Abstract
Among the childhood brain neoplasms, craniopharyngioma constitutes about 2–6% of all primary intracranial tumors. Craniopharyngioma poses a multitude of challenges to the neurosurgeon, endocrinologist, oncologist, intensivist and the anesthesiologist. The morbidity and mortality due to radical surgical treatment is quite high, to the extent of 40–50%. The conservative approach with limited surgical intervention and radiotherapy assistance is taken by some neurosurgeons, but its usefulness is very doubtful. We are reporting a case of craniopharyngioma in an 18-year-old female who had progressive loss of vision and was successfully managed with combined radical surgery and medical therapy.
Keywords: Craniopharyngioma, diabetes insipidus, intracranial neoplasms, visual field defect
INTRODUCTION
Among the childhood brain neoplasms, craniopharyngioma constitutes about 2–6% of all primary intracranial tumors, with an incidence of 0.13 cases per lakh person-years.[1–3] Basically, these are benign neoplasms that are commonly located in the sellar or suprasellar region and very rarely present in the infrasellar area. Craniopharyngioma poses a multitude of challenges to the neurosurgeon, endocrinologist, oncologist, intensivist and the anesthesiologist. The high-recurrence rates after radical surgery, the presence of vital structures like nerves and vessels in the vicinity of the tumor, the numerous pressure effects of the tumor due to brain tissue compression, various postoperative endocrinological sequelae, high morbidity and mortality as well as hazardous effects of radiotherapy are few of the challenging aspects that force many clinicians to adopt a more conservative and palliative approach.[4,5] We are reporting a case of craniopharyngioma in an 18-year-old female who had progressive loss of vision and was successfully managed with combined radical surgery and medical therapy.
CASE REPORT
An 18-year-old short statured female with a height of 129 cm and weighing 33 kg presented to the ophthalmology Outpatient Department (OPD) with chief complaints of progressively increased loss of vision for the last 18 months. Primary amenorrhea was another symptom that was revealed during the elicitation of gynecological history.
On ophthalmic examination, vision of the right eye was found to be severely compromised, with optic atrophy. The left eye vision was found to be 6/6, with normal fundus findings. The right-sided pupil was semi-dilated and was not reacting to light, but consensual light reflex was present. The left-sided pupil was normal in size and elicited normal reaction to direct light, but had absent consensual reflex [Figure 1].
Figure 1.

Visual field examination findings
On physical examination, it was found that patient had poorly developed secondary sexual characteristics. Her endocrinological evaluation revealed the following values of thyroid hormones: thyroid stimulating hormone – 6 mIU/ml, T3 – 1 ng/ml and T4 – 4.36 μ/dl. Her morning cortisol levels were estimated at 294.7 nmol/L, the normal value being in the range of 171–536 nmol/L. Follicle stimulating hormone, luteinizing hormone and prolactin levels were estimated at 8.36 mIU/ml (3.5–12.5), 4.65 mIU/ml (2.4–12.6) and 10 ng/ml (5–23), respectively. Growth hormone levels were also on the lower side.
Magnetic resonance imaging (MRI) of the brain was carried out, which suggested a sellar and suprasellar mass. The sellar portion of the mass was solid while the suprasellar mass was diagnosed to be cystic. T1 and T2 sequences of MRI exhibited hypotense and heterogeneously hypertense mass, respectively. Compression of the optic chiasma by the tumor mass measuring 32 mm Χ 26 mm Χ 23 mm was very much forthright on MRI. A provisional diagnosis of craniopharyngioma was made by the radiologist based on the above radiological evidence [Figures 2 and 3].
Figure 2.
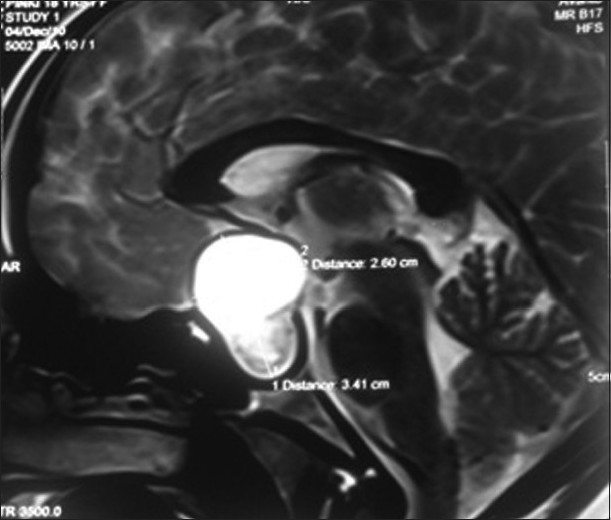
Sagital section of magnetic resonance imaging brain showing the craniopharyngioma
Figure 3.
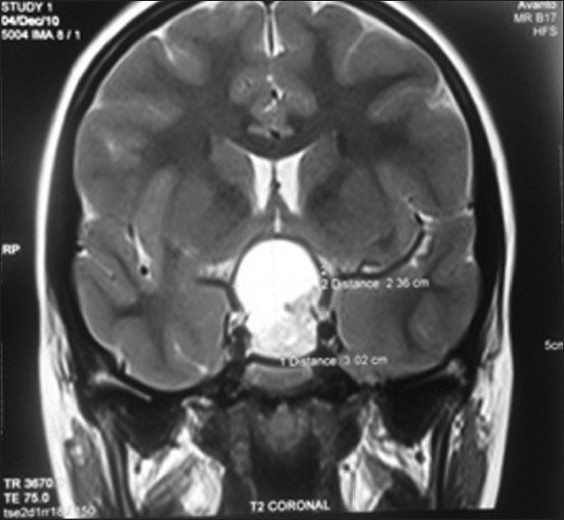
Coronal section of magnetic resonance imaging brain showing the location of craniopharyngioma
The patient was administered medical treatment initially in the form of 50 μg of eltroxin and 10 mg hydrocortisone per day, and the surgical procedure was planned after 2 weeks of medical treatment. In the operation theater, induction of anesthesia was carried out with fentanyl 2 μg/kg, propofol 2 mg/kg, vecuronium 0.1 mg/kg and 50:50 mixture of oxygen and nitrous oxide in isoflurane. Endotracheal intubation was accomplished with a size 7.0 endotracheal tube. Continuous noninvasive monitoring was carried out, which included heart rate (HR), noninvasive blood pressure (NIBP), ECG, pulse oximetry (SpO2) and end tidal carbon dioxide (EtCO2), which was maintained between 30 and 33 mmHg.
Right pterional (fronto temporo sphenoidal) osteoplastic craniotomy was performed and trans-sylvian subtotal tumor removal was carried out. The patient was placed supine on Sugita frame at 20 degree low vertex position and the head was rotated 30 degree to the left to bring malar eminence at the highest point with 30 reverse Trendelenburg position.
Surgical findings revealed a widely opened Sylvian fissure by the tumor, which had a thick capsule. Another remarkable finding was that of lateral displacement and thinning out of the right optic nerve by the tumor invasion. The cystic component of the tumor was containing a straw-colored fluid while the solid component appeared pinkish white with specks of calcification, which could be partly removed by suction. The tumor capsule was adherent to the optic chiasma and, therefore, the floor of the third ventricle was left behind.
At the end of the surgical procedure, reversal of the neuromuscular blockade was carried out with neostigmine and glycopyrrolate and extubation was performed after thorough suctioning and return of protective airway reflexes. Postextubation, the patient responded to verbal commands and had no neurological sequelae. The patient was kept in the recovery room for 2 h, where strict vigil monitoring was carried out. Thereafter, she was shifted to the Intensive Care Unit (ICU) for further observation. The patient had transient diabetes insipidus for the next 3 days, which responded to intranasal desmopressin that was administered three-times daily for 3 days. The rest of the postoperative course was uneventful and surgical sutures were removed on the eighth postop day. The postoperative investigations revealed a TSH level of 2.2 mIU, while postoperative computed tomography scan head revealed a small amount of solid tumor in the sella with left-sided residual capsule. The histopathological examination of the tumor was also carried out, which confirmed the diagnosis of craniopharyngioma. The patient was discharged on the 10th postoperative day and was prescribed tab eltroxin 50 μg/day and tab dexamethasone 0.5 mg/day and was advised regular follow-up.
DISCUSSION
The most common postulated hypothesis for the origin of craniopharyngioma revolves around the theory of neoplastic transformation of embryonic cells of the craniopharyngeal duct during the embryological development.[3] Another theoretical postulate states that it is the metaplastic transformation of adenophysis cells of the pituitary gland that leads to the development of craniopharyngioma.[6] The affected individuals can exhibit both pressure symptoms and endocrine derangements. There is a generalized deficiency of various pituitary hormones like growth hormone, gonadotropin, thyroid stimulating hormone and adrenocorticotropic hormone.[2] In the present case also the compressive symptoms manifested as progressive loss of vision while endocrinological dysfunctions were visible in the form of retarded growth, deranged thyroid hormone levels and primary amenorrhea. The patients typically present to the ophthalmology or surgical OPD with symptoms related to visual field defects, raised intracranial pressure, growth abnormalities and various symptoms related to pituitary deficiency, as was the scenario in our case where the patient presented with progressive loss of vision.[7] MRI is the investigation of choice for precise diagnosis of the tumor location and to supplement the central-type visual field defect diagnosis during ophthalmic examination.[8]
The morbidity and mortality due to radical surgical treatment is quite high, to the extent of 40–50%. In many situations, there is hardly any alternative but to adopt a radical surgical approach for the excision of tumor.[7,9] In the present case also, the patient had severe symptoms that had progressed very rapidly for the last 1 year and therefore the surgical treatment was the only option left. The prognostic criteria for the cure of craniopharyngioma include age greater than 5 years, size of tumor less than 4 cm, complete surgical removal and absence of severe endocrinological dysfunction. The combination of these factors has a favorable prognosis, and the presence of all these factors in our case encouraged us to proceed with radical surgery in our patient. Also, the back-up facilities of intensive care that are being managed by the anesthesiolgists further helped us to plan precisely the overall postoperative management.
Radical surgical intervention is associated with a possible neuronal damage to the parts of the brain including thalamus, mammilo-thalamic tract and basal forebrain. The injury to the vessels during the surgery can result in uncontrolled bleeding, pushing the poor neurosurgeon and the anesthesiologist to the edge of the sword. The ideal surgical conditions were provided in our case as we resorted to hypotensive anesthesia and adopted all the intracranial pressure-lowering interventions. There was also adequate provision of blood in case of uncontrolled hemorrhage. The injury to the nerves during the surgical advancement toward the tumor and its excision can cause permanent neurological impairment necessitating ICU admission of the patient in a comatose state.[10] Even radical surgery fails to achieve the 100% results and does require adjuvant therapy, which warrants the active involvement of the oncologist and the radiotherapist as well.[9,11] The possible advantages of interstitial radiotherapy over radical surgery are still debatable and no general consensus exists regarding the definite treatment of such lesions.[12,13] This patient was also referred to the radiotherapy center after the uneventful postoperative period.
There is another school of thought that lay more stress on a conservative approach with limited surgical intervention, and radiotherapy assistance is taken by some neurosurgeons. The potential complications associated with such measures include delayed effects due to the remnants of the tumor and an inadequate control of the general symptomatology. Because the symptomatology in the present case was of progressive nature despite medical management, we proceeded for surgical removal of the tumor.
The difficulties for the anesthesiologist and the intensivist carries on to the postoperative period also as, along with anterior pituitary impairment, posterior pituitary dysfunction also get accentuated in almost 70–80% of the cases.[14,15] Our patient also suffered from severe diabetes insipidus during the postop period, and the excessive urinary output was controlled only after the administration of intranasal desmopressin. The endocrinological emergencies postoperatively can be very challenging if the patient is not prepared well before proceeding for the surgical and anesthetic interventions.
CONCLUSION
The scope of improvement in the treatment of such lesion is huge provided that there exists a general consensus among different school of thoughts. As the majority of these tumors occur in children, a more comprehensive approach is required at an international level for a better functional outcome as the whole future life of these children is going to be affected by the quality of surgical intervention. There should be regular meetings and discussions at an international level regarding the precise treatment options for such intracranial neoplasms. Only then will we be able to improve our health services for the successful treatment of similar cases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Haupt R, Magnani C, Pavanello M, Caruso S, Dama E, Garrè ML. Epidemiological aspects of craniopharyngioma. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):289–93. [PubMed] [Google Scholar]
- 2.Karavitaki N, Cudlip S, Adams CB, Wass JA. Craniopharyngiomas. Endocr Rev. 2006;27:371–97. doi: 10.1210/er.2006-0002. [DOI] [PubMed] [Google Scholar]
- 3.Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89:547–51. doi: 10.3171/jns.1998.89.4.0547. [DOI] [PubMed] [Google Scholar]
- 4.De Vile C, Grant DB, Kendall BE. Management of childhood craniopharyngioma: Can the morbidity of radical surgery be predicted? J Neurosurg. 1996;85:73–81. doi: 10.3171/jns.1996.85.1.0073. [DOI] [PubMed] [Google Scholar]
- 5.Sands SA, Milner JS, Goldberg J, Mukhi V, Moliterno JA, Maxfield C, et al. Quality of life and behavioural follow-up study of pediatric survivors of craniopharyngioma. J Neurosurg. 2005;103(Suppl 4):302–11. doi: 10.3171/ped.2005.103.4.0302. [DOI] [PubMed] [Google Scholar]
- 6.Sartoretti-Schefer S, Wichmann W, Aguzzi A, Valvanis A. MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR Am J Neuroradiol. 1997;18:77–87. [PMC free article] [PubMed] [Google Scholar]
- 7.Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, et al. Pediatric craniopharyngiomas: Classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106(Suppl 1):3–12. doi: 10.3171/ped.2007.106.1.3. [DOI] [PubMed] [Google Scholar]
- 8.Defoort-Dhellemmes S, Moritz F, Bouacha I, Vinchon M. Craniopharyngioma: Ophthalmological aspects at diagnosis. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):321–4. [PubMed] [Google Scholar]
- 9.Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006;104(Suppl 2):94–102. doi: 10.3171/ped.2006.104.2.5. [DOI] [PubMed] [Google Scholar]
- 10.Hayward RD, Devile C, Brada M. Craniopharyngioma. In: Walker DA, Perilongo G, Punt JA, Taylor RE, editors. Brain and spinal tumors of childhood. London: Arnold; 2004. pp. 370–86. [Google Scholar]
- 11.Scarzello G, Buzzaccarini MS, Perilongo G, Viscardi E, Faggin R, Carollo C, et al. Acute and late morbidity after limited resection and focal radiation therapy in craniopharyngiomas. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):399–405. [PubMed] [Google Scholar]
- 12.Zona G, Spaziante R. Management of cystic craniopharyngiomas in childhood by a transsphenoidal approach. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):381–8. [PubMed] [Google Scholar]
- 13.Szeifert GT, Bálint K, Sipos L, Sarker MH, Czirják S, Julow J. Pathological findings in cystic craniopharyngiomas after stereotactic intracavitary irradiation with yttrium-90 isotope. Prog Neurol Surg. 2007;20:297–302. doi: 10.1159/000100173. [DOI] [PubMed] [Google Scholar]
- 14.De Vile CJ, Grant DB, Hayward RD, Stanhope R. Growth and endocrine sequelae of craniopharyngioma. Arch Dis Child. 1996;75:108–14. doi: 10.1136/adc.75.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poretti A, Grotzer MA, Ribi K, Schönle E, Boltshauser E. Outcome of craniopharyngioma in children: Long term complications and quality of life. Dev Med Child Neurol. 2004;46:220–9. doi: 10.1017/s0012162204000374. [DOI] [PubMed] [Google Scholar]


