Abstract
Asia is home to four of the world's five largest diabetic populations, two of them being South Asian nations, namely, India and Pakistan. This problem is compounded by a substantial rise in the elderly population in Asian countries. On the other hand, the heterogeneous health condition and multiple co-morbidities make the care of chronic disease in the elderly a challenging task. The aim of the South Asian Consensus Guidelines is to provide evidence-based recommendations to assist healthcare providers in the rational management of type 2 diabetes mellitus in the elderly population. Current Guidelines used systematic reviews of available evidence to form its key recommendations. No evidence grading was done for the purpose of this manuscript. The clinical questions of the guidelines, the methodology of literature search, and medical writing strategy were finalized by consultations in person and through mail. The South Asian Consensus guideline emphasizes tailoring of glycemic goals for patients based on age, co-morbid conditions especially that of cardiovascular system, risk of hypoglycemia, and life expectancy. It also recommends cautious use of available pharmacotherapy in geriatric patients with diabetes. The primary principle of diabetes therapy should be to achieve euglycemia, without causing hypoglycemia. Appropriate use of modern insulins and oral drugs, including incretin mimetics will help physicians achieve this aim.
Keywords: Diabetes mellitus, elderly, geriatric, guideline, South Asia
INTRODUCTION
Most developed countries in the world have accepted the chronological age of 65 years as a definition of “elderly” or older person. Because of better healthcare facilities for most by the end of the millennium, the numbers of elderly persons is increasing rapidly. They now constitute a good proportion of the general population. According to one estimate,[1] the elderly constitute 8.3% of general population. In the year 2002, the absolute number of elderly people in India was about 75 million.[2] The percentage of elderly Indians is projected to rise to 9% by 2016. During 1999, 7% of Singapore's population was over the age of 65; however, by 2030, this will increase to 19%.[3] China has about 102 million elderly (those aged 65 and over) or over one-fifth of the world's elderly population. Moreover, the percentage of elderly in China is projected to triple from 8 to 24% between 2006 and 2050, to a total number of 322 million. From a global perspective, elderly will constitute one-third of total population of the world by the year 2050.[3,4]
Asia is home to four of the world's five largest diabetic populations: India with 33 million cases, China with 23 million cases, and Pakistan and Japan with 9 and 7 million cases, respectively.[5] The prevalence of diabetes in Asia is increasing rapidly. There are about 50.8 million people with diabetes in India and the figure will rise to about 70 million by 2025. Every fifth patient visiting a consulting physician is a patient with diabetes, and every seventh patient visiting a family physician has diabetes.
Considering this alarming increase in the incidence and prevalence of diabetics, the World Health Organization (WHO) projects that by 2030 India will have 79.4 million diabetic people and China will have 42.3 million cases. Nepal has a population of 28 million of which 4.2% are 65 and older.[6] In a study of randomly selected participants aged 60 years and above in the urban and rural areas of Kathmandu, 25.9% were found to have diabetes.[7]
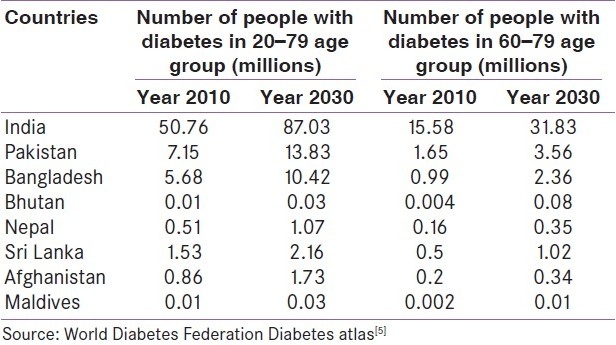
The WHO also projects that by 2030 more than half of diabetes sufferers in the world will live in Asia. More than half of the people with diabetes (53%) are above 60 years and more than 85% are above 45 years. In India, the prevalence of diabetes is 11% in people between 65 and 69 years of age.[1] Furthermore, a recent study suggested that 12% of patients over age 65 with type 2 diabetes have evidence of islet cell autoimmunity, and have disease that is more similar to type 1 diabetes, which may be associated with a greater risk of severe hyperglycemia.[8] An increasing incidence of type 2 diabetes mellitus (T2DM) is also observed in South Asian Association for Regional cooperation (SAARC) countries as shown in Table 1.
Table 1.
Prevalence of diabetes mellitus in South Asian Association for Regional Cooperation countries
THE NEED FOR GUIDELINES
Older adults display extensively heterogeneous health conditions ranging from robust to delicate. This heterogeneity and individual medical complexity makes medical care for older patients particularly challenging. These factors necessitate both careful medical decision-making and a clear understanding of the patients' personal values and individualized goals.
To be realistic and patient-centered, clinical Guidelines that address the needs of older adults with diabetes must address this complexity. Guidelines must go beyond recommending the interventions that target disease-specific conditions. Ideally, they should provide guidance to clinicians for prioritizing medical treatments and preventive care. Guidelines must rank the available interventions according to the impact they are likely to have on the patient's overall health. Experts should clearly acknowledge that geriatric patients' goals and preferences for health care may differ from Guideline recommendations.
Even though no Guidelines achieve this perfection, this South Asian Consensus Statement takes an important step in this direction by providing a rationale for prioritizing and individualizing evidence-based clinical management of older adults with complex health status.
In developing this South Asian Guidelines for the management of diabetes in elderly, we focus our attention on the heterogeneity of health status of the elderly diabetic population. We make an effort to identify and analyze the major health threats to the same population and suggest how physicians can prioritize healthcare recommendations for patients at the extremes of health status and for those in between. In addition to considering the role of traditional components of diabetes care, the proposed Guidelines include recommendations for the screening and management of patients with multiple prescriptions, depression, cognitive impairment, urinary incontinence, injurious falls, and pain. The novelty of these Guidelines lies in this holistic perspective and is at the crux of its utility when translating the recommendations into a patient-centered care plan.
GUIDELINE OBJECTIVES
The aim of the South Asian Consensus Guidelines is to provide evidence-based recommendations to assist healthcare providers in the rational management of T2DM in the elderly population. These Guidelines are intended to be methodology oriented, rather than an exhaustive literature citation on clinical pharmacology of the available drugs for management of diabetes.
Clinical questions
The clinical questions of these Guidelines are:
Who are the elderly with diabetes?
What is the need for Guidelines?
What are the constraints in the management of diabetes in elderly?
What glycemic targets can be recommended?
What treatment options are available for achieving recommended glycemic targets safely and effectively in this elderly population with different co-morbidities?
What systems need to be in place to achieve these recommendations?
Evidence identification and search strategy
The questions to be answered were approved by a group of endocrinologists from various countries of South Asia who met on various occasions in 2010. This group of endocrinologists felt that the unique aspects of geriatric diabetology were not being highlighted in medical texts. The lack of this differentiation led to the use of same therapeutic strategies for elderly diabetic subjects as in younger adults, with suboptimal results.
Therefore, the group felt the need to formulate Guidelines specific for geriatric diabetes management in South Asia, so as to sensitize diabetes care practitioners to the optimal method of geriatric diabetes management. The clinical questions of the Guidelines, the methodology of literature search, and medical writing strategy were finalized by consultations in person and through mail.
A search of the literature was done for systematic reviews (graded by Amstar), randomized controlled trials (graded by Jadad score), observational studies (graded by SIGN 50), and letters to editors and case reports also. The evidence presented in these Guidelines was collated from a systematic review of relevant published literature (up to March 2011) as identified by electronic (e.g. Medline) search and standard textbooks.
The South Asian Consensus Guidelines used systematic reviews of available evidence to form its key recommendations. No evidence grading has been done for the purpose of this manuscript.
Method of development of evidence-based Guidelines
A first-level selection of abstracts was done by the lead author (MPB), followed by a second-level selection of full-text articles, which were distributed to all members of the Consensus group. Quality assessment of these articles was done as detailed above. Data selection and data description was done for the chosen articles.
A first draft of the South Asian Consensus Guidelines was prepared by a core writing group. It was then passed on to all members for their suggestions and recommendations. The corrected draft was circulated to 20 professional leaders from different specialities, 15 lay members of institutional ethics committees, and representatives of the pharmaceutical industry to ensure involvement of all stakeholders across the region for correction and suggestion. Four external reviewers representing basic and clinical sciences, from outside South Asia, then read the Consensus Statement and made recommendations, which were incorporated in the document. Various corrections, additions and suggestions were incorporated, and a fresh draft circulated to all members. A fifth version of the Consensus Guidelines was prepared and circulated again. The final Consensus Guidelines are being presented here, after getting approval from all concerned members.
GENERAL ISSUES IN MANAGEMENT
Constraints
Life expectancy is largely determined by functional status of an individual and presence of different co-morbidities. There is some evidence that diabetes treatment is not pursued as vigorously in older groups, with inadequate treatment offered to individuals aged 85 years and over.[9] There are other conflicting data about the severity of the diabetes in older persons. Many associated co-morbidities like cerebral aging, atherosclerotic changes, compromised cardiorespiratory reserve, blunting of hormone profile, poor hepatic glycogen reserve, cataract, neuropathy, cerebrovascular disease, hyperosmolar nonketotic coma (HONK), hyponatremia, etc. are to be taken into consideration while treating the elderly patients with diabetes.[10]
With increasing age, the pattern of presentation in diabetes changes, with most patients having a fasting plasma glucose (FPG) of 125 mg/dL or less, while their postprandial values mostly remaining above 200 mg/dL,[10] exposing this group of patients to the risk of developing cardiovascular morbidities.[11]
The cognitive function of geriatric patients weakens along with declining levels of glycemic control.[12] Notwithstanding the fact that almost 60% of diabetic patients aged 75 years and above have hypertension, it is very important to treat the same along with other risk factors, i.e. high lipid levels.[13,14] This emphasizes the significance of nonglycemic interventions in this group, and there is apprehension that these treatments may not be that effective in older patients with diabetes. Older individuals with diabetes, however, may be at greater risk of experiencing treatment-related complications than younger persons. For example, metformin treatment, particularly when administered in combination with sulfonylureas (SUs), has been associated with higher mortality,[15] although one cannot be certain if this was due to the presence of more severe underlying illness in patients whose physicians selected this treatment approach. Health economic models have suggested that the benefits of treating glycemia may be somewhat less in older than in younger patients.[16] A reasonable compromise to treating older patients may be to provide all individuals with diabetes, who in the judgment of the treating physician have a reasonable life expectancy, with conventional standard of care to achieve glycemic control even as the complexity of such an endeavor is constantly appreciated.
The American Geriatric Society strongly recommends individualizing the target setting of diabetes care in the elderly and has included in their Guidelines six geriatric syndromes such as polypharmacy, depression, cognitive impairment, urinary incontinence, injurious falls, and pain which should get priority over endeavors to achieve a tight glycemic goal. This Consensus group fully appreciates the view that it takes 8 years for aggressive glycemic control to reduce the risk of diabetic microvascular complication, but only 2 years of treating hypertension and dyslipidemia to reduce the risk of cardiovascular disease; hence, both morbidity and mortality can be reduced more by targeting cardiovascular risk factors than by intensively managing hyperglycemia.[17]
In a recent joint position Statement, the American Diabetes Association (ADA), American Heart Association and American College of Cardiology, based on the findings of Veterans Affairs Diabetes Trial (VADT), Action to Control Cardiovascular Risk in Diabetes (ACCORD), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron-Modified Release Controlled Evaluation (ADVANCE) trials, suggested that the potential risk of intensive glycemic control may outweigh its benefits in patients with a very long duration of diabetes, a known history of severe hypoglycemia, advanced atherosclerosis and advanced age/frailty.[18]
The South Asian Consensus Guidelines reiterate this viewpoint, and emphasize cautious glycemic control strategies, coupled with management of other cardiovascular risk factors, in the geriatric diabetic population. The Guidelines also emphasize tailoring glycemic goals for patients based on age, comorbid conditions, risk of hypoglycemia, and life expectancy.
Patient-centered management design
Approach to management of diabetes in elderly is largely influenced the constraints (please see above). The management issues which are of paramount importance are described in Table 2.
Table 2.
Guidelines and some important tips for designing a patient-specific treatment plan for an elderly patient with diabetes

Diagnostic criteria
ADA uses FPG as the criteria of diagnosis, while WHO uses Oral Glucose Tolerance test (OGTT) as the diagnostic parameter.[7,10,11] FPG increases by 1–2 mg/dL/decade and the postprandial plasma glucose (PPG) increases by 15 mg/dL/decade after 30 years of age. Such physiological changes lead to the overestimation of diabetes in the elderly if WHO parameter is used, while the ADA criteria tend to underdiagnose the same in this population.
The South Asian Consensus Statement endorses WHO Guidelines.
Screening geriatric population
Screening for diabetes complications should be individualized in older adults, but particular attention should be paid to complications that may lead to functional impairment.
The South Asian Consensus Guidelines do not recommend universal screening for all geriatric individuals, but do encourage opportunistic screening. This means that a geriatric person should get blood glucose estimation done whenever the opportunity presents, i.e. during a routine examination or while getting blood test done for fever or any other inter-current illness.
Glycemic control goals
Target glycated hemoglobin (HbA1c) should always be individualized in elderly patients based on their functional status, life expectancy, and cognitive function. While a goal of 7% or lower may be appropriate for most older adults who are healthy, such a target value for other older adults may be more challenging given the issues of hypoglycemia. Most recent information with regard to the HbA1c target set by different agencies is available in Table 3.
Table 3.
Targets of glycemic control in geriatric population as suggested by various premier agencies

For patients with advanced diabetes complications, life-limiting comorbid illness, or substantial cognitive or functional impairment, it is reasonable to set less-intensive glycemic target goals. These patients are less likely to benefit from reducing the risk of microvascular complications and more likely to suffer serious adverse effects from hypoglycemia. However, patients with poorly controlled diabetes may be subject to acute complications of diabetes, including dehydration, poor wound healing, and hyperglycemic hyperosmolar coma. Glycemic goals at a minimum should avoid these consequences.
The South Asian Consensus Guidelines recommend setting tighter glycemic targets for patients with non-healing wounds or inter-current infection such as tuberculosis. The group also endorses the view that older adults who are functional, cognitively intact, and have significant life expectancy should receive diabetes care using goals developed for younger adults. Glycemic goals for older adults not meeting the above criteria may be relaxed using individual criteria, but hyperglycemia leading to symptoms or risk of acute hyperglycemic complications should be avoided in all patients. Other cardiovascular risk factors should be treated in older adults with consideration of the time frame of benefit and the individual patient. Treatment of hypertension is indicated in virtually all older adults, and lipid and anti-platelet therapy may benefit those with life expectancy at least equal to the time frame of primary or secondary prevention trials.
Safety issues and monitoring of therapy for better glycemic control
Elderly people are at higher risk for hypoglycemia due to age-associated decreases in hepatic oxidative enzyme activity and concomitant decline in renal function, polypharmacy, inadequate and/or erratic nutritional intake, hypoglycemic unawareness secondary to loss of counter-regulatory response to hypoglycemia, and cognitive impairment.[21]
Hypoglycemic episodes are associated with a higher rate of injurious falls in older persons, which is a very common geriatric syndrome and affects the quality of life of the elderly.[17] It is also one of the major limiting factors in glycemic control by pharmacological means. In major interventional studies, intensively treated patients experienced twofold to threefold higher incidence of hypoglycemia. The incidence per person year varies from 1.23 to 2.78 depending on the type of pharmacological modality used. The most dangerous form is silent nocturnal hypoglycemia which may present in an atypical manner simulating cerebrovascular accidents.[22]
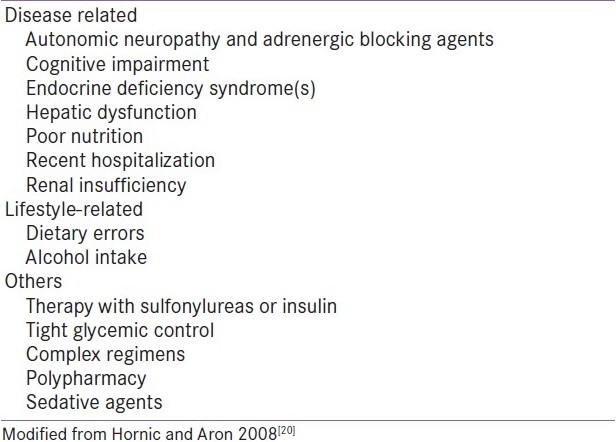
The risk factors for hypoglycemia among elderly patients are tabulated in Table 4. Managing diabetes effectively involves patient and family education regarding signs and symptoms of hypoglycemia, regular home blood glucose monitoring, carrying replacement glucose in pocket, and practicing safe driving. It is important to know that annual self-management training has to be conducted. The clinician must weigh many factors as the risks and benefits of tight glycemic control in elderly patients are evaluated in the context of treatment strategies and priorities.
Table 4.
Risk factors for hypoglycemia in elderly patients
The care of older adults with diabetes is complicated by their clinical and functional heterogeneity. Some older individuals would have developed diabetes years earlier and may have significant complications; others who are newly diagnosed may have had years of undiagnosed diabetes with resultant complications; and yet another subset may have even fewer complications. Some older adults with diabetes are frail and have other underlying chronic conditions, substantial diabetes-related co-morbidity, or limited physical or cognitive functioning. Other older individuals with diabetes have little co-morbidity and are active. Life expectancies are highly variable for this population, but often longer than clinicians realize, or in other words, try to believe.
The South Asian Consensus Guidelines recommend that the aforesaid heterogeneity must be taken into consideration by providers caring for older adults with diabetes while setting and prioritizing treatment goals.
CLINICAL EVALUATION
South Asian Consensus suggests an evaluation scheme to complete all investigations wherever possible. However, treating physician can modify the scheme according to availability of treatment options or financial constraints. The minimum standard of care to be provided for elderly patients with diabetes should be focused on initial evaluation of the health condition and subsequent continuum of care.
Components of the initial evaluation
Complete history and physical examination of the patient
Geriatric assessment
- Laboratory examination:
- FBG
- PPG
- HbA1c
- Lipid profile
- Kidney function tests (at least serum creatinine, blood urea nitrogen, serum sodium, serum potassium)
- Routine urinalysis, urine for microalbumin (spot)
- Liver function tests (at least alanine aminotransferase, aspatate aminotransferase, serum protein and fraction)
- Electrocardiogram (ECG)
Ophthalmological examination including fundoscopy
Dietary assessment
Points to be considered in the continuum of care
Treatment approach needed to meet target blood glucose levels (avoiding complications like hypoglycemia and weight gain): diet, oral agents, incretin-based therapy, or insulin.
Evaluation of blood glucose levels as frequently as needed.
Annual assessment for diabetes complications.
Annual assessment for common geriatric co-morbidities.
SELECTION OF DRUGS IN PHARMACOLOGICAL INTERVENTION
Due to an increase in concomitant disease in the elderly, there is a corresponding rise in multiple medication use. This polypharmacy has the potential to alter responsiveness to medications and increase the incidence of adverse effects among the elderly. Oral hypoglycemic agents comprise drugs that stimulate insulin secretion such as SUs, insulin secretagogues, and incretin mimetics, drugs that reduce hepatic glucose output (biguanides), and drugs that improve insulin sensitivity [biguanides, thiazolidinediones (TZDs)].
ADA and the European Association for the Study of Diabetes (EASD), in their recent joint Consensus Guidelines, highlighted the importance of a stepped approach to therapy. Achieving the glycemic goal remains the most important issue. Therapy should begin with education, then a reduction in dietary fat and an increase in exercise. If control remains inadequate, such steps should be supplemented by addition of metformin. Early addition of potent drugs is suggested if goals are not met. More importantly, initiation of insulin therapy should be early rather then late if such step is required.[22]
The South Asian Consensus, while endorsing the views of ADA/EASD, puts safety as the primary issue in the approach to achieving the glycemic goals in elderly diabetic patients.
Insulin sensitizers
Metformin
The medical fraternity has sufficient clinical experience and evidence to accept metformin as predictable and safe even in the large subset of elderly patients. The remarkable advantage of metformin is its weight neutrality, no tendency of causing weight loss and finally being inexpensive.[24] Metformin is immensely useful in obese diabetic patients with particularly fasting hyperglycemia, and has the ability to reduce HbA1c by 1–2%.[25] Compared with SUs and insulin, it significantly reduces all-cause mortality and diabetes-related end points apart from reducing HbA1c substantially;[26] however, it is inappropriate to use this drug in older patients who are frail, anorexic, or underweight, and in those with congestive cardiac failure (CCF), compromised renal or hepatic function.
To avoid lactic acidosis, a potentially dangerous side effect of biguanides, ADA recommends avoidance of this drug even in borderline azotemic patients (>1.5 mg/dL in male and 1.4 mg/dL in female). One must note that creatinine clearance (CrCl) may be impaired in elderly persons with a high-normal serum creatinine value. In sarcopenic older adults, even these cut-off levels may be little high, leaving only a narrow window of safety. One has to be extremely careful in using it in old persons above 80 years of age. Vitamin B12 deficiency is seen in up to one-third of prolonged users of metformin, a condition otherwise seen quite commonly in elderly. In spite of weight neutral and non-hypoglycemic characteristic, metformin may cause weight loss and hypoglycemia in frail, anorexic older adults on calorie restriction.[27]
The South Asian Consensus recommends conservative initiation with a low dose and a very careful gradual uptitration of the dose specially to avoid gastrointestinal (GI) intolerance. To this end, it is also advisable to use sustained/extended release formulation whenever possible. One has to be extremely careful in using it in persons who are very frail and are above 80 years of age.
This guideline recommends the checking of vitamin B12 every 4–5 years in those who have been using this agent. In the absence of such a facility, and in those on vegetarian diet, supplementation of oral or parenteral form of vitamin B12 should be provided. In this regard, the panel also feels that monitoring renal function initially and periodically, ruling out anoxic conditions such as advanced CCF, chronic obstructive airway disease (COAD), or occlusive peripheral vascular disease (PVD) are to be of foremost consideration.
Thiazolidinediones
These drugs reduce insulin resistance by activating the peroxisome proliferator activated receptor gamma (PPAR-γ). They have been recommended for use as monotherapy and also as a combination with other oral drugs and insulin. They do not cause hypoglycemia on their own but lead to moderate weight gain in most users. However, in the elderly subjects, certain adverse effects like fluid retention and propensity to develop or worsen heart failure, increased risk of fracture, and hepatic toxicity preclude the use of this class of drugs widely. The use of rosiglitazone has been restricted in most countries of the globe because of its potential for increasing morbidity and mortality from cardiac ischemic event. However, pioglitazone is found to be safe in this regard.[27]
In the opinion of the panel, TZDs should not be preferred over metformin as insulin sensitizer to be used as monotherapy or in combination in most of the elderly. It may be preferred in two situations such as azotemia and extreme GI intolerability where metformin is contraindicated.
However, the panel recommends adopting a cautious approach in using pioglitazone in elderly patients with even with mild azotemia (serum creatinine > 2 mg/dL). Similar precautions are required in elderly patients with past history of heart failure, prior coronary ischemic event or angina, hypertension, left ventricular hypertrophy, significant cardiac valve pathology, advanced age (>75 years), long history of diabetes (>10 years), pre-existing edema, concomitant insulin usage, documented osteoporosis or history of osteoporotic fracture. The panel reiterates the use of minimal required dose as most of the side effects are often seen at higher doses of TZDs.
Insulin secretagogues
Sulfonylureas
They are classical prototype of insulin secretagogues. For many decades, they have been the cornerstone of hyperglycemia management in the elderly, with more than 70% of prescriptions of these drugs given for individuals over the age of 60 years.[28] Apart from the risk of hypoglycemia which can be severe, particularly in the elderly, they are generally tolerated well without the GI upset seen with many other agents. In patients with sufficient pancreatic beta cell reserve, they have proven efficacy with reduction of HbA1c by about 1.5%.[24] Of the currently used SUs, glibenclamide (glyburide), due to its longer time action profile, is associated with serious hypoglycemia, particularly in the elderly. Gliclazide has a much safer profile.[29] Glipizide and glimepiride are also considered safer than glibenclamide.[30,31] Hypoglycemia due to SUs may be precipitated/potentiated by various factors [Table 4]. These drugs are metabolized by the liver and excreted through the renal route. As both these organ systems may not function optimally in the elderly, the risk of hypoglycemia is substantial.[27]
South Asian Consensus Guidelines recognize the useful role of the SUs in the management of hyperglycemia in the elderly. The panel suggests avoidance of potent long-acting agent, glibenclamide, in all elderly subjects as safer medicines with similar efficacy are available. It is preferable to use drugs with a single daily dosing schedule, such as gliclazide extended release and glimepiride, to avoid dosing errors to which the elderly are quite prone to. The panel emphasizes conservative approach during initiation (such as 1 mg for glimpiride, 2.5 mg for glipizide and 30–40 mg for gliclazide) and also during uptitration and maintenance. In elderly patients with compromised renal function and in situations where tendency to miss a meal is quite common, SUs may not be the right choice as other safer secretagogues are available.
Meglitinides
They are another class of rapid-acting, non-sulfonylurea insulin secretagogues with a relatively shorter half-life. Because of the physiology of action, they do have the potential to cause hypoglycemia, but the risk is much less than that associated with SUs.[30] These drugs are useful in the large subset of elderly diabetics who present with postprandial hyperglycemia as their primary problem. Due to their short action profile, a dose can be withheld in situations where a meal can be missed, thus giving an advantage over SUs (please see above). As they are metabolized predominantly in the liver, they are considered safe in moderate renal failure. These points make them attractive agents for usage in the elderly.
South Asian Consensus Guidelines advocate use of meglitinides in patients with moderate renal impairment and normal hepatic function, with predominantly postprandial hyperglycemia where uptitration of other agents may lead to post-absorptive or fasting hypoglycemia. Unlike the SUs, conservative dosing is not mandatory. They can be used as an adjunct to long-acting basal insulin.
Drugs retarding the absorption of glucose from gut
With only marginal absorption from the GI tract (0–2%), alpha glucosidase inhibitors (AGIs) are attractive agents to combat postprandial hyperglycemia. Reduction in HbA1c is modest with these agents.[32] This group of drugs scores high on the safety front with negligible number of hypoglycemic events when used as monotherapy, but significant GI side effects limit their use in the elderly who usually possess some form of intestinal dysmotility. However, in selected individuals, these drugs can be used alone or with agents of other classes. Hepatic and severe renal impairments are contraindications.[33]
The panel suggests that these agents should be started on once daily, at the lowest recommended dose, allowing increments by a small quantum over a couple of weeks. When an optimal dose is reached (as indicated by postprandial blood sugar), the frequency of dosing may be increased using the same approach of conservative uptitration to suit the meal pattern and also to achieve the glycemic goals. It is suggested that this class of drugs may be more efficacious in rice-eating populations.
Incretin enhancers
The gut hormone, glucagon like polypeptide-1 (GLP-1), is derived from pro-glucagon which is secreted from intestinal L-cells. The secretion of GLP-1 increases manifold immediately after meal ingestion. Binding of this hormone to the GLP-1 receptor in the pancreatic beta cells results in insulin secretion in a glucose-dependent manner and also suppresses the counter-regulatory glucagon secretion from alpha cells. The short half-life of GLP-1 due to rapid degradation by dipeptidyl peptidase-4 (DPP-4) enzyme necessitated the development of two drug classes, namely, degradation-resistant (injectable) GLP-1 analogues and (oral) inhibitors of DPP-4.[34] These agents have a major advantage, i.e. avoidance of hypoglycemia, which makes them suitable for elderly diabetics.[35]
Oral inhibitors of DPP-IV
Four classes of oral inhibitors of DPP-IV have been approved so far, namely sitagliptin, vildagliptin, linagliptin, and saxagliptin, for use as monotherapy and in combination with other anti-hyperglycemic agents. They are weight neutral, and hypoglycemic events during their use are negligible in number. The National Institute Clinical Excellence (NICE) (UK) guideline recommends their use (instead of SUs) as an add-on to the ongoing metformin therapy if glycemic control is inadequate (HbA1C > 6.5%) and the person is at significant risk of hypoglycemia or its consequences (e.g. older people, those living alone, those working at heights or with heavy machinery).[36]
The usual recommended daily dose for sitagliptin is 100 mg, vildagliptin 50 mg twice daily and saxagliptin 5 mg once daily. No differential dosing is required for elderly. However, in patients (elderly or young likewise) with renal dysfunction, i.e. CrCl <50 mL/min, the dose should be lowered by 50%. In advanced renal failure (CrCl <30ml/min), sitagliptin dose has to be further lowered to 25 mg a day. Saxagliptin requires a single-step approach, i.e. 2.5 mg daily, in all patients with CrCl <50 mL/min. Vildagliptin has not been studied in advanced renal failure. Dose adjustment is not required for sitagliptin and saxagliptin for use in mild to moderate hepatic impairment.[27,37] Though vildagliptin is not recommended in patients with hepatic dysfunction at present, a recent meta-analysis of 8000 odd patients treated with this drug belonging to all age groups including elderly diabetics did not indicate any association with increase risk of elevation of liver enzyme or drug-related hepatic events.[38] Current recommendation prohibits the use of vildagliptin in patients with a CrCl <50 mL/min. It is, however, expected that large clinical trials which are underway to evaluate the long-term safety of vildagliptin in patients with moderate or severe renal impairment will provide favorable evidence in this subgroup of patients also.[39] Only vildagliptin has been specifically studied in elderly diabetic patients (both >65 and >75 years). In both the instances, their safety and efficacy were well documented.[27,40]
GLP-1 receptor agonists
Exenatide has been used extensively through most part of the last decade as an add-on to metformin, TZD, and SUs.[48] In some countries (including India), it has been used with insulin in some instances. Liraglutide was approved for use in 2009 (2010 in USA and India). Both the drugs reduce HbA1c significantly when used as monotherapy or in combination, with an excellent safety profile. They have been shown to cause significant weight reduction by their anorexigenic property. Not much of data are available on the use of these agents particularly in the elderly. Notwithstanding the disadvantage of daily injections and significant GI side effects, this group holds promise as a treatment option in elderly diabetic population. Liraglutide provides multiple benefits like better glycemic control compared to SUs, reduction in systolic blood pressure, improvement in lipid profile, reduction in cardiovascular risk factors, and weight reduction, requiring once daily administration.[42]
The panel recommends that oral DPP-IV inhibitors are a natural choice in the management of hyperglycemia in the elderly with normal or mildly impaired renal function, i.e. CrCl > 50 mL/min (corresponding approximately to serum creatinine of 1.7 mg/dL). They can be used as monotherapy for initiation or as second-line add-on to metformin. They may be used along with insulin specifically when postprandial targets are not met. The panel considers that in the subgroup of patients >75 years of age, vildagliptin can be preferred based on current literature, but does not limit the use of other options in this class. These drugs are suitable irrespective of body structure (frail and robust alike). In relatively younger elderly (<75 years) who are either overweight or obese and have poor dietary compliance, injectable GLP-1 analogue may a reasonable option. As regards the choice of the agent, it is the discretion of the physician; but ease of administration (like number of injections), safety (like risks of hypoglycemia, cardiac events and GI friendliness), and efficacy – all these issues favor liraglutide over its predecessors in the same group.
Novel agents
A new class of oral glucose lowering agents called selective inhibitor of sodium glucose transporter 2 (SGLT-2) acts by decreasing renal glucose reabsorption. As these drugs target hyperglycemia independently of insulin, risk of hypoglycemia should be negligible. They have been shown to have pleotropic beneficial effects such as reducing blood pressure, lipid profile, body weight, waist circumference, serum uric acid and high-sensitivity C-reactive protein levels. These effects should have beneficial outcome on the cardiovascular system. Simultaneously, potential negative effects such as higher rate of urinary and genital infection, hyperparathyroidism, and increased hematocrit have been shown to occur with the use of SGLT-2 inhibitors. There has not been any focused study on elderly diabetics.[43]
Bromocriptine, an extensively used dopamine 2 modulator, is a novel drug which has recently been approved for use in T2DM. The rationale of using this agent is that modulating dopamine, which is the most abundant adrenergic neurotransmitter in brain, might counterbalance stress hyperglycemia. It may be useful in elderly patients with diabetes and parkinsonsian symptoms, obese, depressed (anhedonic) patients with limited mobility and features of insulin resistance. As a glucose lowering agent, bromocriptine therapy is initiated in a dose of 0.8 mg once daily, early morning, as monotherapy or in combination.[44,45]
The South Asian Consensus panel feels that at this time, the use of these drugs in T2DM patients in general and elderly T2DM patients in particular seems promising, but more evidence is needed to have conclusive evidence in this regard.
INSULIN THERAPY IN THE ELDERLY
Considerations before initiation of insulin therapy in older adults
Even as the 10 classes of agents have been used to treat diabetes (please see above) over the last 50 years or so, insulin remains the most cost-effective treatment for diabetic patients, both old as well as young. The advent of novel insulin analogues has improved the safety and convenience of insulin therapy.[46] The ADA has recommended that the approach to drug therapy of diabetes consider insulin a first-tier therapy.[47] Insulin has no upper dose limit and, unlike other anti-diabetic agents, it has no contraindications to its use.[48] Nevertheless, there is a general reluctance among physicians and patients alike to accept insulin. Apart from the fear of hypoglycemia, loss of independence, weight gain, and cost, other common patient-identified barriers to insulin therapy include beliefs that insulin is a personal failure, that insulin injections are painful, that insulin is not effective, or that insulin causes complications or even death.[49] In addition to the anxiety about potential side effects and learning self-injection techniques, patients may be concerned about the complexity of regimens. Finally, some patients have the misperception that the need to start insulin therapy is a signal that their diabetes has advanced to a more serious stage or that they have failed in their efforts to achieve glycemic control.[50] Although patient-identified barriers are the most common reasons cited for delay in initiating insulin therapy, many physicians also are hesitant to initiate insulin. Because provider attitudes are crucial for patient acceptance of insulin, it is important to determine to what extent “clinician inertia” is influencing the decision in clinical practice. Physicians may be concerned about the possible side effects (i.e. weight gain, hypoglycemia), as well as having limited time for patient education regarding proper insulin administration techniques.[51]
Co-morbidity caused by multiple pathology, as well as ill-health associated with specific conditions such as impaired cognitive function, depression, inflammatory arthropathy, and cardiac or renal failure, make insulin use in the elderly a challenging task. Age-related changes result in functional disability, affecting the patient's ability to administer insulin, monitor blood glucose and manage hypoglycemia.[52]
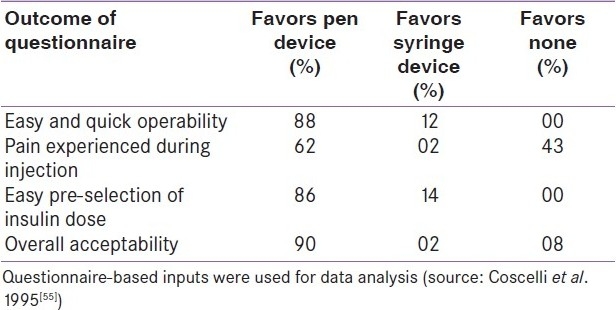
One more important technical consideration in making insulin treatment less complex to the elderly is the accurate and acceptable injection device. In fact, non-compliance with complex insulin injection procedures is well known.[53] On the other hand, it is known that older patients make significant errors in drawing the insulin dose prescribed by the physician with a syringe.[54] One elegant study found significantly higher acceptability of disposable pen device at the end of 6 weeks of transition from conventional syringe to such devices [Table 5]. Such a transition did not compromise the medical issues like safety and efficacy.[55] It is of paramount importance on the part of the caregiver to provide technically superior and user-friendly devices for injecting insulin to all patients in general and elderly diabetic patients in particular.[56]
Table 5.
Comparison of acceptability of injection device versus conventional syringes by elderly diabetic patients
The South Asian Consensus group recognizes that the initiation of insulin therapy is a challenging task, especially in older adults who often have multiple co-morbidities and physical limitations. It, however, strongly believes that physicians should not avoid usage of insulin, using age as a bar. Essentially, the need to start insulin in older patients is not different from younger patients. Technical superiority of a product (analogue vs. conventional), or an injection device (pen vs. syringes), should play a role in planning and executing insulin therapy in the elderly to make it more comfortable and acceptable as well. It is recommended the all patients must be sensitized to the importance of self and structured monitoring of blood glucose at home. In frail and very old patients, it essential to ensure the presence of responsible attendants/family members during selecting the right dose of insulin, injecting that dose, and monitoring by a glucometer. This panel recognizes the importance of assessing the possibility of increased incidence of hypoglycemic episodes, as this age group could be using medication (e.g. beta blockers) which can mask some of the symptoms of hypoglycemia.
Indications for initiation of insulin therapy in the elderly
Insulin is usually started when full doses of oral hypoglycemic agents do not achieve acceptable glycemic control or well-being, and if the deterioration of glycemic status is associated with substantial weight loss. This clinical picture is often referred to as “sulfonylurea failure” though it reflects disease progression and insulin deficiency. The indications of insulin therapy in older adults with T2DM are summarized in Table 6.
Table 6.
Indications of insulin therapy in older adults with T2DM

Using insulin in elderly diabetic: Role of basal insulin
Initiation with bedtime basal, simple way to start
It is difficult to outline a uniform strategy to make a transition from oral agents to insulin therapy in elderly type 2 diabetics. However, it is easier to persuade patients to combine oral drugs with a single dose of bedtime intermediate-acting insulin which can be taken at the privacy of their home without disturbing their daytime routine. In assisted care also, such a routine is easier to follow for the caregiver (family member/private nurse). The patient would normally continue the oral agents because although they have “failed” in the situation of the secondary failure, they may be still exerting considerable effect. Once the patient is familiar with insulin usage, the acceptability of more complex regimens increases.[58,59]
A starting dose of intermediate-acting insulin [neutral protamine Hagedorn (NPH) or detemir] or the long-acting insulin glargine at 0.1–0.2 U/kg or 10 U at bedtime is a reasonable first step for patients with fasting hyperglycemia.[60] Smaller doses (say 0.1 U/kg) might be started in frail, underweight and malnourished patients, and those with significant co-morbidity.[58] The challenge of starting basal insulin at bedtime is nocturnal hypoglycemia, which is more common among those with type 2 diabetes treated with NPH compared with insulin glargine (28.8% vs. 12.6%, respectively; P = 0.011).[61] Such a phenomenon is not at all surprising because the peak activity of NPH, which usually occurs at 6-8 hours following the injection, might coincide with the most insulin sensitive period of the day, i.e. midnight. Low cortisol is the most important contributing factor. As the greatest efficacy of NPH weans off, i.e. toward dawn, insulin resistance rises due to surge of cortisol, leading to hyperglycemia. Such factors necessitate the injection of NPH as late as possible, preferably before midnight. Technically, it is quite disadvantageous for the elderly who might prefer to retire early. Insulin analogues like glargine and detemir, being virtually peakless can be given even early, and hence have been emerging as natural choices in the elderly.
Initiation with basal bolus: Ideal but too complex
A combination of long-acting insulin once a day and preprandial rapid-acting insulin is considered an ideal regimen since it mimics basal and prandial endogenous insulin secretion. However, it is a very intense and complex regimen. It may require four to five injections daily and frequent monitoring of blood glucose levels at least three times daily, and it requires special skills in carbohydrate counting and in adding insulin correction doses for preprandial hyperglycemia. It may be a necessity in type 1 diabetics and in very special situations such as pregnancy, preoperative patients or patients hospitalized for other medical morbidities. Because of the complexity of this regimen, it may not be appealing to older adults for domiciliary use on long-term basis. The initial starting total daily dose of insulin is estimated to be 0.6 U/kg. The insulin regimen should subsequently be modified on the basis of the individual's response to therapy.[47] In the Treating to Target in Type 2 diabetes (4-T) study, up to 81.6% of patients who were initiated on basal analogue detemir required additional prandial insulin during 3 years of follow-up when titrations were done to achieve a tight glycemic control.[62]
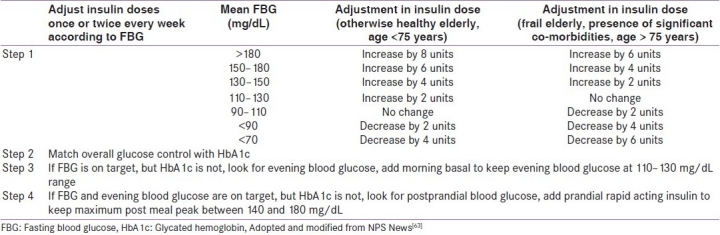
The South Asian Consensus group recommends that in patients already undergoing treatment with adequate doses of two or more oral anti-diabetic drugs (OADs), addition of bedtime basal insulin may be considered when FPG is >150 mg/dL and PPPG is >200 mg/dL and/or HbA1c is >8.5%. Long-acting analogues are preferred over NPH basal insulin. The best time to inject both analogues and NPH is in the evening; however, the former can be given at any time of the day depending on the patients’ (or attendants’) convenience. The physician may continue the ongoing secretagogues, but nighttime SUs are to be avoided. Metformin should be continued along with basal insulin therapy. The panel prefers a conservative initial starting dose of 0.1 U/kg/day. After initiation, the dose should be titrated once or twice every week on the basis of glucose monitoring results, targeting FBG. If HbA1c targets are not achieved, it may be due to hidden rise in postprandial blood sugar which has to be identified and treated according to a pre-set protocol [Table 7].
Table 7.
Protocol of intensification of basal insulin therapy in elderly diabetic patients
Using insulin in elderly diabetics: Role of premixed insulin
Conventionally, premixed insulins are used twice daily, with breakfast and supper. Premixed insulin preparations are more convenient and less prone to errors in dosing, two pertinent points in the elderly; but they limit the flexibility in diet and lifestyle. Among the patients who have round the clock hyperglycemia, i.e. fasting/pre-meal and post-meal hyperglycemia, premixed insulin can be used especially in those not preferring multiple injections and those who cannot perform frequent self-monitoring of blood glucose, thus making the prescription of the basal bolus regimen redundant (see above). Premix insulin is the preferred insulin in the social situation prevailing in the South Asian countries because of the following reasons: simple start, option to intensify with same insulin, coverage of both FPG and PPG, and eventually effective HbA1c control.[57]
Initiation with premix insulin: Simple and effective way to start
In the recent times, evidence has accumulated favoring the use of premix insulin as an option for initiation in the primary care setting. The INITIATE (INITiation of Insulin to reach A1c TargEt) study found the addition of premix insulin to OADs as a more effective option than adding basal insulin for treatment of type 2 diabetes. The glycemic targets were attained in 66% of patients treated with premix insulin as compared to 40% in group treated with basal insulin.[64] The PREFER study comparing premix insulin therapy with basal bolus therapy has shown that the effect of premixed analogue is equivalent to that of basal bolus therapy in insulin naοve patients.[65] The DURABLE study post-hoc analysis also favored premixed analogue (biphasic lispro) over basal analogue (glargine) in terms of efficacy in lowering HbA1c in type 2 diabetes in elderly. More number of hypoglycemic events seen in the premixed arm in this study was attributed to use of more number of doses (twice) compared to basal insulin and also the lack of titration of concomitant OAD doses.[66] A pilot multicenter study from India examining the role of Designer Insulin Regimen in Clinical Practice has depicted that both premix insulin analogues (two injections per day) and basal bolus analogue regimen (four injections per day) can be used in an equally effective way for initiating insulin therapy in T2DM. However, premix insulin analogue fared better than the basal bolus regimen in lowering HbA1c (1.58 vs. 1.16%; P < 0.05). Better adherence and compliance to the therapy with premixed insulin group was the main reason behind this.[67] In a sub-analysis of Korean elderly patients with type 2 diabetes inadequately controlled on their previous therapies, treatment with BIAsp30 offered improvements in glycemic control and was well tolerated. Body weight gain was minimal with BIAsp30, and treatment satisfaction among these patients appeared to be high.[68] Premixed analogue has certain advantages over human premixed insulin, such as better postprandial control, less hypoglycemia, and meal time flexibility, which make it the preferred choice for use in the elderly.[57]
Intensification of therapy with premix insulin
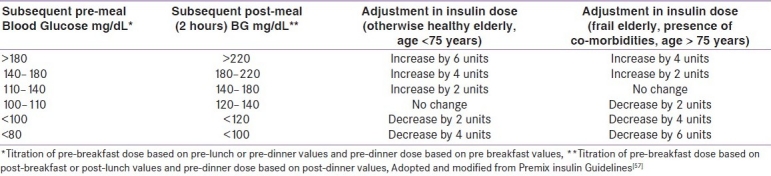
The South Asian Consensus group recommends initiation of insulin therapy with premix insulin in those elderly diabetics who have round the clock hyperglycemia (both fasting and postprandial blood glucose high). Moreover, switching over to premix insulin from basal insulin is preferred when goals remain unmet.[69] An useful algorithm is provided in Figure 1. The intensification protocol for premixed insulin in given in Table 8. It is important to remember the following pertinent points while titrating the dose during intensification: (i) the lowest of the most recent pre-meal levels should be used; (ii) the meal time dose preceding the measurement should be titrated; e.g. if the referred measurement is pre-lunch or pre-dinner, change of pre-breakfast insulin dose is required; (iii) the dose should not be increased if hypoglycemia occurs during these days; (iv) dose adjustments can be made once a week until the target is reached; and (iv) only one dose at a time should be changed.
Figure 1.
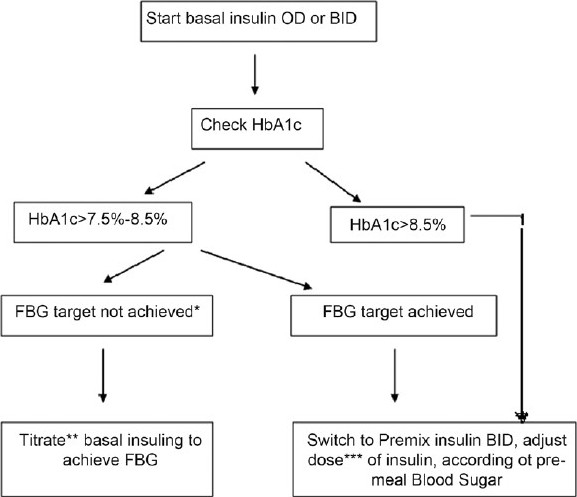
A simple approach of switching over to premix insulin from basal insulin is shown here. Such a stepped approach is preferred when goals remain unmet (*To achieve FBG 90–110 mg/dL and HbA1c <7.5%. Less stringent cutoff such as FBG 110–130, HbA1c <8% for patients who are frail, aged >75 years, having significant co-morbidities, **Please refer to the protocol shown in Table 7, ***Please refer to the protocol shown in Table 8, OD: Once daily, BID: Twice daily, HbA1c: Glycated hemoglobin, FBG: Fasting blood glucose, Adopted and modified from Unnikrishnan et al.[69])
Table 8.
Protocol of intensification of premixed insulin therapy in elderly diabetics
The South Asian Consensus Guidelines recommend cautious use of all pharmacotherapy in geriatric diabetes. The primary principle of diabetes therapy should be to achieve euglycemia, without causing hypoglycemia. Appropriate use of modern insulins and other drugs will help physicians achieve this aim.
SIGNIFICANCE OF OTHER PARADIGMS
Diabetes education
Persons with diabetes mellitus, and if appropriate, family members and caregivers, should be given information about hypo- and hyperglycemia at diagnosis, with reassessment and reinforcement periodically as needed. The need to understand the importance of the precipitating factors, prevention, symptoms and monitoring, treatment, and notifying a healthcare professional about hypo- and hyperglycemia should be elaborated.[70,71]
Multidisciplinary interventions that provide education on medication use, monitoring, and recognizing hypo- and hyperglycemia can significantly improve the glycemic control.[72]
The individuals should be informed about the benefits of exercise and available resources for becoming more active. The older adult with diabetes and any caregiver should receive education about the risk factors for foot ulcers and amputation. Physical ability to provide proper foot care should be evaluated.[71]
Dietary therapy
Nutrition for persons with diabetes must include the importance on improving metabolic outcomes by modifying nutrient intake and lifestyle. Major objectives should be to achieve and sustain normal or as close to normal range as is safely possible of blood glucose, blood pressure, and lipid/lipoprotein levels. Dietary therapy in elderly diabetics is a problem in itself because of various co-existing features. The following should be specially considered for older adults:
financial difficulty;
difficulty with shopping because of transportation or mobility problems;
poor food preparation skills (especially of elderly widowed men);
ingrained dietary habits;
difficulty following the dietary instructions because of impaired cognitive function;
decreased taste due to loss of taste buds;
increased frequency of constipation; and
problems with chewing because of loss of teeth.
Keeping in view all these considerations in the elderly, the quality, quantity and frequency have to be modified as per the person concerned. However, the total calories and its distribution should more or less correspond to the standard dietary therapy. It is important that physicians understand the general principles of medical nutrition therapy and support the elderly persons with diabetes. In most people, nutrition recommendations are similar to those of the general population.
One should ensure, however, that the diet prescription is not only accurate, but also appropriate. The diet should be available/accessible, acceptable, attractive, achievable and affordable. The advised meals should be absorbable/digestible as well.[73] One should keep in mind these eight principles of a patient-friendly diet while counseling persons with diabetes. Following these basic rules will ensure better concordance with the diet prescription and help in achieving good glycemic control. The South Asian Consensus Guidelines reiterate the “eight As” of dietary prescription in geriatric people with diabetes.
Physical activity
Exercise improves insulin resistance and has beneficial effects in preventing and treating type 2 diabetes. However, aerobic exercise is hindered in many type 2 diabetic patients because of advancing age, obesity, and other comorbid conditions.
Evidence has accumulated suggesting that the progressive decrease in fitness and muscle mass and strength with aging is in part preventable by maintaining regular exercise. The decrease in insulin sensitivity with aging is also partly due to lack of physical activity. Lower levels of physical activity are especially likely in the population at risk for type 2 diabetes. It is likely that maintaining better levels of fitness in this population will lead to less chronic vascular disease and an improved quality of life.
Before beginning an exercise program, the individual with diabetes mellitus should undergo a detailed medical evaluation with appropriate diagnostic studies. This examination should carefully screen for the presence of macrovascular and microvascular complications which may be worsened by the exercise program.[74] The potential benefits of exercise for elderly people are improved exercise tolerance, improved glucose tolerance, improved maximum oxygen consumption, increased muscle strength, decreased blood pressure, decreased body fat, improved lipid profile and improved sense of well-being. Sometimes, the patients face some risks due to exercise such as sudden cardiac death, foot and joint injuries, hypoglycemia, etc. Because of co-existence of other ailments like osteoarthritis, Parkinson's disease, visual impairment, poor vital reserve, etc., exercises should be carried out in familiar surroundings and should be isotonic rather than isometric.[70,74]
The South Asian Consensus recognizes difficulties faced by patients in gaining access to diabetes education, physical activity, appropriate diet, and medical care. It suggests concerted action by all stakeholders to achieve optimal health for elderly diabetics.
CONCLUSION
Physicians treating elderly diabetes patients are now better placed then before with the multiple options available in the treatment armamentarium. What needs to be emphasized is to choose the treatment goals appropriately and use the available pharmacological agents judiciously. This panel believes that the aim of treatment in this group of patients is to achieve goals steadily while doing “no harm”. One needs to underscore the importance of a “shared healthcare approach” involving counselors, community workers, local doctors and specialist geriatric diabetologists/endocrinologists. Such an approach is of critical importance for ensuring good health for these patients. South Asian cultures pride themselves for their values of respect and care for elders. The South Asian Consensus Guidelines hope to justify this pride by improving the quality of care for the elderly diabetic population, not only in South Asia, but across the globe as well.
Acknowledgments
Authors acknowledge the writing and editorial assistance of Dr. B. S. Mohan in manuscript preparation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gupta V, Suri P. Diabetes in Elderly patients. JK Pract. 2002;91:258–9. [Google Scholar]
- 2.Census of India 1991 (Report) New Delhi: Registrar General, India; 1996. Population projection for India and states - 1996-2016. [Google Scholar]
- 3.Singapore: Ministry of Community Development; 1999. Inter-Ministerial Committee on Ageing Report. [Google Scholar]
- 4.India: M/s Banarsidas Bhanot Publishers; 2007. Park’s Text Book of Preventive and Social Medicine. [Google Scholar]
- 5.IDF Diabetes Atlas. ©International Diabetes Federation. (4th ed) 2009 [PubMed] [Google Scholar]
- 6.Chhetri MR, Chapman RS. Prevalence and determinants of diabetes among the elderly population in the Kathmandu Valley of Nepal. Nepal Med Coll J. 2009;11:34–8. [PubMed] [Google Scholar]
- 7. [cited in 2010]. Available from: https://www.cia.gov/library/publications/the-worldfactbook/geos/np.html .
- 8.Pietropaolo M, Barinas-Mitchell E, Pietropaolo SL, Kuller LH, Trucco M. Evidence of islet cell autoimmunity in elderly patients with type 2 diabetes. Diabetes. 2000;49:32–8. doi: 10.2337/diabetes.49.1.32. [DOI] [PubMed] [Google Scholar]
- 9.Glynn RJ, Monane M, Gurwitz JH, Choodnovskiy I, Aging Avorn J. comorbidity, and reduced rates of drug treatment for diabetes mellitus. J Clin Epidemiol. 1999;52:781–90. doi: 10.1016/s0895-4356(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 10.Resnick HE, Harris MI, Brock DB, Harris TB. American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2000;23:176–80. doi: 10.2337/diacare.23.2.176. [DOI] [PubMed] [Google Scholar]
- 11.Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in Europe. Lancet. 1999;354:617–21. [PubMed] [Google Scholar]
- 12.Wandell PE, Tovi J. The quality of life of elderly diabetic patients. J Diabetes Compl. 2000;14:25–30. doi: 10.1016/s1056-8727(99)00066-5. [DOI] [PubMed] [Google Scholar]
- 13.Goriya Y, Suzuki T, Takizawa S, Natto M. Follow-up of elderly diabetics with or without hypertension and hyperlipidemia. J Environ Pathol Toxicol Oncol. 2000;19:159–65. [PubMed] [Google Scholar]
- 14.Vidt DG. Good news for the older patient with diabetes: added cardiovascular risk reduction. Curr Hypertens Rep. 1999;1:379–80. doi: 10.1007/s11906-999-0051-4. [DOI] [PubMed] [Google Scholar]
- 15.Fisman EZ, Tenenbaum A, Benderly M, Goldbourt U, Behar S, Motro M. Antihyperglycemic treatment in diabetics with coronary disease: Increased metformin-associated mortality over a 5-year follow-up. Cardiology. 1999;91:195–202. doi: 10.1159/000006909. [DOI] [PubMed] [Google Scholar]
- 16.de Lissovoy G, Ganoczy DA, Ray NF. Relationship of hemoglobin A1c, age of diabetes diagnosis, and ethnicity to clinical outcomes and medical costs in a computer-simulated cohort of persons with type 2 diabetes. Am J Manag Care. 2000;6:573–84. [PubMed] [Google Scholar]
- 17.Brown AF, Mangione CM, Saliba D, Sarkisian CA. California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes: Guidelines for Improving the Care of the Older Person with Diabetes Mellitus. J Am Geratr Soc. 2003;51:S265–80. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 18.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA diabetes trials.A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–92. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935–40. doi: 10.1001/jama.295.16.1935. [DOI] [PubMed] [Google Scholar]
- 20.Hornick T, Aron DC. Managing diabetes in the elderly: Go easy, individualize. Cleve Clin J Med. 2008;175:5–9. doi: 10.3949/ccjm.75.1.70. [DOI] [PubMed] [Google Scholar]
- 21.Barnett AH, Owens DR. Insulin analogues [published correction appears in Lancet 1997;349:656] Lancet. 1997;349:47–51. doi: 10.1016/S0140-6736(96)06032-1. [DOI] [PubMed] [Google Scholar]
- 22.Thomson FJ, Masson EA, Leeming JT, Boulton AJ. Lack of knowledge of symptoms of hypoglycaemia by elderly diabetic patients. Age Ageing. 1991;20:404–6. doi: 10.1093/ageing/20.6.404. [DOI] [PubMed] [Google Scholar]
- 23.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycemia in type 2 di-abetes: A consensus algorithm for the initia-tion and adjustment of therapy. Diabetes Care. 2006;29:1963–72. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 24.Sinclair AJ. Aging and diabetes mellitus. In: Defranzo RA, Ferrannini E, Keen H, Zimmet P, editors. International textbook of Diabetes mellitus. 3rd ed. Vol. 2. Chichester, England: John Wiley & Sons Ltd; 2004. pp. 1579–624. [Google Scholar]
- 25.Haas L. Management of diabetes mellitus medications in the nursing home. Drugs Aging. 2005;22:209–18. doi: 10.2165/00002512-200522030-00003. [DOI] [PubMed] [Google Scholar]
- 26.The UKPDS group. Effect of intensive blood glucose control with metformin on combinations in overweight patients with type2 diabetes. Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 27.Joshua J, Neumiller JJ, Setter SM. Pharmacologic management of the older patient with type 2 diabetes mellitus. Am J Geriatr Pharmacother. 2009;7:324–34. doi: 10.1016/j.amjopharm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy DL, Piper JM, Baum C. Trends in the use of oral hypoglycemic agents. Diabetes Care. 1988;11:558–62. doi: 10.2337/diacare.11.7.558. [DOI] [PubMed] [Google Scholar]
- 29.Tessie D, Dawson K, Tetrault JP, Bravo G, Meneilly GS. Glibenclamide vs Gliclazide in type 2 diabetes of the elderly. Diabetic Med. 1994;11:974–80. doi: 10.1111/j.1464-5491.1994.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 30.Chelliah A, Burge MR. Hypoglycemia in elderly patients with diabetes mellitus: Causes and strategies for prevention. Drugs Aging. 2004;21:511–30. doi: 10.2165/00002512-200421080-00003. [DOI] [PubMed] [Google Scholar]
- 31.Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev. 2001;17:467–73. doi: 10.1002/dmrr.235. [DOI] [PubMed] [Google Scholar]
- 32.Josse RG, Chausson JL, Ryan EA, Lau DC, Ross SA, Yale JF, et al. Acarbose in the treatment of elderly patients with Type 2 diabetes. Diabetes Res Clin Pract. 2003;59:37–42. doi: 10.1016/s0168-8227(02)00176-6. [DOI] [PubMed] [Google Scholar]
- 33.Odegard PS, Setter SM, Neumiller JJ. Considerations for the pharmacological treatment of diabetes in older adults. Diabetes Spectrum. 2007;20:239–47. [Google Scholar]
- 34.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–9. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 35.Mathieu C, Bollaerts K. Antihyperglycaemic therapy in elderly patients with type 2 diabetes: potential role of incretin mimetics and DPP-4 inhibitors. Int J Clin Pract. 2007;61(Suppl.154):29–37. doi: 10.1111/j.1742-1241.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 36.NICE clinical guideline 87: Type 2 diabetes: The management of type 2 diabetes. [cited in May 2009]. Available from: http://www.nice.org.uk .
- 37.Shubrook J, Colucci R, Guo A, Schwartz F. Saxagliptin: A selective DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Med Endocr Diabetes. 2011;4:1–12. doi: 10.4137/CMED.S5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ligueros-Saylan M, Foley JE, Schweizer A, Couturier A, Kothny W. An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of phase II and III clinical trials. Diabetes Obes Metab. 2010;12:495–509. doi: 10.1111/j.1463-1326.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- 39.Halimi S, Raccah D, Schweizer A, Dejager S. Role of vildagliptin in managing type 2 diabetes mellitus in the elderly. Curr Med Res Opin. 2010;26:1647–56. doi: 10.1185/03007995.2010.485881. [DOI] [PubMed] [Google Scholar]
- 40.Pratley RE, Rosenstock J, Pi-Sunyer FX, Banerji MA, Schweizer A, Couturier A, et al. Management of type 2 diabetes in treatment-naive elderly patients: Benefits and risks of vildagliptin monotherapy. Diabetes Care. 2007;30:3017–22. doi: 10.2337/dc07-1188. [DOI] [PubMed] [Google Scholar]
- 41.Schweizer A, Dejager S, Foley JE, Shao Q, Kothny W. Clinical experience with vildagliptin in the management of type 2 diabetes in a patient population ≥75 years: A pooled analysis from a database of clinical trial. Diabetes Obesit Metab. 2011;13:55–64. doi: 10.1111/j.1463-1326.2010.01325.x. [DOI] [PubMed] [Google Scholar]
- 42.Edavalath M, Stephens JW. Liraglutide in the treatment of type 2 diabetes, mellitus: Clinical utility and patient perspectives. Patient Pref Adherence. 2010;4:61–8. doi: 10.2147/ppa.s6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katsiki N, Papanas N, Mikhailidis DP. Dapagliflozin: More than just another oral glucose lowering agent? Expert Opin Investig Drugs. 2010;19:1581–9. doi: 10.1517/13543784.2011.539558. [DOI] [PubMed] [Google Scholar]
- 44.Via MA, Chandra H, Araki T, Potenza MV, Shamgas M. Bromocriptine approved as the first medication to target dopamine activity to improve glycemic control in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2010;3:43–8. doi: 10.2147/dmsott.s9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalra S. Bromocriptine: A revolution in diabetes care? (Editorial) Int J Clin Cases Investig. 2010;1:1–2. [Google Scholar]
- 46.Bott U, Ebrahim S, Hirschberger S, Skovlund SE. Effect of the rapid-acting insulin analogue insulin as part on quality of life and treatment satisfaction in patients with type 1 diabetes. Diabetes Med. 2003;20:626–34. doi: 10.1046/j.1464-5491.2003.01010.x. [DOI] [PubMed] [Google Scholar]
- 47.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nathan DM. Clinical practice: Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342–9. doi: 10.1056/NEJMcp021106. [DOI] [PubMed] [Google Scholar]
- 49.Funnell MM, Kruger DF. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ. 2004;30:274–80. doi: 10.1177/014572170403000220. [DOI] [PubMed] [Google Scholar]
- 50.Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients‘ fears and hopes about insulin therapy: The basis of patient reluctance. Diabetes Care. 1997;20:292–8. doi: 10.2337/diacare.20.3.292. [DOI] [PubMed] [Google Scholar]
- 51.Dailey G. A timely transition to insulin: identifying type 2 diabetes patients failing oral therapy. Formulary. 2005;40:114–30. [Google Scholar]
- 52.Hendra TJ. Starting insulin therapy in elderly patients. J R Soc Med. 2002;95:453–5. doi: 10.1258/jrsm.95.9.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kreinhofer M, Aufseesser-Stein M, Assal JP. Insulin injections: Mistakes and errors made by patients and/or health care providers. Diabetes Res Clin Pract. 1988;(Suppl.I):35–40. doi: 10.1016/0168-8227(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 54.Berger AS, Saubrey N, Puxty JA, Hunter DH, Burr WA. Accuracy of insulin injection in elderly patients. Br Med J. 1983;287:1762. doi: 10.1136/bmj.287.6407.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coscelli C, Lostiab S, Lunettac M, Nosarid I, Coronele GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28:173–7. doi: 10.1016/0168-8227(95)01092-r. [DOI] [PubMed] [Google Scholar]
- 56.Baruah MP. Insulin pens: the modern delivery devices. J Assoc Physic India. 2011 Apr;59(Suppl):38–40. [PubMed] [Google Scholar]
- 57.Premix Insulin: Initiation and Continuation -Guidelines for Management of Diabetes in Primary Care: Indian National Consensus Group. J Assoc Physic India. 2009;57(suppl):42–6. [Google Scholar]
- 58.Wong J, Yue D. Starting insulin treatment in type 2diabetes. Aust Preser. 2004;27:93–6. [Google Scholar]
- 59.Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. UK Prospective Diabetes Study Group.. Sulfonylurea inadequacy: Efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57) Diabetes Care. 2002;25:330–6. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- 60.Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2007;55:182–8. doi: 10.1111/j.1532-5415.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 61.Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes: HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–6. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- 62.Holman RR, Farmer AJ, Melanie J, Davies MJ, Jonathan C, Levy JC, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–47. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 63.National Prescribing Service. NPS News 56: Managing hyperglycaemia in type 2 diabetes. [cited in Feb 2008]. Available from: http://www.nps.org.au/_data/assets/pdUle/0019/25219/news56.pdf .
- 64.Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, et al. Initiating insulin therapy in type 2 diabetes: A comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–5. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 65.Liebl A, Prager R, Kaiser M, Binz K, Gallwitz B. Biphasic insulin as part 30 (BIAsp30), Insulin Detemir (IDet) and Insulin Aspart (IAsp) allow patients with type 2 diabetes to reach A1c target: The PREFER study. Diabetes. 2006;55(Suppl.1):A123. [Google Scholar]
- 66.Wolffenbutte BH, Bhushan LJ, Klaff J, Fahrbach JL, Jiang H, Martin S. Initiating insulin therapy in elderly patients with Type 2 diabetes: efficacy and safety of lispro mix 25 vs.basal insulin combined with oral glucose-lowering agents. Diabetes Med. 2009;26:1147–55. doi: 10.1111/j.1464-5491.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- 67.Joshi SR, Kalra S, Badgandi M, Rao YS, Chawla M. Designer insulins regimens in clinical practice - pilot multicenter indian study. J Assoc Physic India. 2005;53:775–9. [PubMed] [Google Scholar]
- 68.Jang HC, Lee SR, Vaz JA. Biphasic insulin aspart 30 in the treatment of elderly patients with type 2 diabetes: A subgroup analysis of the PRESENT Korea NovoMix study. Diabet Obestet Metab. 2009;11:20–6. doi: 10.1111/j.1463-1326.2008.00891.x. [DOI] [PubMed] [Google Scholar]
- 69.Unnikrishnan AG, Tibaldi J, Hadley-Brown M, Krentz AJ, Ligthelm R, Damci T, et al. Practical guidance on intensification of insulin therapy with BIAsp 30: A consensus statement. Int J Clin Pract. 2009;63:1571–7. doi: 10.1111/j.1742-1241.2009.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baruah MP, Kalra S, Mathew J, Unnikrishnan AG, Ganapathi B, Sahay R. Diabetes in the elderly: Critical appraisal in management. Int J Clin Cases Investig. 2010;1:7–14. [Google Scholar]
- 71.Kalra S, Ganie A, Agrawal N. Geriatric diabetology: An overview. Int J Geriatr Gerontol. 2009;5:1. [Google Scholar]
- 72.Chiniwala N, Jabbour S. Management of diabetes mellitus in elderly. Curr Opin Endocr Diabetes Obes. 2011;18:148–52. doi: 10.1097/MED.0b013e3283444ba0. [DOI] [PubMed] [Google Scholar]
- 73.Kalra S, Kalra B, Saluja S. Dietary management in geriatric patients of diabetes mellitus: Special considerations. Int J Geriatr Gerontol. 2009;5:1. [Google Scholar]
- 74.Kalra S, Kalra B, Gandhi A, Agarwal N, Sirka M. Exercise prescription in diabetes. Int J Chiropract. 2009;1:1. [Google Scholar]


