Abstract
Context:
Rapidly rising prevalence of obesity is alarming. Obesity predisposes to co-morbidities like hypertension, type 2 diabetes mellitus, dyslipidemias, thus substantially rising healthcare expenditure. Lifestyle modifications alone have very limited success, necessitating the addition of pharmacotherapy to it.
Objective:
Present study was carried out to evaluate the efficacy and safety of orlistat in obese patients.
Materials and Methods:
Eighty obese (BMI>30) patients according to inclusion and exclusion criteria were randomized into either of the two groups. Group 1 received orlistat 120 mg three times a day and group 2 received placebo three times a day. Weight, waist circumference, BMI, total cholesterol, triglycerides, HDL, LDL were measured at baseline and then at 8th, 16th and 24th week. ADR reported by patients were recorded. For safety evaluation various hematological and biochemical parameters were assessed. Z test was used for analysis of data.
Results:
Compared to placebo, orlistat caused significant reduction (P<0.05) in weight (4.65 kg vs 2.5 kg; orlistat vs placebo, respectively), BMI (1.91 kg/m2 vs 0.64 kg/m2) and waist circumference (4.84 cm vs 2 cm), cholesterol (10.68 mg vs 6.18 mg) and LDL level (5.87 mg vs 2.33 mg). In the orlistat group, the GI side effects like loose stools, oily stools/spotting, abdominal pain and fecal urgency were observed.
Conclusion:
Orlistat is an effective and well-tolerated antiobesity drug, which can be employed as an adjunct to therapeutic lifestyle changes to achieve and maintain optimal weight.
Keywords: Antiobesity agents, obesity, orlistat, weight loss
INTRODUCTION
Obesity is a state of excess adipose tissue mass.[1] Prevalence is approximately 30% and is rapidly rising.[2] Obesity leads to increased morbidity more than mortalityand increases the prevalence of co-morbidities like hypertension, type 2 diabetes mellitus, dyslipidemia, endocrinal abnormalities and higher mortality from some cancers like esophagus, colon, rectum and breast.[1]
Primary measures taken to control obesity like dietary restriction and exercise are met with short-term success and are often inadequate alone, therefore there is need for pharmacotherapy as adjunct to lifestyle changes in these patients.[3,4]
Most antiobesity drugs act on central nervous system to suppress appetite and reduce food intake. Serious adverse effects of older drugs (e.g. amphetamine) and association of fenfluramine with cardiovascular side effects led to their withdrawal.[5] Sibutramine is inhibitor of reuptake of 5HT and NA, causes 4–6% weight loss after one year treatment but has adverse cardiovascular effects like slight increase in blood-pressure, heart rate which restricts its use in obese patients with coexisting cardiovascular morbidity.[4] A new drug orlistat is a lipase inhibitor which reduces fat absorption from intestines. It is reported to be effective in reducing weight.[4–6] Therefore present study was carried out to compare safety and efficacy of orlistat with placebo in reducing weight in obese patients.
MATERIALS AND METHODS
This was a prospective, randomized, single blind, parallel group study carried out at outpatient department of an endocrinology hospital over the period of 24 weeks. The study was carried out after obtaining approval from Institutional Ethics Committee.
The inclusion criteria were patients with BMI ≥ 30 kg/m2 of either sex between the age of 18 and 60 years. The following categories of patients were excluded: patients with endocrinologic obesity; patients using other medications that can alter body weight or lipid levels; patients with clinically significant cardiovascular; respiratory or hepatobiliary disorder; chronic diarrhea and pregnant and lactating women.
Total 80 patients were enrolled in the study according to inclusion and exclusion criteria and were randomized into two groups of 40 patients each. Group 1 patients received orlistat 120 mg three times a day, 1 hour before breakfast, lunch and dinner. A standard dose of orlistat 120 mg capsule three times a day was selected from the previous studies. Group 2 patients received placebo three times a day, 1 hour before breakfast, lunch and dinner. Placebo, similar in appearance to orlistat capsule was used.
After enrolment into the study, baseline clinical examination (weight, BMI) and laboratory investigations (lipid profile, BSL) were carried out. In the same visit, irrespective of group allotted, all the patients were educated about the importance of lifestyle changes including healthy dietary habits and exercise in weight reduction and maintenance. Patients were given information about nutritional value of various foods and few simple exercises for decreasing and maintaining near normal body weight. Their compliance for lifestyle change advice was checked through verbal questions at each visit. Biochemical examinations assessed were Hb, total leukocyte count (TLC), serum creatinine, SGPT SGOT at baseline and at the 24th week of study period.
The efficacy of drugs was assessed by primary efficacy parameters i.e. body weight (kg), body mass index (kg/m2) and waist circumference (in cm measured at midpoint between the lower border of rib cage and iliac crest). Other parameters assessed were total serum cholesterol (mg/dl), serum triglyceride (mg/dl), serum low-density lipoprotein (mg/dl), serum high-density lipoprotein (mg/dl), random blood glucose level (mg/dl) and blood pressure (mm Hg).
Safety was assessed in terms of both subjective and objective adverse effects. Subjective symptoms such as loose stools were assessed by questioning patients at each visit and objective signs were assessed by clinical and biochemical examination.
Follow-up was performed at 8th, 16th and 24 weeks of study period, and during each visit all the efficacy parameters were measured and safety evaluation was done.
Quantitative data was analyzed by Z test for difference between two means, while qualitative was analyzed by Z test for difference between two proportions. P value <0.05 was taken as significant while P value >0.05 was taken as insignificant.
RESULTS
Baseline values of both the groups were comparable with respect to age, sex, weight, height, BMI, waist circumference, total cholesterol, triglycerides, LDL, HDL, random blood glucose level and blood pressure.
In orlistat-treated group, the mean weight at day 0 was 94.26 ± 13.45 kg, which reduced to 92.67 ± 8.71 kg, 89.61 ± 7.39 kg and 89.01 ± 7.39 kg at 8, 16 and 24 weeks, respectively [Table 1]. When values of mean weight at 8, 16 and 24 weeks were compared with baseline it was found that the mean weight at 16 and 24 weeks was significantly lesser (P <0.05) while at 8 weeks the difference was insignificant (P >0.05). In placebo-treated group, mean weight at day 0 was 94.54 ± 15.75 kg, it reduced to 93.05 ± 9.56 kg, 92.81 ± 6.88 kg and 92.04 ± 6.39 kg at 8, 16 and 24 weeks respectively [Table 2]. When values of mean weight at 8, 16 and 24 weeks were compared with baseline, it was found that the difference in mean weight at 8, 16 and 24 weeks was statistically insignificant (P > 0.05).
Table 1.
Effect of orlistat on various parameters at 8, 16 and 24 weeks
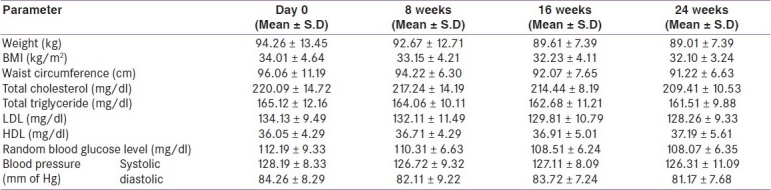
Table 2.
Effect of placebo on various parameters at 8, 16 and 24 weeks
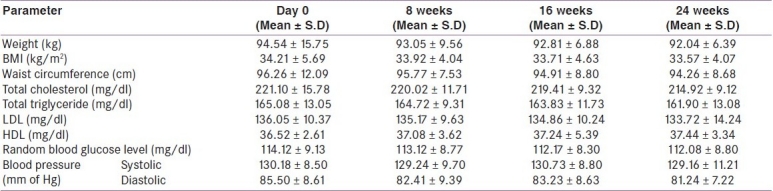
Mean BMI in orlistat-treated group at day 0 was 34.01 ± 4.64 kg/m2, which reduced to 33.15 ± 4.21 kg/m2,32.23 ± 4.11 kg/m2 and 32.10 ± 3.24 kg/m2 at 8, 16 and 24 weeks, respectively [Table 1]. When values of mean BMI at 8, 16 and 24 weeks were compared with baseline it was found that the mean BMI at 16 and 24 weeks was significantly lesser (P <0.05) while at 8 weeks the difference was insignificant (P >0.05). In placebo-treated group, the mean BMI at day 0 was 34.21 ± 5.69 kg/m2 which reduced to 33.92 ± 4.04 kg/m2, 33.71 ± 4.63 kg/m2 and 33.57 ± 4.07 kg/m2 at 8, 16 and 24, weeks respectively [Table 2]. When values of mean BMI at 8, 16 and 24 weeks were compared with baseline it was found that the difference in mean BMI at 8, 16 and 24 weeks was statistically insignificant (P >0.05).
Mean waist circumference in orlistat-treated group at day 0 was 96.06 ± 11.19 cm, which reduced to 94.22 ± 6.30 cm, 92.07 ± 7.65 cm and 91.22 ± 6.63 cm at 8, 16 and 24 weeks, respectively [Table 1]. When values of mean waist circumference at 8, 16 and 24 weeks were compared with baseline it was found that the mean waist circumference at 16 and 24 weeks was significantly lesser (P <0.05) while at 8 weeks the difference was insignificant (P >0.05). In placebo-treated group, the mean waist circumference at day 0 was 96.26 ± 12.09 cm which reduced to 95.77 ± 7.53 cm, 94.91 ± 8.80 cm, 94.26 ± 8.68 cm at 8, 16, 24 weeks, respectively [Table 2]. When values of mean waist circumference at 8, 16 and 24 weeks were compared with baseline it was found that the difference in mean waist circumference at 8, 16 and 24 weeks was statistically insignificant (P >0.05).
When orlistat and placebo group were compared for absolute reduction in weight (4.65 kg vs 2.5 kg; orlistat vs placebo, respectively as observed in Figure 1), BMI (1.91 kg/m2 vs 0.64 kg/m2) and waist circumference (4.84 cm vs 2 cm) at 24 weeks the difference was found to be statistically significant (P <0.05).
Figure 1.
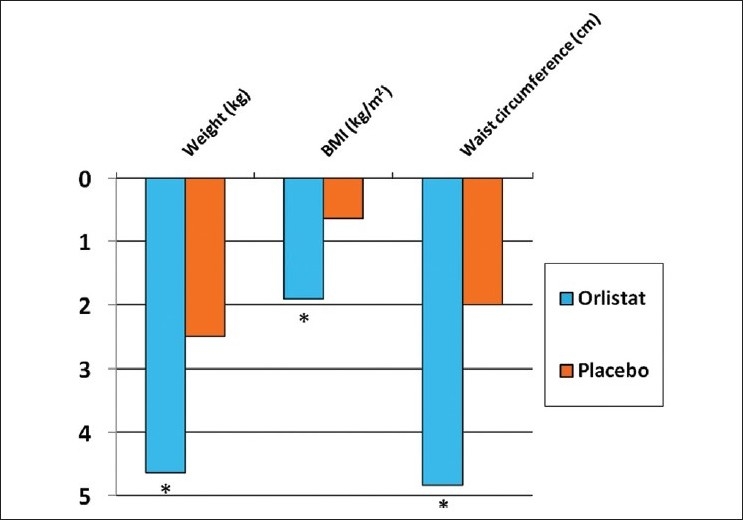
Comparison between effect of orlistat and placebo on efficacy parameters at 24 weeks (*P<0.05)
Mean total cholesterol concentration in orlistat-treated group at day 0 was 220.09 ± 14.72 mg/dl, which reduced to 217.24 ± 14.19 mg/dl, 214.44 ± 8.19 mg/dl and 209.41 ± 10.53 mg/dl at 8 ,16 and 24 weeks, respectively [Table 1]. When values of mean total cholesterol concentration at 8, 16 and 24 weeks were compared with baseline it was found that the mean total cholesterol concentration at 16 and 24 weeks was significantly lesser (P <0.05), while at 8 weeks the difference was insignificant (P >0.05). In placebo group mean total cholesterol concentration at day 0 was 221.10 ± 15.78 mg/dl which reduced to 220.02 ± 11.71 mg/dl at 8 weeks and to 219.41 ± 9.32 mg/dl and 214.92 ± 9.12 mg/dl at 16 and 24 weeks, respectively [Table 2]. When values of mean total cholesterol concentration at 8, 16 and 24 weeks were compared with baseline it was found that the difference in mean total cholesterol concentration at 8, 16 and 24 weeks was statistically insignificant (P >0.05).
In orlistat-treated group mean triglyceride concentration at day 0 was 165.12 ± 12.16 mg/dl which reduced to 164.06 ± 10.11 mg/dl, 162.68 ± 11.21 mg/dl and 161.51 ± 9.88 mg/dl at 8, 16 and 24 weeks, respectively [Table 1]. When values of mean triglyceride concentration at 8, 16 and 24 weeks were compared with baseline it was found that the difference in mean triglyceride concentration at 8, 16 and 24 weeks was statistically insignificant (P >0.05). Placebo-treated group had day 0 mean value of 165.08 ± 13.05 mg/dl which reduced to 164.72 ± 9.31 mg/dl, 163.83 ± 11.73 mg/dl, 161.90 ± 13.08 mg/dl at 8, 16 and 24 weeks, respectively [Table 2]. When values of mean triglyceride concentration at 8, 16 and 24 weeks were compared with baseline it was found that the difference in mean triglyceride concentration at 8, 16 and 24 weeks was statistically insignificant (P >0.05).
Mean LDL concentration in orlistat-treated group at day 0 was 134.13 ± 9.49 mg/dl, which reduced to 132.11 ± 11.49 mg/dl, 129.81 ± 10.79 mg/dl and 128.26 ± 9.33 mg/dl at 8, 16 and 24 weeks, respectively [Table 1]. When values of mean LDL concentration at 8, 16 and 24 weeks were compared with baseline it was found that the mean LDL concentration at 16 and 24 weeks was statistically significantly lesser (P <0.05), while at 8 weeks the difference was insignificant (P >0.05). Mean LDL concentration in placebo group at day 0 was 136.05 ± 10.37 mg/dl which reduced to 135.17 ± 9.63 mg/dl at 8 weeks and to 134.86 ± 10.24 mg/dl and 133.72 ± 14.24 mg/dl at 16 and 24 weeks, respectively [Table 2]. When values of mean LDL concentration at 8, 16 and 24 weeks were compared with baseline it was found that the difference in mean LDL concentration at 8, 16 and 24 weeks was statistically insignificant (P >0.05).
Orlistat-treated group had mean baseline HDL concentration of 36.05 ± 4.29 mg/dl which changed to 36.71 ± 4.29 mg/dl at 8 weeks, 36.91 ± 5.01 mg/dl at 16 weeks and 37.19 ± 5.61 mg/dl at 24 weeks [Table 1]. When values of mean HDL concentration at 8, 16 and 24 weeks were compared with baseline it was found that the difference in mean HDL concentration at 8, 16 and 24 weeks was statistically insignificant (P >0.05). Mean HDL concentration in placebo group at day 0 was 36.52 ± 2.61 mg/dl, 37.08 ± 3.62 at 8 weeks, 37.24 ± 5.39 mg/dl at 16 weeks and 37.44 ± 3.34 mg/dl at 24 weeks [Table 2]. When values of mean HDL concentration at 8, 16 and 24 weeks were compared with baseline it was found that the difference in mean HDL concentration at 8,16 and 24 weeks was statistically insignificant (P >0.05).
When orlistat- and placebo-treated group were compared for absolute reduction in cholesterol (10.68 mg vs 6.18 mg; orlistat vs placebo, respectively) and LDL level (5.87 mg vs 2.33 mg) at 24 weeks the difference was statistically significant (P <0.05). But when the comparison of change in triglyceride (3.61 mg vs 3.18 mg) and HDL level (1.14 mg vs 0.92 mg) at 24 weeks was done, the difference was found to be insignificant (P >0.05).
There was no significant change found in blood glucose level, systolic and diastolic blood pressure at 24 weeks in orlistat- and placebo-treated group when compared with respective baseline. Also there was no significant (P >0.05) difference in the values of these parameters in both the groups when compared at 24 weeks.
In the present study the incidence of adverse drug reactions like loose stools (28.5%), oily stools/spotting (25.65%), abdominal pain (31.35%) and fecal urgency (34.2%) was significantly more (P <0.05) in orlistat-treated group compared to placebo group but most of these adverse effects were mild and transient. Other adverse effects noted were nausea, vomiting, dyspepsia in both the groups. Orlistat had no adverse impact on Hb, TLC, serum creatinine, SGOT and SGPT level at the end of study.
DISCUSSION
A sedentary lifestyle coupled with an increased intake of energy dense food is contributing to the increased prevalence of obesity worldwide. Obesity increases mortality and risk of morbidity from cardiovascular disease, dyslipidemia, hypertension, stroke, osteoarthritis, sleep apnea, type 2 diabetes mellitus and several types of cancer.[1] A range of strategies can be employed for weight loss which includes lifestyle changes (diet, exercise), behavioral therapy, pharmacotherapy and surgery. Pharmacotherapy is recommended for obese patients in whom lifestyle interventions have failed, while surgery is reserved for morbidly obese patients.[1,4,6]
A new drug orlistat is reversible inhibitor of gastric and pancreatic lipases that acts in the lumen of stomach and small intestine, preventing absorption of fat significantly.[7] Therefore this study was undertaken to evaluate efficacy of orlistat along with its safety compared to placebo in obese patients.
Absolute weight reduction in orlistat group in this study was 4.65 kg which is comparable with the previous studies; Bakris et al.,[8] showed weight loss of 5.4 kg, Broom et al.,[9] showed mean weight loss of 5.8 kg, Lindgarde et al.,[10] found mean weight loss of 5.6 kg while Swinburn et al.,[11] reported 4.7 kg mean weight loss. Effect on BMI in orlistat-treated group in present study is comparable to the previous trials; Bakris et al.,[8] found mean reduction in BMI by 1.9 kg/m2 while Krempf et al.,[12] showed reduction by 2.3 kg/m2. In the present study in orlistat group reduction seen in waist circumference was 4.84 cm; many randomized clinical trials carried previously have shown similar results. Sari et al.,[13] found reduced waist circumference by 4.5 cm, Bakris et al.,[8] showed mean reduction in waist circumference by 5.2 cm. Guy-Grand et al.,[14] reported mean waist circumference reduction of 5.0 cm.
In the present study, mean percentage weight reduction seen in orlistat-treated group was 5.63% compared to 2.3% in placebo-treated group. Previous studies like Bakris et al.,[8] found 5.3% reduction, Broom et al.,[9] showed reduction of 5.8% while Lindgarde et al.,[10] found of 5.9% weight reduction. It has been proved that a weight loss of 5–10% of initial body weight has been shown to improve cardiovascular risk factors[12,15] so orlistat has an advantage in this regard.
In the present study, the absolute reduction in cholesterol concentration was 10.68 mg/dl, which is comparable to results of previous studies; Davidson et al.,[6] showed mean reduction of 12 mg/dl, Finer et al.,[5] found mean reduction of 11.6 mg/dl. In present study LDL concentration was reduced by 5.87 mg/dl, which is in accordance with the previous studies; Derosa et al.,[16] showed mean decrease by 7.8 mg/dl, Goley et al.,[17] reported mean fall in LDL concentration by 3.9 mg/dl while Finer et al.,[5] found mean reduction of 4.2 mg/dl.
We observed no significant change in triglyceride and HDL level, also there was no significant effect on random blood glucose level, systolic and diastolic blood pressure in orlistat-treated group. This findings correlate with the previous studies carried out by Finer et al.,[5] Davidson et al.,[6] Goley et al.[17]
In the present study, orlistat was well tolerated and adverse drug reactions were mostly gastrointestinal like loose stools, oily stools/spotting, abdominal pain and fecal urgency and these were significantly more common in orlistat-treated group compared to placebo group reflecting its mechanism of action. Orlistat did not produce any adverse impact on the Hb, TLC, serum creatinine, SGPT and SGOT. This again indicates a good safety profile of orlistat.
Efficacy of orlistat has been demonstrated in diverse group of obese patients including adolescent, adults with metabolic syndrome, pre-diabetics, type 2 diabetic. Patients with binge eating disorder, nonalcoholic fatty liver disease, postmenopause, depression or heart failure have been shown to derive modest benefit.[7] Since the prevalence of obesity in children and adolescents is increasing at alarming rate, the beneficial effects of orlistat in this group are encouraging and represent a potentially promising treatment modality in conjunction with lifestyle changes for obese adolescents in future. Orlistat is known to reduce the incidence of development of diabetes in prediabetics, this effect is desirable as prevention of diabetes is definitely a more cost effective option compared to its treatment.
It is widely known that any long-term weight reduction program involving any strategy has two phases: initial weight reduction followed by stabilization. Initial phase of around 9 months is of rapid weight loss followed by weight stabilization even with continuation of the same therapy. As the present study was of 6 months it may well be a part of the initial rapid weight reduction phase which may stabilize afterwards, emphasizing the need for longer duration studies to check for any sustained or incremental effect on weight reduction and to look for any detrimental effect on any organ system apart from gastrointestinal tract.
In India the cost of orlistat 120 mg three times a day comes up to ![]() 105/-, which may seem to be quite high but well-designed pharmacoeconomic analyses have shown that it is a cost-effective treatment option for obese patients with or without diabetes.[18,19]
105/-, which may seem to be quite high but well-designed pharmacoeconomic analyses have shown that it is a cost-effective treatment option for obese patients with or without diabetes.[18,19]
It is known that even moderate weight loss achieved by healthy diet and adequate exercise with or without adjuvant pharmacotherapy significantly reduces the obesity associated co-morbidities and future complications.[15] In the present study, we observed that placebo group patients also showed improvement in all the efficacy parameters under consideration, which might be because of the lifestyle changes adopted by the patients.
Given the burden of obesity and its comorbidities on the healthcare system of developing country like ours, it follows that pharmacotherapy that reduces morbidity and mortality from obesity-related complications may provide economic as well as health benefits.
Thus from the present study it can be concluded that orlistat is an effective and well-tolerated antiobesity drug, which can be employed as an adjunct to therapeutic lifestyle changes to achieve and maintain optimal weight.
Acknowledgments
Authors wish to acknowledge the help extended by Dr. Mangala Murthy and Dr. Girish Raparti, Department of Pharmacology, GMC, Miraj.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 3.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of weight- loss drug rimonabant: A meta-analysis of randomized trials. Lancet. 2007;370:1706–13. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 4.Padwal RS, Majumdar SR. Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–7. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 5.Finer N, James WP, Kopelman PG, Lean ME, Williams G. One year treatment of obesity: A randomized, double blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord. 2000;24:306–13. doi: 10.1038/sj.ijo.0801128. [DOI] [PubMed] [Google Scholar]
- 6.Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: A randomized controlled trial. JAMA. 1999;281:235–42. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 7.Henness S, Perry CM. Orlistat: A review of its use in the management of obesity. Drugs. 2006;66:1625–56. doi: 10.2165/00003495-200666120-00012. [DOI] [PubMed] [Google Scholar]
- 8.Bakris G, Calhoun D, Egan B, Hellmann C, Dolker M, Kingma I. Orlistat and resistant hypertension investigators.Orlistat improves blood pressure control in obese subjects with treated but inadequately controlled hypertension. J Hypertens. 2002;20:2257–67. doi: 10.1097/00004872-200211000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Broom I, Wilding J, Stott P, Myers N. UK Multimorbidity Study Group.Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. Int J Clin Pract. 2002;56:494–9. [PubMed] [Google Scholar]
- 10.Lindgärde F. The effect of Orlistat on body weight and coronary heart disease risk profile in obese patients: The Swedish Multimorbodity study. J Intern Med. 2000;248:245–54. doi: 10.1046/j.1365-2796.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 11.Swinburn BA, Carey D, Hills AP, Hooper M, Marks S, Proietto J, et al. Effect of Orlistat on cardiovascular disease risk in obese adults. Diabetes Obes Metab. 2005;7:254–62. doi: 10.1111/j.1463-1326.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 12.Krempf M, Louvet JP, Allanic H, Miloradovich T, Joubert JM, Attali JR. Weight reduction and long-term maintenance after 18 months treatment with orlistat for obesity. Int J Obes Relat Metab Disord. 2003;27:591–7. doi: 10.1038/sj.ijo.0802281. [DOI] [PubMed] [Google Scholar]
- 13.Sari R, Balci MK, Cakir M, Altunbas H, Karayalcin U. Comparison of efficacy of sibutramine or Orlistat versus their combination in obese women. Endocr Res. 2004;30:159–67. doi: 10.1081/erc-200027356. [DOI] [PubMed] [Google Scholar]
- 14.Guy-Grand B, Drouin P, Gin H, Joubert JM, Valensi P. Effects of orlistat on obesity-related diseases - A six-month randomized trial. Diabetes Obes Metab. 2004;6:375–83. doi: 10.1111/j.1462-8902.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 15.Hofbauer KG, Nicholson JR, Boss O. The obesity epidemic: Current and future pharmacological treatments. Annu Rev Pharmacol Toxicol. 2007;47:565–92. doi: 10.1146/annurev.pharmtox.47.120505.105256. [DOI] [PubMed] [Google Scholar]
- 16.Derosa G, Cicero AF, Murdolo G, Ciccarelli L, Fogari R. Comparison of metabolic effects of orlistat and sibutramine treatment in type 2 diabetic obese patients. Diabetes Nutr Metab. 2004;17:222–9. [PubMed] [Google Scholar]
- 17.Golay A, Laurent-Jaccard A, Habicht F, Gachoud JP, Chabloz M, Kammer A, et al. Effect of orlistat in obese patients with binge eating disorder. Obes Res. 2005;13:1701–8. doi: 10.1038/oby.2005.208. [DOI] [PubMed] [Google Scholar]
- 18.Foxcroft DR. Orlistat for the treatment of obesity: Cost utility model. Obes Rev. 2005;6:323–8. doi: 10.1111/j.1467-789X.2005.00211.x. [DOI] [PubMed] [Google Scholar]
- 19.Maetzel A, Ruof J, Covington M, Wolf A. Economic evaluation of orlistat in overweight and obese patients with type 2 diabetes mellitus. Pharmacoeconomics. 2003;21:501–12. doi: 10.2165/00019053-200321070-00005. [DOI] [PubMed] [Google Scholar]


