.1136/jnnp.2008.154633. [DOI] [PubMed] [Google Scholar]
- 8.Helenius J, Soinne L, Salonen O, Kaste M, Tatlisumak T. Leukoaraiosis, ischemic stroke, and normal white matter on diffusion-weighted MRI. Stroke. 2002;33(1):45–50. doi: 10.1161/hs0102.101228. [DOI] [PubMed] [Google Scholar]
- 9.Viana-Baptista M, Bugalho P, Jordão C, et al. Cognitive function correlates with frontal white matter apparent diffusion coefficients in patients with leukoaraiosis. Journal of Neurology. 2008;255(3):360–366. doi: 10.1007/s00415-008-0661-9. [DOI] [PubMed] [Google Scholar]
- 10.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 11.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journals of Gerontology. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Elton RL. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ, USA: Macmollan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 13.Lezak MD. Neuropsychological Assessment. 2nd edition. New York, NY, USA: Oxford Univer Press; 1983. [Google Scholar]
- 14.Inzitari D, Simoni M, Pracucci G, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Archives of Internal Medicine. 2007;167(1):81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Rosano C, Kuller LH, Chung H, et al. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. Journal of the American Geriatrics Society. 2005;53(4):649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 16.Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57(6):990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- 17.Guttmann CRG, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54(6):1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 18.Kerber KA, Enrietto JA, Jacobson KM, Baloh RW. Disequilibrium in older people: a prospective study. Neurology. 1998;51(2):574–580. doi: 10.1212/wnl.51.2.574. [DOI] [PubMed] [Google Scholar]
- 19.Tell GS, Lefkowitz DS, Diehr P, Elster AD. Relationship between balance and abnormalities in cerebral magnetic resonance imaging in older adults. Archives of Neurology. 1998;55(1):73–79. doi: 10.1001/archneur.55.1.73. [DOI] [PubMed] [Google Scholar]
- 20.Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74(1):94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. Journal of the Neurological Sciences. 2005;232(1-2):23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Bäzner H, Oster M, Daffertshofer M, Hennerici M. Assessment of gait in subcortical vascular encephalopathy by computerized analysis: a cross-sectional and longitudinal study. Journal of Neurology. 2000;247(11):841–849. doi: 10.1007/s004150070070. [DOI] [PubMed] [Google Scholar]
- 23.Longstreth WT, Jr., Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the cardiovascular health study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 24.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS Study. Neurology. 2008;70(12):935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 25.Srikanth V, Beare R, Blizzard L, et al. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40(1):175–180. doi: 10.1161/STROKEAHA.108.524355. [DOI] [PubMed] [Google Scholar]
- 26.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS Study. Neurology. 2008;70(12):935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 27.Shenkin SD, Bastin ME, MacGillivray TJ, et al. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovascular Diseases. 2005;20(5):310–318. doi: 10.1159/000087930. [DOI] [PubMed] [Google Scholar]
- 28.Rosano C, Sigurdsson S, Siggeirsdottir K, et al. Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiology of Aging. 2010;31(7):1197–1204. doi: 10.1016/j.neurobiolaging.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouw AA, Van Der Flier WM, Van Straaten ECW, et al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. Journal of Neurology. 2006;253(9):1189–1196. doi: 10.1007/s00415-006-0193-5. [DOI] [PubMed] [Google Scholar]


