Abstract
Background:
Purine compounds are special types of alkaloids. Caffeine and aminophylline are considered the most important members of purines due to their wide use in therapeutics.
Aims:
To detect any potential antibacterial effects on pathogenic bacteria of the widely prescribed members of purines caffeine and aminophylline.
Materials and Methods:
Two species of gram-positive bacteria and five species of gram-negative bacteria were exposed to these purine agents. Antibacterial effects of the tested purines were determined using the spectrophotometer method to assess the minimum inhibition concentrations (MIC).
Results:
Among the strains of bacteria tested, Bacillus subtilis showed the most susceptibility to purine agents. Staphylococcus aureus and Bacillus subtilis were found to be more susceptible to caffeine than the other strains. Aminophylline showed inhibitory action on many isolates, especially at the concentration of 10mg/ml. Paracoccus yeei demonstrated resistance to all tested purine compounds up to a concentration of 10.5mg/ml.
Conclusions:
Caffeine and aminophylline had the ability to inhibit many strains of pathogenic bacteria.
Keywords: Aminophylline, Antibacterial effect, Caffeine, Purine
INTRODUCTION
Purine compounds of the alkaloid group consist of three main chemical agents: caffeine, theophylline, and theobromine. Some amounts of these compounds can be found in many species of plants, with the amount present varying with the plant's age and the parts tested. Thus, the level of endogenous caffeine in leaves of Coffea arabica L. is much higher in buds and young leaves than in developed leaves.[1] Such accumulation of caffeine in young leaves may play a role in protecting the tender tissues of the plant against attack by predators, which include insect larvae and beetles.[2] The leaves of Thea sinensis that are commonly used to prepare tea for popular drinking also contain caffeine and small amounts of theophylline.[3]
Aminophylline synthesized from theophylline and ethylenediamine is widely used in pharmaceutical preparations due to its high solubility in water as compared to theophylline.[4] Aminophylline and theophylline have been used in the treatment of asthma and chronic obstructive airway diseases for more than 70 years.[5] Caffeine is formulated in combination with many pharmaceutical drugs; for example, it is used in combination with ergotamine for treatment of migraine headaches and in combination with non-steroidal anti-inflammatory drugs (NSAIDs) for relief of painful conditions.[6]
The action of purine on the metabolic pathways of bacteria may range from harmful to beneficial, depending upon the species of bacteria. Some species of bacteria use caffeine as a major source of carbon for their nutritional requirement, while others are inhibited in the presence of caffeine. Pseudomonas putida can degrade 20% of caffeine incorporated in culture media (1 g/L) after 9 hours of incubation, and it uses this purine compound as its sole source of carbon and nitrogen.[7]
Purines can also have an effect on the sporulation rate and morphological development of bacterial cells. The conversion of the rod shape of Escherichia coli into a filamentous form has been observed upon addition of 10 mM of theophylline into the culture media.[8] Aerobacter aerogenes and Aerobacter cloaca developed a long filamentous form in the presence of 1mg/ml of caffeine. In addition, the rate of spore production by Clostridium perfringens was increased after culture on casein digest media containing 100–1000 μg/ml of caffeine.[9]
On the other hand, many studies have illustrated no activity of purine compounds on the viability of bacteria. Aminophylline and caffeine (up to 4mg/ml aminophylline and 8mg/ml caffeine) did not show any antibacterial effects on Staphylococcus aureus and Pseudomonas aerogenosa.[10] Oral microorganisms (including seven strains) were also not affected by caffeine at concentrations of up to 400 μg/ ml.[11] Furthermore, theophylline exhibited no action on the persistence of Serratia marcescens that was successfully isolated from theophylline bottles given to neonates.[12]
In this study we attepted to detect the potential antibacterial effects of the widely prescribed members of the purine group – caffeine and theophylline (in the form of aminophylline). These compounds were tested against several pathogenic bacteria.
MATERIALS AND METHODS
Organisms
Seven strains of bacteria were clinically isolated from patients (30–38 years of age) with respiratory and gastrointestinal tract infections at AL-Hussein General Hospital of Karbala province. Throat swabs and stool samples were cultured on Mueller-Hinton agar (HiMedia, Mumbai, India) and incubated at 35°C for 24 hours. In addition to gram-staining and morphological criteria, we used the API-20 system (Biomérieux, Netherlands-France) to identify the bacterial species. For further confirmation, the isolates were sent to the bacteriological bank of the College of Science, University of Basraha, Iraq.
The isolated strains (one strain for each species) included two species of gram-positive bacteria (S aureus and Bacillus subtilis) and five species of gram-negative bacteria (E coli, Enterobacter aerogenes, Enterobacter cloacae, Salmonella typhi, and Paracoccus yeei).
Chemical agents
Caffeine and aminophylline were purchased from HiMedia, Mumbai, India. Ampicillin sodium and cefotaxime sodium were supplied by KonTam Pharmaceuticals Co. (Zhongshan, China).
Antibacterial assays
For antibacterial assay, total count of isolated strains were standardized to equivalent a 0.5 MacFarland Nephelometer standard (1 × 108 cfu/ml) by Mueller-Hinton broth (HiMedia, Mumbai, India) and then diluted as 1:10.
The spectrophotometer technique was applied to determine the antibacterial activities of purine agents. Briefly, various concentrations of caffeine and aminophylline (0.312, 0.625, 1.25, 2.5, 5, and 10mg/ml) were singly mixed in tubes containing Mueller-Hinton broth. These tubes were inoculated with previously prepared cultures of each strain (50 μl to each milliliter of broth). The tubes were then incubated at 35°C for 24 hours. The optical density of bacterial growth was measured using a spectrophotometer (Optima-SP-300; Karzma Co. Tokyo, Japan) at a wavelength of 450 nm.[13]
Ampicillin sodium and cefotaxime sodium were dissolved in sterile distilled water to prepare concentrations of 20, 39, and 78 μg/ml. Both of these drugs and media without any added chemicals were used as controls.
Determination of minimum inhibitory concentration
Determination of minimum inhibitory concentration (MICs) were determined by the microdilution method recommended by the NCCLS.[14] Briefly, the tested chemical agents were two-fold diluted in Mueller-Hinton broth. A volume of 100 μl of each dilution was dispensed into the wells of 96-well microdilution plates. The filled well was inoculated with 50 μl of previously prepared of standardized count of bacteria. The inoculated trays were incubated at 35°C for 24 hours and examined for visible growth in order to determine the MIC. Three previous controls were also included.
Statistical analysis
Each experiment was repeated three times with triplicates of each concentration. The data were statistically analyzed using two-way analysis of variance (ANOVA) with least significant difference (LSD) at P<0.05.
RESULTS
The purine compounds caffeine and aminophylline were investigated at various concentrations for antibacterial effects against seven strains of bacteria using the spectrophotometer method.
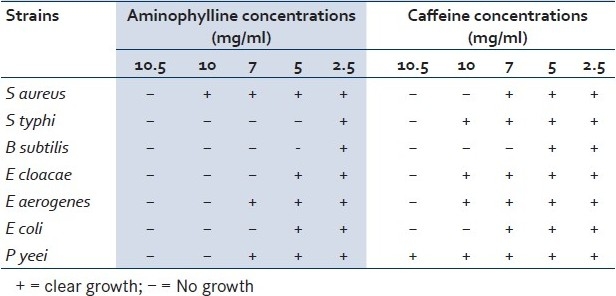
B subtilis was found to be more susceptible to the tested purines than the other species of bacteria. The growth rate of six of the seven strains of bacteria (the exception being Staphylococcus aureus) significantly decreased in the presence of a high level of aminophylline (10mg/ml). S typhi and B subtilis needed only 5mg/ml of aminophylline to reduce their ability to grow in culture media [Figures 1 and 2] [Table 1].
Figure 1.
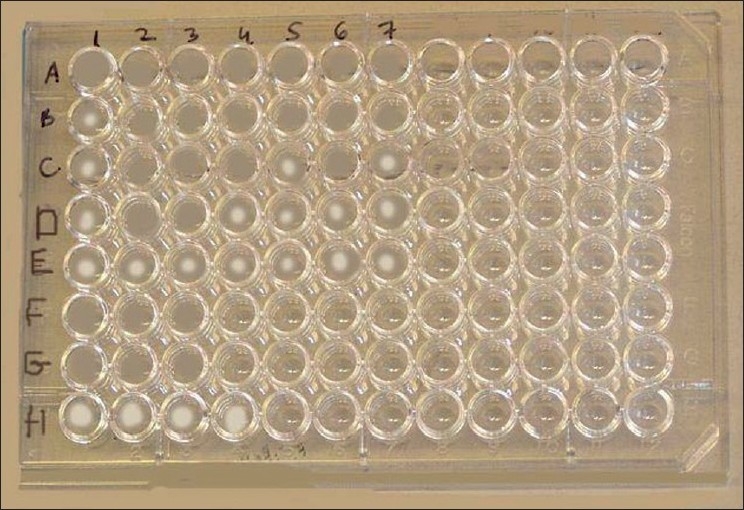
Microdilution plate for MIC of aminophylline in tested bacteria. (A) 10.5mg/ml, (B) 10mg/ml, (C) 7mg/ml, (D) 5mg/ml, (E) 2.5mg/ml, (F) Ampicillin, (G) Cefotaxime, and (H) Control (free medium). Bacterial strains are (1) S aureus, (2) S typhi, (3) B subtilis, (4) E cloacae, (5) E aerogenes, (6) E coli, and (7) P yeei
Figure 2.
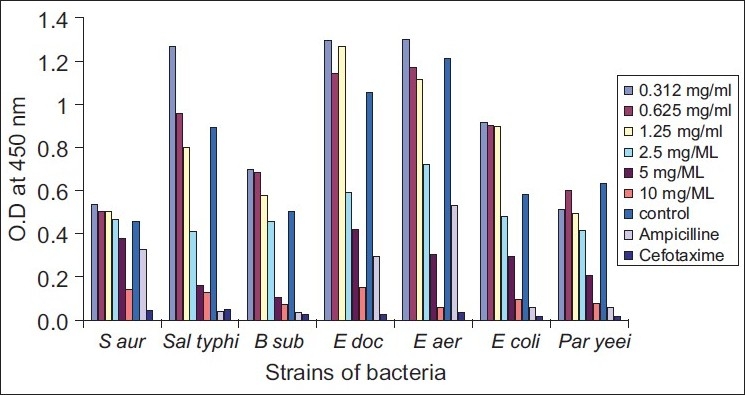
Effects of different concentrations of aminophylline on the growth of bacteria
Table 1.
MICs of caffeine and aminophylline in the bacterial isolates
As Figure 3 shows, bacterial strains exhibited more variable susceptibility to different concentrations of caffeine. The gram-positive bacteria, S aureus and B subtilis, were more susceptible to the action of caffeine than the gram-negative bacteria. The turbidity of the broth culture of Paracoccus yeei, and the elevated optical density ratio, pointed to a very clear resistance to the toxic action of caffeine. Statistical analysis showed that the purine compounds had significant effect (P<0.05) in reducing the growth of Paraccocus yeei [Figures 2 and 3]. The efficacy of aminophylline in decreasing bacterial growth (as compared to growth in the control media) was significantly more than that of caffeine.
Figure 3.
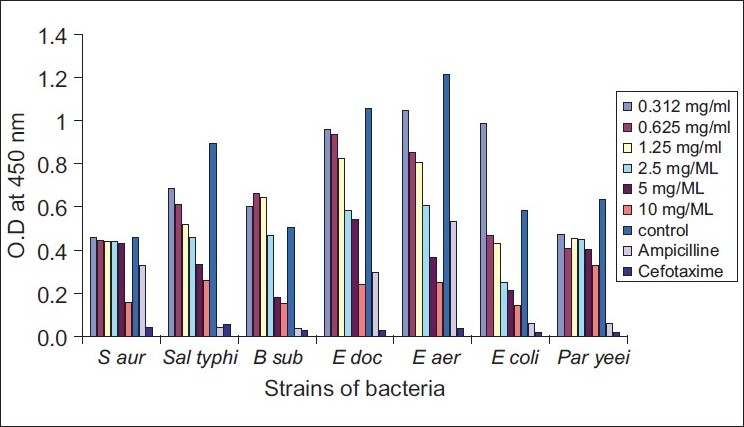
Effects of different concentrations of caffeine on the growth of bacteria
Caffeine and aminophylline, at concentrations of 10 mg/ ml, revealed greater ability to inhibit most of tested strains (especially S aureus, E cloacea, and E aerogenes) than the standard antibiotic ampicillin, which was used as a control. However, cefotaxime showed greater effect on the isolated bacteria than the purine compounds [Figures 2 and 3].
DISCUSSION
Caffeine and theophylline are important purine compounds for human beings as large amounts are consumed daily in foods, beverages, and drugs.
Caffeine is the most widely consumed behaviorally active substance in the world. In the USA alone, the daily intake of caffeine among adults approaches an average of approximately 200mg.[15] Dietary surveys in North America indicate that 80%–90% of children and adults have weekly or more frequent consumption of caffeine-containing foods.[16]
Theophylline is still commonly prescribed in the treatment of asthma and chronic obstructive airway disease because it is inexpensive.[5] The similarities between purine alkaloids and nucleic acids in structure encourage the use of these compounds as antimicrobial agents.
The results of this study revealed that high concentrations are required for an antibacterial action, with aminophylline proving to be much more effective than caffeine against the bacterial isolates. In contrast, Raj and Dhala[17] reported that caffeine at concentrations up to 5mg/mL had more antibacterial activity than theophylline.
Among the seven strains used in this study, the spore-forming bacteria (B subtilis) was most susceptible to the action of the tested purine substances. Salmonella typhi was susceptible to aminophylline only. Concordant with our findings, a previous study also found that B subtilis and Salmonella typhi were inhibited by 5mg/ml of theophylline and caffeine.[17]
In the present study, some strains of bacteria, including S aureus and P yeei, exhibited resistance to both purine compounds. Hosseinzadeh et al.[10] also recorded such resistance to caffeine and aminophylline after culturing S aureus and P aerogenosa in media containing these compounds.
Antimicrobial drugs act in one of several ways: by selective toxicity, by inhibition of cell membrane synthesis and function, by inhibition of protein synthesis, or by inhibition of nucleic acid synthesis. Resistance to cephalosporins had been observed in many strains of bacteria due to the production of a stable cephalosporinase.[18] This enzyme is related to the β-lactamase group that is expressed by plasmids.[19] It is considered the most important mechanism of development of drug resistance in Enterobacter strains.[20] However, the E cloacea and E aerogenes that showed resistance to ampicillin in the present study were found to be susceptible to the purine compounds.
The main action of caffeine in bacterial cells can be explained by many mechanisms: for example, interaction of caffeine with bacterial nucleic acid[9] or inhibition of the synthesis of DNA (as noted in E coli K2).[21] Caffeine also can interact with the enzyme responsible for repair of bacterial DNA damage by inhibition of the ATP-dependent enzyme.[22]
Three important mechanisms of theophylline action in mammalian cells are proposed: inhibition of cAMP phosphodiesterase enzyme,[23] adenosine receptor antagonisism,[24] and inhibition of histone deacetylase (HDACs).[25] In bacterial cells, the inhibition of cAMP phosphodiesterase resembles the accepted mechanism of theophylline.[8]
A previous study has shown that purine compounds have both in vitro and in vivo efficacy in inhibiting a special group of skin pathogenic fungi (dermatophytes).[26] Recent data suggests that these compounds also have inhibitory activity on other groups of microorganisms such as bacteria. These results indicate that purine agents, with their ability to destroy different types of pathogenic organisms, may be promising drugs for treatment of many diseases.
Judging on the basis of their pharmaceutical characteristics, caffeine and aminophylline have the potential to be useful drugs for treatment of bacterial infection. The beneficial characteristics include the following: these compounds are toxic to humans only at relatively high concentrations;[4] the mode of action in microorganisms is suspected to be an interaction with the nucleic acids of the pathogen;[8,9,21] development of resistance in microorganism to these purine agents arises slowly because of usage of high concentrations of the drug; few adverse effects are associated with administration of these agents;[3] caffeine and aminophylline are inexpensive drugs, so large numbers of patients can be benefitted.[5] Most of the known antibacterial drugs lack one or more of these characteristics. Thus, caffeine and aminophylline have the potential to become one of the prefered drugs for treatment of bacterial infection.
CONCLUSIONS
The purine compounds demonstrated potential antibacterial activities in high concentrations, with aminophylline showing greater ability than caffeine to decrease bacterial growth. However, more studies are needed to investigate the antimicrobial activity of purine compounds and their potential for use in clinical situations. Certainly, present knowledge regarding the characteristics of purine compounds suggest that they could find use as antimicrobials.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ashihara H, Crozier A. Caffeine: A well known but little mentioned compound in plant science. Trends Plant Sci. 2001;6:407–13. doi: 10.1016/s1360-1385(01)02055-6. [DOI] [PubMed] [Google Scholar]
- 2.Ashihara H, Monteriro AM, Gillies FM, Crozier A. Biosynthesis of caffeine in leaves of coffee. Plan Physiol. 1996;111:747–53. doi: 10.1104/pp.111.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunton LL, Lazo JS, Parker KL. The pharmacological basis of therapeutics. Goodman and Gilmansth. 11th ed. New York: McGraw-Hill; 2006. pp. 727–30. [Google Scholar]
- 4.Parfitt K. Martindale, the complete drug reference, U. 32nd ed. U.S.A.: Pharmaceutical Press (PhP); 1999. [Google Scholar]
- 5.Barnes PJ. Theophylline in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:334–9. doi: 10.1513/pats.200504-024SR. [DOI] [PubMed] [Google Scholar]
- 6.Sawynok J. Pharmacological rationale for the clinical use of caffeine. Drugs. 1995;49:37–50. doi: 10.2165/00003495-199549010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Yamaoka-Yano DM, Mazzafera Catabolism of caffeine and purification of a xanthine oxidase responsible for methyluric acids production in Pseudomonas putida L. Revista. 1999;30:62–70. [Google Scholar]
- 8.Kawamukai M, Murao K, Utsumi R, Himeno M, Komano T. Cell filamentation in an Escherichia coli K-12 fic mutant caused by theophylline or an adenylate cyclase gene (cya)-containing plasmid. FEMS Microb Lett. 1986;34:117–20. [Google Scholar]
- 9.Sacks LE, Thompson PA. Increased spore yields of Clostridium perfringens in the presence of methylxanthines. Appl Environ Microbiol. 1977;34:189–93. doi: 10.1128/aem.34.2.189-193.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosseinzadeh H, Bazzaz BS, Sadati MM. In vitro evaluation of methylxanthines and some antibiotics: Interaction against Staphylococcus aureus and Pseudomonas aeruginosa. Iran Biomed J. 2006;10:163–7. [Google Scholar]
- 11.Cogo K, Montan MF, Bergamaschi C, Andrade ED, Rosalen PL, Groppo FC. In vitro evaluation of the effect of nicotine, cotinine and caffeine on oral microorganisms. Can J Microbiol. 2008;54:501–8. doi: 10.1139/w08-032. [DOI] [PubMed] [Google Scholar]
- 12.Fleisch F, Zimmermann-Baer U, Zbinden R, Bischoff G, Arlettaz R, Waldvogel K, et al. Three consecutive outbreaks of Serratia marcescens in a neonatal intensive care unit. Clin Infect Dis. 2002;34:767–73. doi: 10.1086/339046. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez MC, de la Rosa M, Borobio MV. Application of a spectrophotometric method for the determination of post-antibiotic effect and comparison with viable counts in agar. J Antimicrob Chemother. 2001;47:391–8. doi: 10.1093/jac/47.4.391. [DOI] [PubMed] [Google Scholar]
- 14.NCCLS. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically; approved standard-sixth edition. NCCLS document M7-A6. Pennsylvania: NCCLS, Wayne; 2003. [Google Scholar]
- 15.Holtzman SG. Caffeine as a model drug of abuse. Trends Pharmacol Sci. 1990;11:355–6. doi: 10.1016/0165-6147(90)90175-8. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths RR, Juliano LM, Chausmer AL. Principles of addiction medicine. 3rd ed. Philadelphia: American society of addiction medicine; 2003. pp. 193–224. [Google Scholar]
- 17.Raj CV, Dhala S. Effect of naturally occurring xanthines on bacteria: 1- antimicrobial action and potentiating effect on antibiotic spectra. Appl Microbiol. 1965;13:432–6. doi: 10.1128/am.13.3.432-436.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stearne LE, van Boxtel D, Lemmens N, Goessens WH, Mouton JW, Gyssens IC. Comparative study of the effects of ceftizoxime, piperacillin, and piperacillin-tazobactam concentrations on antibacterial activity and selection of antibiotic-resistant mutants of Enterobacter cloacae and Bacteroides fragilis in vitro and in vivo in mixed-infection abscesses. Antimicrob Agents Chemother. 2004;48:1688–98. doi: 10.1128/AAC.48.5.1688-1698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikonomidis A, Spanakis N, Poulou A, Pournaras S, Markou F, Tsakris A. Emergence of carbapenem-resistant Enterobacter cloacae carrying VIM-4 metallo-β-lactamase and SHV-2a extended-spectrum β-lactamase in a conjugative plasmid. Microb Drug Resist. 2007;13:221–6. doi: 10.1089/mdr.2007.768. [DOI] [PubMed] [Google Scholar]
- 20.Ehrhardt AF, Sanders CC. Beta-lactam resistance amongst Enterobacter species. Antimicrob Agents Chemother. 1993;32:1–11. doi: 10.1093/jac/32.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- 21.Sandlie I, Solberg K, Kleppe K. The effect of caffeine on cell growth and metabolism of thymidine in Escherichia coli. Mutat Res. 1980;73:29–41. doi: 10.1016/0027-5107(80)90133-5. [DOI] [PubMed] [Google Scholar]
- 22.Selby CP, Sancar A. Molecular mechanisms of DNA repair inhibition by caffeine. Proc Natl Acad Sci U S A. 1990;87:3522–5. doi: 10.1073/pnas.87.9.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banner KH, Page CP. Theophylline and selective phosphodiesterase inhibitors as anti-inflammatory drugs in the treatment of bronchial asthma. Eur Respir J. 1995;8:996–1000. [PubMed] [Google Scholar]
- 24.Biaggioni I, Paul S, Puckett A, Arzubiaga C. Caffeine and theophylline as adenosine receptor antagonists in humans. J Pharmacol Exp Ther. 1991;258:588–93. [PubMed] [Google Scholar]
- 25.Ito K, Lim S, Caramori G, Cosio B, Fan Chung K, Adock IM, et al. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci U S A. 2002;99:8921–6. doi: 10.1073/pnas.132556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AL-Janabi AS. Treatment of dermatophytoses by drugs containing some purine compounds. Iraq: Ph.D. Thesis. AL-Mustansiryia Unvi., College of Science; 2004. [Google Scholar]


