Abstract
Objective:
Since its discovery in the western hemisphere in 1999, West Nile virus (WNv) has caused extensive bird mortality across North America, especially in American crows (Corvus brachyrhynchos) which are highly susceptible to WNv. In this study, antigenic distribution of WNv among different organs of American crows was studied, using the immunohistochemistry technique (IHC).
Materials and Methods:
Dead crows reported by residents were collected, transported on ice, and were necropsied for heart, lung, brain, intestine, kidney, liver, spleen, pancreas, and gonad tissues. Gross examination was performed on brain, heart, lung, liver, kidney, spleen, bursa of fabricius, gastrointestinal tract, skeletal muscle, pancreas, reproductive tract, and skin. Gross hemorrhage of brain, splenomegaly, meningoencephalitis, myocarditis, and trauma were sporadically observed in some of the infected carcasses. Formalin-fixed paraffin-embedded tissue sections were stained with IHC technique followed by counter staining with hematoxylin and eosin.
Results:
WNv antigen was detected in brain, spleen, heart, kidney, liver, gonads, intestine, lung, and pancreas. The spleen was found to be positive in all infected crows, followed by kidney, liver, and duodenum (95% each). Heart and pancreas were positive in 63% while brain was positive in 36.5% of the infected crows.
Conclusion:
More than one tissue sample is suggested to screen WNv infection using IHC technique. IHC has the advantage of correlating the visual destruction of tissue architecture with the presence of stained WNv antigen but as compared to PCR, IHC has the disadvantage of longer turnaround time, which is critical when used as a surveillance tool.
Keywords: American crows, Distribution of WNv, Immunohistochemistry, West Nile virus
INTRODUCTION
WNv is the most widespread arbovirus in the world. Birds are its natural reservoir (amplifying) hosts and in nature it is maintained in mosquito–bird–mosquito transmission cycle. For the first time in the western hemisphere, it was detected in August 1999 during an epidemic of meningoencephalitis in residents of New York City with 62 residents falling sick (7 fatalities) during that year.[1] Since then it has rapidly spread across the North American continent into all 48 continental states, seven Canadian provinces, and throughout Mexico. In addition, WNv activity has been detected in Puerto Rico, the Dominican Republic, Jamaica, Guadeloupe, and El Salvador. According to the U.S. Centers for Disease Control and Prevention (CDC), 29,624 people in the United States have tested positive for WNv infection with 1161 fatalities, during years 1999–2009. (http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm). The 1999 New York outbreak also resulted in fatal neurological disease in a variety of birds and horses. The incidence of this disease was particularly high in American crows (herein referred as crows). Dead crows were found to serve as excellent sentinel surveillance system for this virus.[2] To study the distribution of this virus in different organs of dead crows, immunohistochemistry (IHC) technique, electron microscopy (EM), virus isolation, etc. can be used. In this study, we preferred IHC as EM facility is expensive, highly specialized knowledge is required and we were not having access to it. Compared to virus isolation (VI), IHC has comparable diagnostic ability to detect WNv antigen.[3] IHC positive birds, in this study, were further confirmed by testing with RT-PCR. Additionally as compared to RT-PCR and VI, IHC is less expensive, less hazardous to lab workers, and tissues can be visually examined for type and extent of architectural damage.
The objectives of this study were to determine relevant gross diagnostic lesions, antigenic distribution of this virus in different organs of wildly infected crows and to find out appropriate tissue sample for screening of WNv with IHC technique.
MATERIALS AND METHODS
Dead birds reported by the public to the Northwest Mosquito and Vector Control District (NWMVCD) Corona, West Valley Mosquito and Vector Control District (WVMVCD), Ontario and Riverside Animal Shelter, Riverside were used for this study. As these birds died in the wild, their route of infection for WNv was not known. Infection could have been acquired orally by eating infected carcasses/drinking contaminated water/roosting with infected crows or by mosquito bites. All the dead birds collected/submitted were identified to sex and species. Tissues from WNv infected 31 American Crows were included in this study. Gross examination was performed on brain, heart, lung, liver, kidney, spleen, bursa, gastrointestinal tract, skeletal muscle, pancreas, reproductive tract, and skin. Gross hemorrhage of brain, splenomegaly, meningoencephalitis, myocarditis, and trauma were sporadically observed in some of the infected carcasses. Since autolysis can obscure subtle lesions, tissues with minimal autolysis were chosen for microscopic evaluation. Samples of heart, lung, brain, intestine, kidney, liver, spleen, pancreas, and gonads were collected in 10% buffered formalin for making histological sections. Paraffin tissue blocks were sectioned at 5 μm thickness and mounted on positively charged glass slides. All tissue sections were analyzed for WNv with immunoperoxidase method using polyclonal antibodies against WNv (procured from DAKO corporation, USA).[1] All immunostained slides were counterstained with hematoxylin. A snippet of kidney tissue collected from each bird was stored without any additive at –80°C in cryogenic vials for screening with singleplex RT-PCR Taqman assay. Any carcass which was positive for WNv in at least one organ was considered as infected. After staining, all the slides were evaluated under light microscope and positive tissue slides were digitally photographed for record.
Ethics
No experimentation was done on live animals. Dead birds collected from the field were used for this study.
Statistics
The results obtained were analyzed for percentage proportions.
RESULTS
In general, most of the carcasses were in good physiological state. Out of 31 crows, 21(67.74%) were male and 10(32.26%) were females. None of the carcasses had evidence of musculoskeletal trauma, but 9(42.8%) carcasses had subcutaneous (SQ) hemorrhages on the head. No gross lesions on the examined internal organs were found to be specific in nature and distribution for WNv infection. Myocardial lesions included pallor and hemorrhage while splenomegaly was evident in some of them. WNv antigen was detected in brain, spleen, heart, kidney, liver, gonads, duodenum, lung, and pancreas. As is evident from Table 1, the spleen was found to be positive in all the infected crows followed by kidney, liver, and duodenum (95% each).
Table 1.
Distribution of WNv antigen in organs of wildly infected American crows
![]()
Microscopically, staining pattern in the spleen was diffuse type and the cells showing presence of WNv antigen were reticuloendothelial cells [Figure 1]. Liver showed diffuse type staining pattern with Kupffer cells constituting the majority of the stained cells [Figure 2]. Staining of the kidney was multifocal and was centered around collecting ducts. WNv antigen was stained mostly in macrophages and in the walls of excretory tubules in tubular epithelial cells [Figure 3]. The duodenum showed intense focal staining pattern, and WNv antigen was stained throughout the payer patches [Figure 4]. Lungs were positive in 92% of the infected carcasses, showed staining around airways and blood vessels [Figure 5] and the stained cells were macrophages and endothelium of the vessels and airways. The heart was found to be positive in 63% of the crows tested which was faint, diffuse, and scattered all over the myocardium. Stained cells were macrophages, myofibers, and occasionally endothelial cells were also stained [Figure 6]. Pericardium was also showing marked sections of intense staining of macrophages. The brain was found positive in the 36.5% of the infected crows and WNv antigen was found in Purkinje cells, neurons, and Glial cells [Figure 7]. In 70% of the infected carcasses, gonads stained positive for WNv. In the ovaries, the parenchymal, interstitial, and stromal cells were stained [Figure 8]. No WNv antigen was stained in the epithelium of the wall of oviduct and Zona pellucida. In testes [Figure 9], the sertoli cells stained positive for WNv and the staining pattern was focal type. Pancreas was positive in 63% of the tested carcasses, and it showed strong multifocal as well as diffuse staining pattern for WNv antigen [Figure 10].
Figure 1.
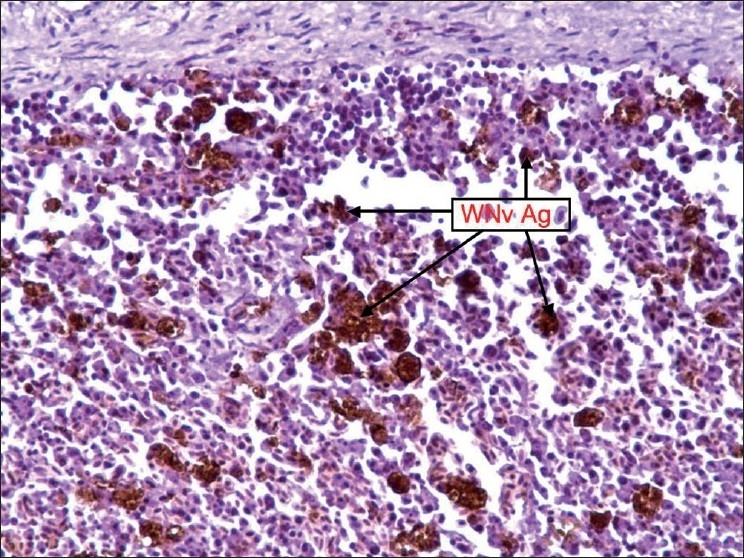
Spleen: Staining was diffuse and WNv antigen-stained cells were reticuloendothelial cells
Figure 2.
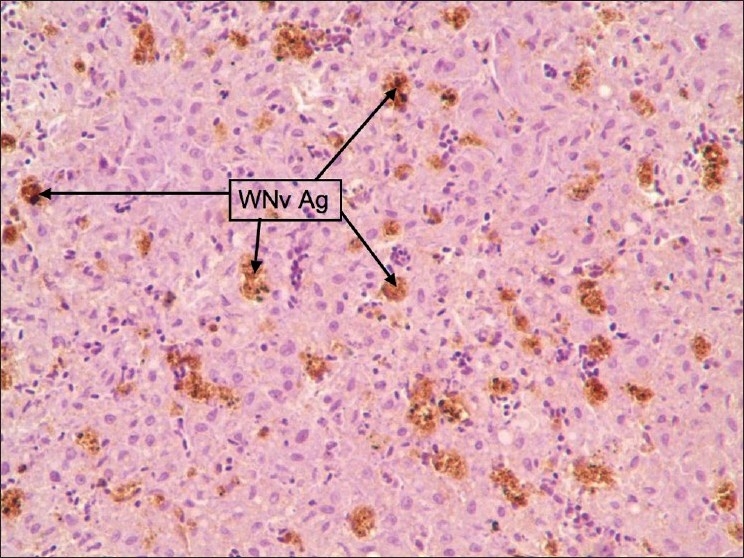
Liver: Staining was diffuse type and WNv antigen-stained cells were Kupffer cells
Figure 3.
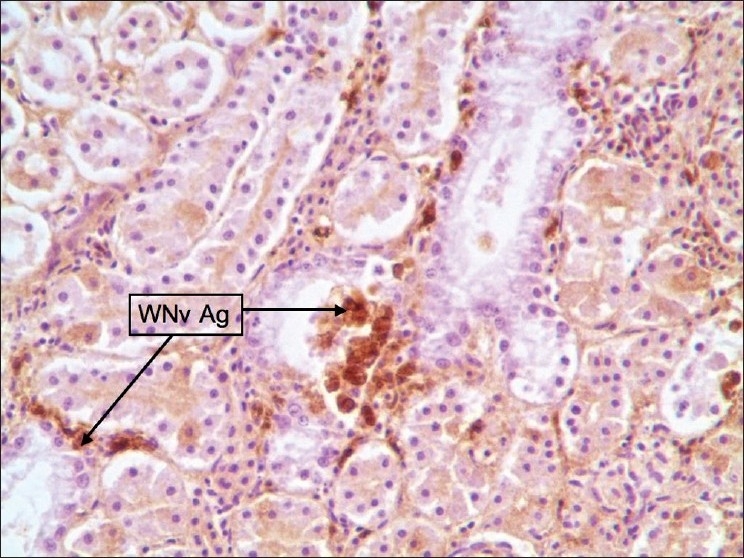
Kidney: Staining was multifocal and centered around collecting ducts. Stained cells are macrophages and tubular epithelial cells
Figure 4.
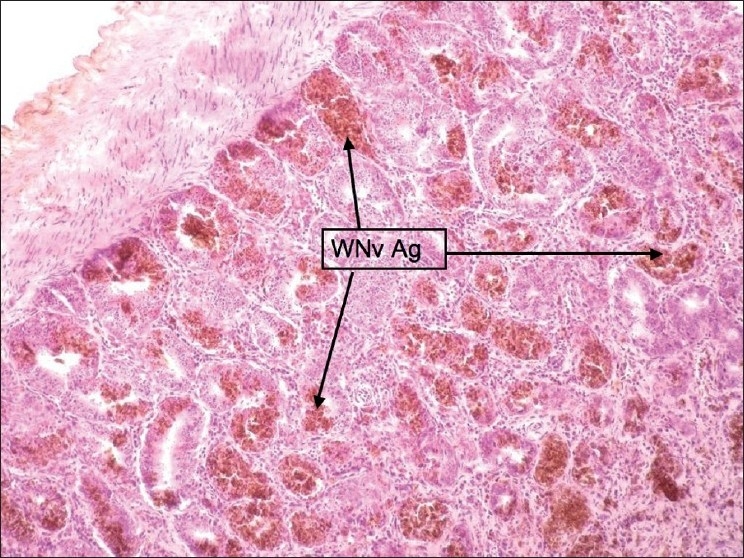
Duodenum: Staining was intense and focal. Stained cells were payer patches
Figure 5.
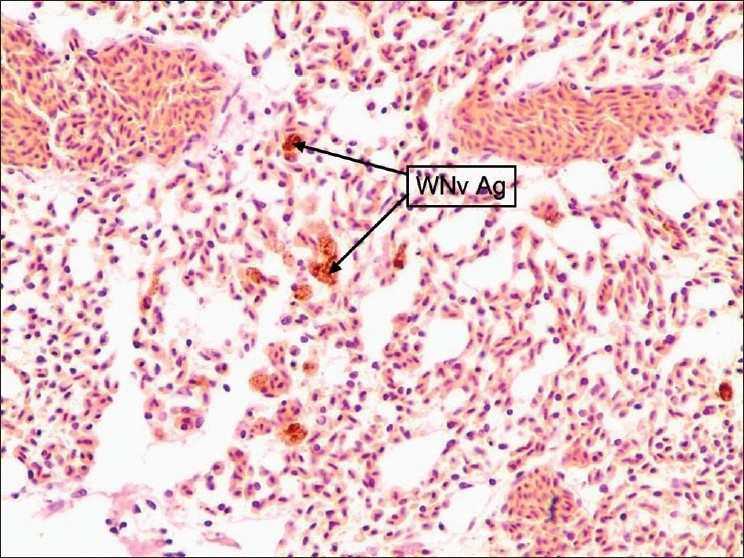
Lung: Patchy staining around airways and vessels. Stained cells were macrophages and endothelium of vessels and airways
Figure 6.
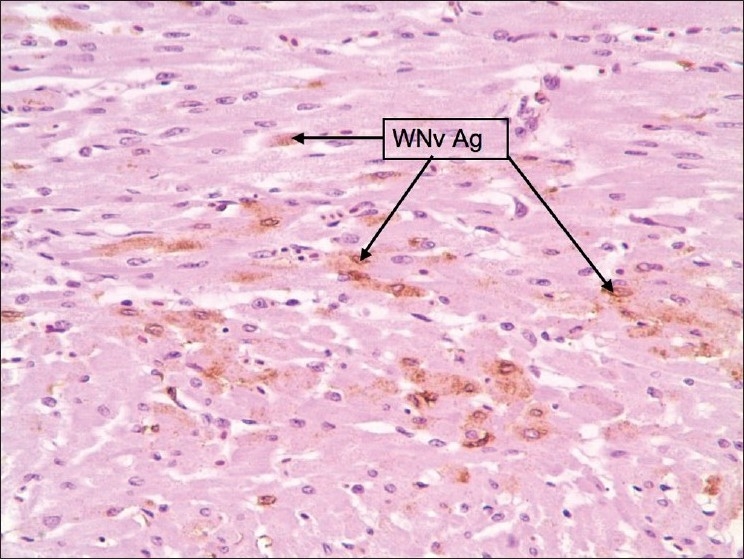
Heart: Staining was faint, diffuse, and scattered. Stained cells were macrophages, myofibers, and occasionally endothelial cells. Pericardium showed sections of intense staining of macrophages
Figure 7.
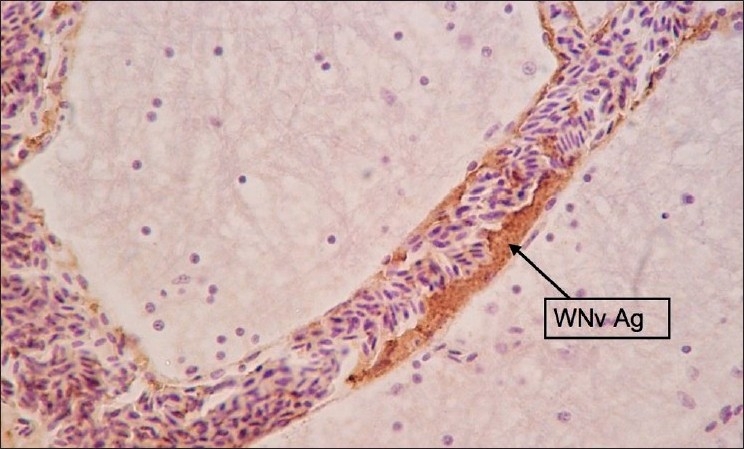
Brain: Staining was focal or multifocal. Stained cells were Purkinje cells, Neurons and Glial cells
Figure 8.
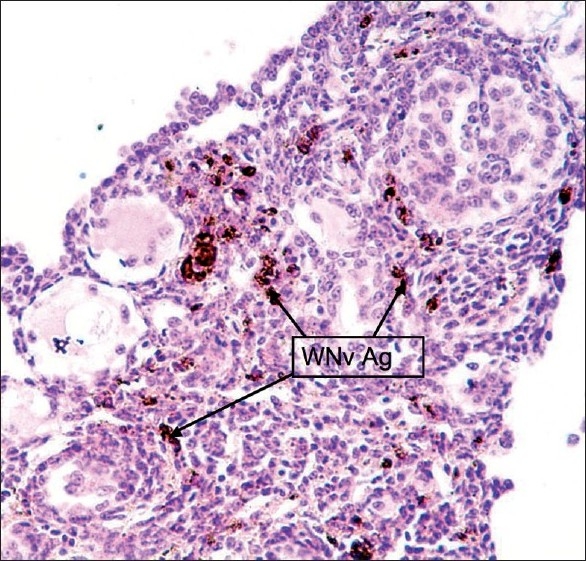
Ovaries: Stained cells were parenchymal, interstitial, and stromal cells. No WNv antigen was stained in the epithelium of the wall of oviduct and Zona pellucida
Figure 9.
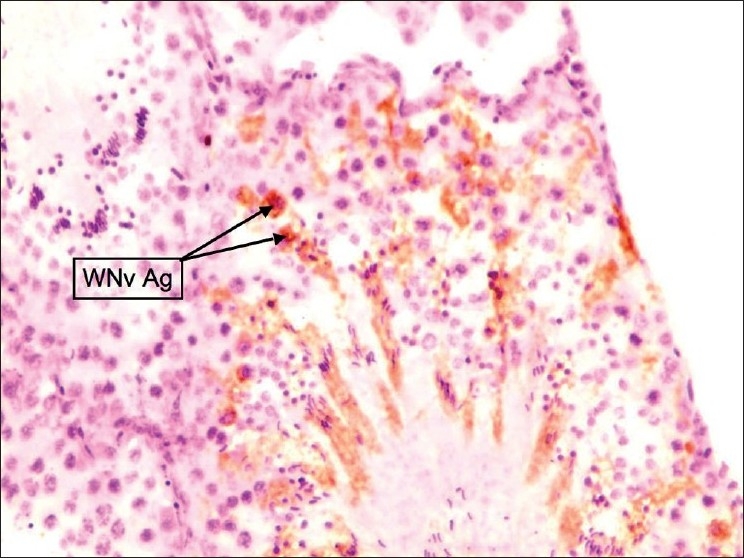
Testes: Staining pattern was focal. Stained cells were Sertoli cells
Figure 10.
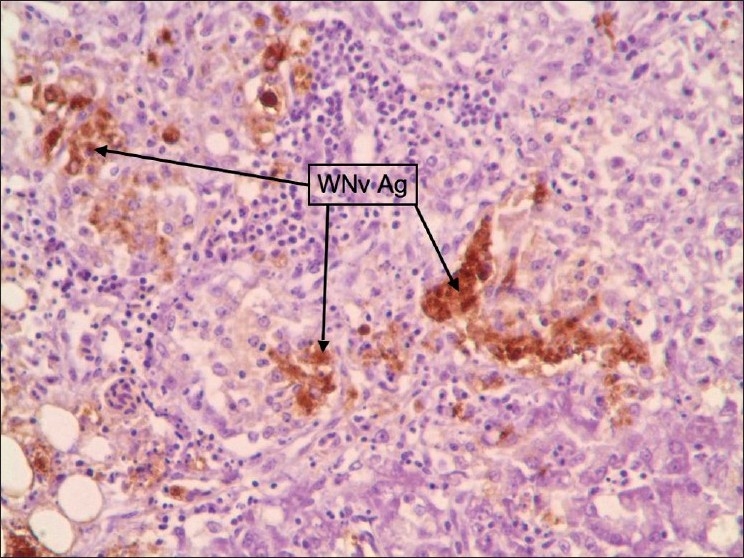
Pancreas: Multifocal and strong diffuse staining pattern was observed
DISCUSSION
Since crows are highly sensitive to WNv infection with experimental studies showing 100% mortality rate, they probably did not get sufficient time to respond back in terms of gross pathological changes. Inflammation of optic tectum and meningitis might had resulted in loss of perception for depth of vision and thus were not able to avoid the obstacles in their path of flight resulting in SQ hemorrhages due to trauma or may be these hemorrhages could have been caused by falling dead straight from the sky as was reported by some residents. The presence of WNv antigen in the excretory tubules, duodenum, and lungs suggests shedding of this virus in the urinary, intestinal as well as respiratory secretions. As some organs showed intense focal rather than diffused staining pattern, appropriate size of the tissue sample is also important to avoid false negatives. With IHC, multiple organs can be screened at the same time, actual extent of damage to tissue architecture can be viewed directly and correlated with staining pattern to rule out false positives.
CONCLUSIONS
Although studies showed that brain tissue[4] and intestine[5] have been the most reliable organ for VI and RT-PCR testing of crows for WNv, this study showed that the spleen appears to be the most reliable organ for IHC testing. Since in terms of practicality, multiple organs can be screened with the same expense and time, it is recommended that snippets of liver, spleen, kidney, lung, and intestine be included to increase the accuracy of result. In comparison to EM, VI or RT-PCR facilities, no expensive or specialized equipment is required to set up an IHC lab. Moreover, as lab workers deal with formalin-fixed virus, it is much less hazardous to them as compared to other diagnostic tests. The IHC method is much faster than VI but slower than EM as well as RT-PCR testing because of long turnaround time which is critical when IHC is used for disease surveillance purpose. Additionally, formalin-fixed or paraffin-embedded tissue samples can be archived and re-sectioned and re-stained for confirmations or for second opinions or for time travel in the past to look for newly emerged pathogens of future.
Acknowledgments
Sincere thanks to Dr. Steven Su at West Valley Mosquito and Vector Control District for supplying dead American crows and to Bryan Haynes and Gregory Williams for providing the necessary logistic support in collecting carcasses from field.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Steele KE, Linn MJ, Schoepp RJ, Komar N, Geisbert TW, Manduca RM, et al. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet Pathol. 2000;37:208–24. doi: 10.1354/vp.37-3-208. [DOI] [PubMed] [Google Scholar]
- 2.Eidson M, Komar N, Sorhage F, Nelson R, Talbot T, Mostashari F, et al. Crow deaths as sentinel Surveillance System for West Nile Virus in the Northeastern United States, 1999. Emerg Infect Dis. 2001;7:615–20. doi: 10.3201/eid0704.010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis AE, Mead DG, Allison AB, Gibbs SE, Gottdenker NL, Stallknecht DE, et al. Comparison of immunohistochemistry and virus isolation for diagnosis of west nile virus. J Clin Microbiol. 2005;43:2904–8. doi: 10.1128/JCM.43.6.2904-2908.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panella NA, Kerst AJ, Lanciotti RS, Bryant P, Wolf B, Komar N. Comparative West Nile virus detection in organs of naturally infected American Crows (Corvus brachyrhynchos) Emerg Infect Dis. 2001;7:754–5. doi: 10.3201/eid0704.010430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tewari D, Kim H, Feria W, Russo B, Acland H. Detection of West Nile virus using formalin fixed paraffin embedded tissues in crows and horses: Quantification of viral transcripts by real-time RT-PCR. J Clin Virol. 2004;30:320–5. doi: 10.1016/j.jcv.2004.01.003. [DOI] [PubMed] [Google Scholar]


