Abstract
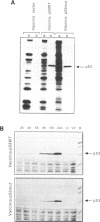
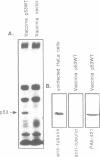
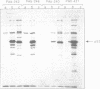
To facilitate the purification of wild type p53 protein, we established a recombinant p53 vaccinia viral expression system. Using this efficient eukaryotic expression vector, we found that the expressed p53 proteins retained their specific structural characteristics. A comparison between wild type and mutant p53 proteins showed the conservation of the typical subcellular localization and the expression of specific antigenic determinants. Furthermore, wild type p53 exhibited a typical binding with large T antigen, whereas no binding was detected with mutant p53. Both wild type and mutant p53 proteins were highly stable and constituted 5-7% of total protein expressed in the infected cells. These expression recombinant viruses offer a simple, valuable system for the purification of wild type and mutant p53 proteins that are expressed abundantly in eukaryotic cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison C., Jenkins J. R., Stürzbecher H. W. The p53 nuclear localisation signal is structurally linked to a p34cdc2 kinase motif. Oncogene. 1990 Mar;5(3):423–426. [PubMed] [Google Scholar]
- Ahuja H., Bar-Eli M., Advani S. H., Benchimol S., Cline M. J. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N., Nomura D., Yokota K., Wolf D., Brill E., Shohat O., Rotter V. Immunologically distinct p53 molecules generated by alternative splicing. Mol Cell Biol. 1986 Sep;6(9):3232–3239. doi: 10.1128/mcb.6.9.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Sturzbecher H. W., Addison C., Palmer C., Rudge K., Jenkins J. R. Mouse p53 inhibits SV40 origin-dependent DNA replication. Nature. 1987 Oct 1;329(6138):458–460. doi: 10.1038/329458a0. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Chen Y. M., Bookstein R., Lee W. H. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990 Dec 14;250(4987):1576–1580. doi: 10.1126/science.2274789. [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Chen P. L., Arnaiz N., Goodrich D., Lee W. H. Expression of wild-type p53 in human A673 cells suppresses tumorigenicity but not growth rate. Oncogene. 1991 Oct;6(10):1799–1805. [PubMed] [Google Scholar]
- Ciechanover A., DiGiuseppe J. A., Bercovich B., Orian A., Richter J. D., Schwartz A. L., Brodeur G. M. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):139–143. doi: 10.1073/pnas.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Nuclear and nucleolar targeting sequences of c-erb-A, c-myb, N-myc, p53, HSP70, and HIV tat proteins. J Biol Chem. 1989 Oct 25;264(30):18019–18023. [PubMed] [Google Scholar]
- Deppert W., Buschhausen-Denker G., Patschinsky T., Steinmeyer K. Cell cycle control of p53 in normal (3T3) and chemically transformed (Meth A) mouse cells. II. Requirement for cell cycle progression. Oncogene. 1990 Nov;5(11):1701–1706. [PubMed] [Google Scholar]
- Diller L., Kassel J., Nelson C. E., Gryka M. A., Litwak G., Gebhardt M., Bressac B., Ozturk M., Baker S. J., Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Selby M. J., Mobley W. C., Weinrich S. L., Hruby D. E., Rutter W. J. Processing and secretion of nerve growth factor: expression in mammalian cells with a vaccinia virus vector. Mol Cell Biol. 1988 Jun;8(6):2456–2464. doi: 10.1128/mcb.8.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexner C., Broyles S. S., Earl P., Chakrabarti S., Moss B. Characterization of human immunodeficiency virus gag/pol gene products expressed by recombinant vaccinia viruses. Virology. 1988 Oct;166(2):339–349. doi: 10.1016/0042-6822(88)90504-1. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987 Oct 1;329(6138):456–458. doi: 10.1038/329456a0. [DOI] [PubMed] [Google Scholar]
- Ginsberg D., Michael-Michalovitz D., Ginsberg D., Oren M. Induction of growth arrest by a temperature-sensitive p53 mutant is correlated with increased nuclear localization and decreased stability of the protein. Mol Cell Biol. 1991 Jan;11(1):582–585. doi: 10.1128/mcb.11.1.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Halevy O., Hall A., Oren M. Stabilization of the p53 transformation-related protein in mouse fibrosarcoma cell lines: effects of protein sequence and intracellular environment. Mol Cell Biol. 1989 Aug;9(8):3385–3392. doi: 10.1128/mcb.9.8.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Frey A. B., Levine A. J. Immunological evidence for the association of p53 with a heat shock protein, hsc70, in p53-plus-ras-transformed cell lines. Mol Cell Biol. 1987 Aug;7(8):2863–2869. doi: 10.1128/mcb.7.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Plowman G. D., Sridhar P., Stevenson U. S., Brown J. P., Estin C. D. Characterization of a recombinant vaccinia virus expressing human melanoma-associated antigen p97. J Virol. 1988 Jan;62(1):176–180. doi: 10.1128/jvi.62.1.176-180.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänggi M., Bannwarth W., Stunnenberg H. G. Conserved TAAAT motif in vaccinia virus late promoters: overlapping TATA box and site of transcription initiation. EMBO J. 1986 May;5(5):1071–1076. doi: 10.1002/j.1460-2075.1986.tb04324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman Z., Prokocimer M., Peller S., Kahn Y., Rechavi G., Manor Y., Cohen A., Rotter V. Rearrangements in the p53 gene in Philadelphia chromosome positive chronic myelogenous leukemia. Blood. 1989 Nov 15;74(7):2318–2324. [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Baker S. J., Nigro J. M., Rotter V., Levine A. J., Friedman P., Prives C., Vogelstein B. Mutant p53 proteins bind DNA abnormally in vitro. Oncogene. 1991 Jan;6(1):131–136. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990 Jan;4(1):1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Lin J. Y., Simmons D. T. Transformation by simian virus 40 does not involve the mutational activation of p53 to an oncogenic form. Virology. 1990 May;176(1):302–305. doi: 10.1016/0042-6822(90)90258-s. [DOI] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- May E., Jenkins J. R., May P. Endogenous HeLa p53 proteins are easily detected in HeLa cells transfected with mouse deletion mutant p53 gene. Oncogene. 1991 Aug;6(8):1363–1365. [PubMed] [Google Scholar]
- Mercer W. E., Amin M., Sauve G. J., Appella E., Ullrich S. J., Romano J. W. Wild type human p53 is antiproliferative in SV40-transformed hamster cells. Oncogene. 1990 Jul;5(7):973–980. [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Oren M., Reich N. C., Levine A. J. Regulation of the cellular p53 tumor antigen in teratocarcinoma cells and their differentiated progeny. Mol Cell Biol. 1982 Apr;2(4):443–449. doi: 10.1128/mcb.2.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhasi-Kimhi O., Michalovitz D., Ben-Zeev A., Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986 Mar 13;320(6058):182–184. doi: 10.1038/320182a0. [DOI] [PubMed] [Google Scholar]
- Prokocimer M., Shaklai M., Bassat H. B., Wolf D., Goldfinger N., Rotter V. Expression of p53 in human leukemia and lymphoma. Blood. 1986 Jul;68(1):113–118. [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter V., Boss M. A., Baltimore D. Increased concentration of an apparently identical cellular protein in cells transformed by either Abelson murine leukemia virus or other transforming agents. J Virol. 1981 Apr;38(1):336–346. doi: 10.1128/jvi.38.1.336-346.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G., Ben-Ze'ev A., Rotter V. Subcellular distribution of the p53 protein during the cell cycle of Balb/c 3T3 cells. Oncogene. 1990 Nov;5(11):1707–1711. [PubMed] [Google Scholar]
- Shaulsky G., Goldfinger N., Ben-Ze'ev A., Rotter V. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol Cell Biol. 1990 Dec;10(12):6565–6577. doi: 10.1128/mcb.10.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G., Goldfinger N., Peled A., Rotter V. Involvement of wild-type p53 in pre-B-cell differentiation in vitro. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8982–8986. doi: 10.1073/pnas.88.20.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G., Goldfinger N., Peled A., Rotter V. Involvement of wild-type p53 protein in the cell cycle requires nuclear localization. Cell Growth Differ. 1991 Dec;2(12):661–667. [PubMed] [Google Scholar]
- Shaulsky G., Goldfinger N., Rotter V. Alterations in tumor development in vivo mediated by expression of wild type or mutant p53 proteins. Cancer Res. 1991 Oct 1;51(19):5232–5237. [PubMed] [Google Scholar]
- Stunnenberg H. G., Lange H., Philipson L., van Miltenburg R. T., van der Vliet P. C. High expression of functional adenovirus DNA polymerase and precursor terminal protein using recombinant vaccinia virus. Nucleic Acids Res. 1988 Mar 25;16(6):2431–2444. doi: 10.1093/nar/16.6.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürzbecher H. W., Brain R., Maimets T., Addison C., Rudge K., Jenkins J. R. Mouse p53 blocks SV40 DNA replication in vitro and downregulates T antigen DNA helicase activity. Oncogene. 1988 Oct;3(4):405–413. [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B. Cancer. A deadly inheritance. Nature. 1990 Dec 20;348(6303):681–682. doi: 10.1038/348681a0. [DOI] [PubMed] [Google Scholar]
- Wang E. H., Friedman P. N., Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation functions of SV40 large T antigen. Cell. 1989 May 5;57(3):379–392. doi: 10.1016/0092-8674(89)90913-6. [DOI] [PubMed] [Google Scholar]
- Wilcock D., Lane D. P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991 Jan 31;349(6308):429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- Wolf D., Harris N., Goldfinger N., Rotter V. Isolation of a full-length mouse cDNA clone coding for an immunologically distinct p53 molecule. Mol Cell Biol. 1985 Jan;5(1):127–132. doi: 10.1128/mcb.5.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Gannon J. V., Lane D. P. Monoclonal antibody analysis of p53 expression in normal and transformed cells. J Virol. 1986 Aug;59(2):444–452. doi: 10.1128/jvi.59.2.444-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]