Abstract
Objectives:
The pathogenesis of neuropathy in type 2 diabetes mellitus is multifactorial.Dyslipidemia may contribute to the development of diabetic neuropathy. This study aimed to assess the atherogenic lipid indices in type 2 diabetic patients with neuropathy.
Material and Methods:
Fifty-one patients with type 2 diabetes mellitus and 31 healthy subjects were studied in the Unit of Neurophysiology at the University Hospital of Medical College, Al-Nahrin University in Baghdad, Iraq, from January 2002 to January 2003. Neuropathy total symptom score (NTSS), neuropathy impairment score in the lower leg (NIS-LL), and electrophysiological study of sensory (ulnar and sural) and motor (ulnar and common peroneal) nerves were used to assess nerve function. Fasting venous blood was obtained from each participant for determination of lipid profile and atherogenic lipid ratios.
Results:
The frequency of high blood pressure was significantly higher in neuropathic patients. The electrophysiology study revealed significant decrease in conduction velocity of ulnar (sensory and motor components), sural, and common peroneal nerves. The minimum F-wave latency of motor nerve was significantly prolonged. Among the lipid fractions, only high-density lipoprotein–cholesterol was significantly reduced by 14% of healthy participant's value. Atherogenic lipid ratios were significantly higher in diabetic patients than corresponding healthy ratios.
Conclusion:
Metabolic lipid disturbances in terms of atherogenicity co-existwith neuropathy in type 2 diabetes mellitus, irrespective of duration of disease.
Keywords: Atherogenic ratios, neuropathy, type 2 diabetes
INTRODUCTION
Diabetic neuropathy is the most common neuropathy and is associated with a wide range of clinical manifestations. It tends to occur after 50 years of age, and mostly in patients with long-standing diabetes mellitus.[1] It results from microvascular injury involving small blood vessels that supply nerves , but macrovascular pathogenesis is also involved.[2] The pathogenesis of sensorimotor neuropathy in diabetes mellitus type 2 is multifactorial and related metabolic disturbances, such as hyperglycemia, dyslipidemia, oxidative and nitrosative stress, and growth factor deficiency all contribute to the development of this complication.[3] Several metabolic products, such as advanced glycated end products, activation of protein kinase C enzyme, and sorbitol aldose reductase (polyol) pathway flux are claimed to have a role in pathogenesis of neuropathy.[4]
Dyslipidemia is a significant contributor to the development of diabetic neuropathy via inducing oxidative stress in root ganglia sensory neurons.[5,6] Fujita et al. reported that short-term glycemic control in patients with type 2 diabetes mellitus can improve vibratory sensation, metabolic changes in glucose and lipids (total cholesterol, triglyceride, and free fatty acids) being the factors responsible for impairment of peripheral nerve function.[7] On the other hand, a previous study showed that there was no significant difference in serum cholesterol of type 2 diabetic patients with negative and positive sensorimotor neuropathy.[8] Atherogenic lipid indices including total cholesterol/high-density lipoprotein cholesterol, low-density lipoprotein–cholesterol, triglycerides/high-density lipoprotein and other ratios are predictors for atherosclerosis in type 2 diabetes mellitus but not for neuropathy.[9,10]
This study aimed to explore the status of some atherogenic lipid indices in type 2 diabetes neuropathy assessed by neuropathy total symptom score (NTSS), neuropathy impairment score in the lower leg (NIS-LL), and electrophysiological studies of sensory and motor nerves.
MATERIALS AND METHODS
This study was conducted in the Unit of Neurophysiology, the University Hospital of Medical College, Al-Nahrin University in Baghdad, Iraq, from January 2002 to January 2003. The study is approved by the local scientific committee of Medical College, Al-Nahrin University. A consent form was obtained from each participant prior to the study. A total number of 51 patients with a history of type 2 diabetes mellitus, presenting with subjective symptoms of peripheral neuropathy, as well as 31 healthy subjects, serving as control, were enrolled in the study.
Each patient and subject was clinically examined and the severity as well as the functional impairment were assessed using neuropathy total symptom score (NTSS)[11] and neuropathy impairment score in the lower leg (NIS-LL).[12]
Neuropathy total symptom score is a questionnaire that measures the frequency and intensity of numbness, pickling, aching pain, burning pain, lacinating pain, and allodynia). In this measure, the symptom frequency is graded as occasional, frequent, and continuous while the symptom intensity is graded as absent, slight, moderate, and severe. Therefore, the score ranged between 0 (absent symptom) and 3.66 (severe continuous symptom).
The neuropathy impairment score in lower limbs has 14 items and evaluates the muscle power, reflexes, and sensory modalities. The total score ranged between 0 and 88 points. The age factor is considered in scoring the reflexes using NTSS.
Electrophysiological measurements, using DANTEC counterpoint clinical four-channel electromyography system, included nerve conduction velocity, minimum F-wave latency and sensory refractory period. The electrophysiological study of sensory (ulnar and sural) and motor (ulnar and common peroneal nerves were assessed.
None of participants was used lipid lowering agents, and patients with hypertension were on angiotensin converting enzyme inhibitors).
A fasting venous blood sample were obtained from participants and sera were separated for determination of glucose and lipid profile, including serum total cholesterol (TC), triglycerides (TG), and high-density lipoprotein–cholesterol (HDL–c). Very low–density lipoprotein is equal to 1/5 TG level and the LDL–c is calculated according to the Friedewald equation {LDL = TC – (HDL + VLDL)}. The following atherogenic lipid indices were calculated: (TC – HDL–c)/HDL, LDL/HDL, and log (TG/HDL).The later represented small dense LDL particles.
Statistical analysis
The results were analyzed using Excel 2003. The results are presented as number, percent, odds ratio, and mean ± SE. The data were analyzed using two-tailed unpaired Student's t test, difference between percentages test and simple correlation test taking P ≤ 0.05 as the lowest limit of significance.
RESULTS
Table 1 shows the characteristics of the study participants. There is nonsignificant difference (P > 0.05) between nondiabetic and diabetic groups regarding the age. A history of high blood pressure was significantly reported more often in the diabetic group compared with nondiabetic participants (50.9% vs 22.6%, respectively, P < 0.01). Fasting serum glucose and duration of illness of diabetic patients at the time of the study were ranged from 115 to 360 mg/dL and 3 months to 23 years, respectively. Forty-six out of 51 diabetics scored TSS (maximum score was 11.32) compared with 5 out of 31 healthy individuals (maximum score was 4.66) (odds ratio 47.8), and 39 out of 51 diabetics scored NIS-LL (maximum score was 28) compare with 3 out of 31 healthy individuals (maximum score was 9.7) (odds ratio 30.33).
Table 1.
The characteristics of the study
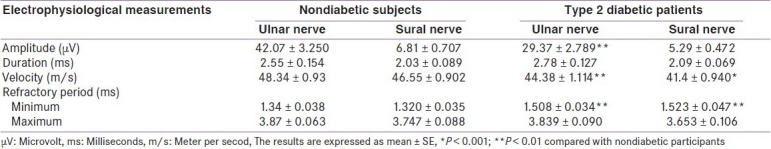
Table 2 shows the electrophysiological study of sensory component of ulnar nerve and sural nerve. The means of amplitude (29.37 ± 2.789 μV) and conduction velocity (44.38 ± 1.114 m/s) of ulnar nerve impulse among diabetics were significantly (P < 0.01) less than those observed with healthy individuals (42.07 ± 3.250 μV and 48.34±0.93 m/s, respectively). The minimum value of refractory period of ulnar nerve impulse was significantly prolonged among diabetics compared with healthy participants (1.508 ± 0.034 vs 1.34 ± 0.038 ms, respectively, P < 0.01). The significant changes in the electrophysiological study; decreased conduction velocity (41.4 ± 0.940 vs 46.55 ± 0.902 m/s, P < 0.001); and prolonged the minimum refractory period (1.523 ± 0.047 vs 1.320 ± 0.035 ms, P < 0.01) of sural nerve were observed [Table 2].
Table 2.
Electrophysiological findings of sensory nerves (ulnar and sural) in patients with type 2 diabetes mellitus compared with nondiabetic subjects
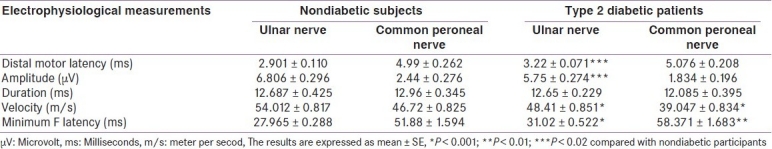
The significant changes in terms of decreased conduction velocity and prolonged of minimum F latency were observed in both motor component of ulnar nerve and common peroneal nerve in diabetic patients compared with healthy individuals [Table 3]. The prolonged distal motor latency (3.22 ± 0.071 ms) and the reduction in the amplitude of nerve impulse (5.75 ± 0.274 μV) were significantly (P < 0.001) observed in motor component of ulnar nerve.
Table 3.
Electrophysiological findings of motor nerves (ulnar and common peroneal) in patients with type 2 diabetes mellitus compared with nondiabetic subjects
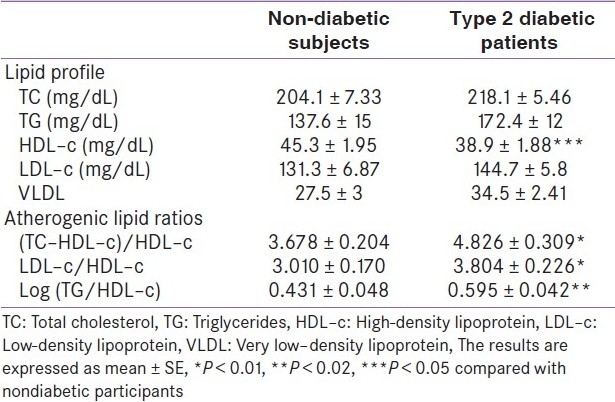
Although the changes in the lipid profile were in terms of high serum TC, triglycerides, LDL–c, and VLDL in diabetic patients, they did not reach to a statistically significant level [Table 4]. The significant decrease in serum HDL–c in diabetic patients was an approximated 14% of mean healthy participants, was observed. Significantly high values of atherogenic lipid ratios were observed in diabetic patients compared with healthy participants [Table 4].
Table 4.
Fasting serum lipid profile and atherogenic lipid ratios
DISCUSSION
The results reported in this study revealed the existence of disturbances in lipid metabolism in type 2 diabetic patients presented with peripheral neuropathy.
The significant high ratios of atherogenicity in diabetic patients compared with healthy subjects explained the significant high frequency of diabetics having high blood pressure. This observation is in agreement with Shera et al., who reported the association of microvascular complications with hypertension.[1]
Neuropathic pain and other symptoms occur in about 6%–7% of the general population and this explains the scoring of NTSS and NIC–LL in healthy subjects. The nerve conduction study revealed a significant decrease in conduction velocities of both sensory and motor nerves and prolonged the minimum refractory periods of sensory nerves and minimum F-wave latency of motor nerves. These findings are commonly observed in clinical practice in diabetic patients. It is important to mention here that using of multivitamins, including vitamin B12 as over-the-counter drugs may alter the nerve conduction study. Previous studies showed that vitamins may reverse the symptoms and pathophysiology of diabetic neuropathy.[13] Moreover, patients on oral hypoglycemic agents notably metformin may develop vitamin B12 deficiency[14] and vitamin B12 is effective in alleviating the symptoms of neuropathy.[15]
The patterns of lipid profile as well as the atherogenic lipid ratios reported in this study were in agreement with earlier and current studies. Abdul-Ghani et al. reported significant association of metabolic syndrome (TG and HDL–c contributed in metabolic syndrome criteria) in type 2 diabetes mellitus with a high risk (odds ratio 1.77) of neuropathy,[16] while serum cholesterol did not differ in diabetic patients with neuropathy compared with those without neuropathy.[8] Moreover, Agrawal et al. found that neuropathy did not have any significant correlation with lipid profile abnormalities in type 2 diabetes mellitus.[17] It is unlikely that the observed results are related to the medications because none of our patients received lipid lowering agents and the concomitant use of angiotensin converting enzyme inhibitors did not alter the electrophysiological properties and lipid profile.
Diabetic peripheral neuropathy as well as diabetic autonomic neuropathy did not correlate with lipid profile.[18] The significant high Log(TG/HDL–c) ratio, which indicates the presence of small-density low-density lipoprotein may herald the existence of atherosclerosis. Shoji et al.[19] demonstrated high levels of small dense, low-density lipoprotein in patients with type 2 diabetes mellitus and it can be used as marker in the risk assessment for atherosclerosis. Therefore, the lipid profiles are not adequate in studying the metabolic disturbances in type 2 diabetes mellitus and should be supplemented with determination of atheogenic lipid ratios, which explore the hidden atherosclerosis. The findings of this study reinforce an important message to clinicians to assess the lipid profile , including lipid ratios and Log(TG/HDL–c) ratio and evaluate for , preclinical atherosclerosis in diabetic patients who complain of neuropathic signs and symptoms.
We conclude that metabolic lipid disturbances in terms of atherogenicity co-exists with neuropathy in type 2 diabetes mellitus despite duration of disease and is a useful marker for preclinical atherosclerosis. Therefore, each diabetic neuropathy patient need to be investigated thoroughly.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shera AS, Jawad F, Maqsood A, Jamal S, Azfar M, Ahmed U. Prevalence of chronic complications and associated factors in type 2 diabetes. J Pak Med Assoc. 2004;54:54–9. [PubMed] [Google Scholar]
- 2.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinik A. The approach to the management of the patient with neuropathic pain. J Clin Endocrinol Metab. 2010;95:4802–11. doi: 10.1210/jc.2010-0892. [DOI] [PubMed] [Google Scholar]
- 4.Duby JJ, Campbell RK, Setter SM, White JR, Rasmussen KA. Diabetic neuropathy: An intensive review. Am J Health Syst Pharm. 2004;61:160–73. doi: 10.1093/ajhp/61.2.160. [DOI] [PubMed] [Google Scholar]
- 5.Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: A new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14:257–67. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: The role of oxLDL/LOX-1. Diabetes. 2009;58:2376–85. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita Y, Fukushima M, Suzuki H, Taniguchi A, Nakai Y, Kuroe A, et al. Short-term intensive glycemic control improves vibratory sensation in type 2 diabetes. Diabetes Res Clin Pract. 2008;80:e16–9. doi: 10.1016/j.diabres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Jarmuzewska EA, Ghidoni A, Mangoni AA. Hypertension and Sensorimotor Peripheral Neuropathy in Type 2 Diabetes. Eur Neurol. 2006;57:91–5. doi: 10.1159/000098058. [DOI] [PubMed] [Google Scholar]
- 9.Temelkova-Kurktschiev T, Hanefeld M. The lipid triad in type 2 diabetes - prevalence and relevance of hypertriglyceridaemia/low high-density lipoprotein syndrome in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2004;112:75–9. doi: 10.1055/s-2004-815753. [DOI] [PubMed] [Google Scholar]
- 10.Décary S, Dumont G, Lamarche B, Hogue JC, Tremblay AJ, Bergeron J, et al. Assessment of the validity of the frequently used lipid indices for predicting LDL peak particle diameter in a large cohort of 1955 normal and dyslipidemic subjects. Clin Biochem. 2010;43:401–6. doi: 10.1016/j.clinbiochem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schütte K, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid.A 3-week multicentre randomized controlled trial (ALADIN Study) Diabetologia. 1995;38:1425–33. doi: 10.1007/BF00400603. [DOI] [PubMed] [Google Scholar]
- 12.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O′Brien PC. Variables influencing neuropathic endpoints: The Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology. 1995;45:1115–21. doi: 10.1212/wnl.45.6.1115. [DOI] [PubMed] [Google Scholar]
- 13.Walker MJ, Morris LM, Cheng D. Improvement of cutaneous sensitivity in diabetic peripheral neuropathy with combination L-methylfolate, methylcobalamin, and pyridoxal 5´-phosphate. Rev Neurol Dis. 2010;7:132–9. [PubMed] [Google Scholar]
- 14.Bell DS. Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathy. South Med J. 2010;103:265–7. doi: 10.1097/SMJ.0b013e3181ce0e4d. [DOI] [PubMed] [Google Scholar]
- 15.Talaei A, Siavash M, Majidi H, Chehrei A. Vitamin B12 may be more effective than nortriptyline in improving painful diabetic neuropathy. Int J Food Sci Nutr. 2009;60:71–6. doi: 10.1080/09637480802406153. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Ghani M, Nawaf G, Nawaf F, Itzhak B, Minuchin O, Vardi P. Increased prevalence of microvascular complications in type 2 diabetes patients with the metabolic syndrome. Isr Med Assoc J. 2006;8:378–82. [PubMed] [Google Scholar]
- 17.Agrawal RP, Sharma P, Pal M, Kochar A, Kochar DK. Magnitude of dyslipidemia and its association with micro and macro vascular complications in type 2 diabetes: A hospital based study from Bikaner (Northwest India) Diabetes Res Clin Pract. 2006;73:211–4. doi: 10.1016/j.diabres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Meyer C, Milat F, McGrath BP, Cameron J, Kotsopoulos D, Teede HJ. Vascular dysfunction and autonomic neuropathy in Type 2 diabetes. Diabet Med. 2004;21:746–51. doi: 10.1111/j.1464-5491.2004.01241.x. [DOI] [PubMed] [Google Scholar]
- 19.Shoji T, Hatsuda S, Tsuchikura S, Shinohara K, Kimoto E, Koyama H, et al. Small dense low-density lipoprotein cholesterol concentration and carotid atherosclerosis. Atherosclerosis. 2009;202:582–8. doi: 10.1016/j.atherosclerosis.2008.04.042. [DOI] [PubMed] [Google Scholar]


