Abstract
Progress towards Taenia solium control is evident in the development of new technologies and in increasing regional coordination, yet disease eradication remains unlikely in the near future. In the meantime, translation of research advances into functioning control programs is necessary to address the ongoing disease burden in endemic areas. Multiple screening assays, effective treatments for both human and porcine infection, and vaccines blocking transmission to pigs are currently available. Strategies based on identification and treatment of T. solium adult tapeworms, as well as interventions that block cysticercosis acquisition in pigs have temporarily reduced transmission. Building on these successes with controlled community trials in varying endemic scenarios will drive progress towards regional elimination.
Keywords: Control, Cysticercosis, Elimination, Eradication neurocysticercosis, Taenia solium
INTRODUCTION
Cysticercosis is an emerging zoonosis causing debilitating brain lesions in humans, widespread compromises in food safety and important economic losses from contaminated pork. It is caused by the pork tapeworm, Taenia solium, which infects both humans and pigs. As the most common helminthic infection of the central nervous system, T. solium is a leading cause of late-onset epilepsy in Latin America, Asia and sub-Saharan Africa;[1] in Latin America alone over 400,000 people have symptomatic disease.[2]
The International Task Force for Disease Eradication targeted cysticercosis as a potentially eradicable disease in 1992.[3] Yet despite the availability of multiple screening tools, effective treatment for humans and pigs, development of candidate pig vaccines and increased knowledge of local transmission dynamics, translation into operating control projects has been limited. Effective, affordable and sustainable interventions on local and regional levels are urgently needed to reduce the immediate burden of the disease.
ETIOLOGY AND TRANSMISSION
Cysticercosis is a parasitic tissue infection by the larval form of the pork tapeworm, T. solium. Humans and pigs acquire cysticercosis by ingesting T. solium eggs shed in the feces of a human infected with the adult intestinal tapeworm. Once ingested, these eggs release onchospheres, which invade the intestinal wall and disseminate to form cysts throughout the body. Neurocysticercosis occurs when onchospheres invade the central nervous system. The natural lifecycle completes when a human consumes pork contaminated by T. solium larval cysts, as these may then develop into adult egg-producing intestinal tapeworms. While both pigs and humans can acquire cysticercosis, only humans can harbor a T. solium tapeworm. This endemic lifecycle occurs in regions where sanitation is poor and where pigs can access raw sewage. Small landowners in impoverished areas are therefore the most affected, although migrants and travelers in developed nations are also increasingly at risk.[4–7] Worldwide cysticercosis is a common disease, with over 50 million people affected.[1]
CONSEQUENCES OF ENDEMIC TRANSMISSION
Neurocysticercosis is a leading cause of preventable epilepsy responsible for 30% of adult onset epilepsy in endemic regions.[9–12] In these regions, 10–20% of the general population can have brain lesions consistent with neurocysticercosis on CT scans.[10,13–15] Seizures, precipitated by inflammation around degenerating or calcified cysts, occur in up to 70% of symptomatic diagnoses.[16] Young adults are frequently afflicted contributing to decreased productivity and unemployment among primary household wage-earners.[17] The stigma associated with epilepsy, along with direct healthcare costs related to diagnosis and management compound the problem by contributing to delayed diagnosis and undertreatment.[18] Chronic headaches, stroke syndromes, cognitive impairment and death also occur, but the associated health and economic burden remains unknown.
Porcine cysticercosis also harms local economies where domestically raised pigs provide an important source of cash or protein. Small landowners allow their pigs to roam free through villages to scavenge on waste, thereby reducing the amount of feed purchased before slaughter. However, when T. solium eggs are present in the environment, scavenging pigs become infected resulting in reduced sale price or confiscation of these animals. On a regional scale these losses can be significant. In China, 200,000,000 kg of contaminated pork meat is destroyed each year representing $120,000,000 USD in losses.[19] T. solium thus perpetuates conditions for continued transmission by further impoverishing those affected.
THE ECONOMIC ENGINE: DRIVING TRANSMISSION, LIMITING INTERVENTION
Local economic factors ultimately drive disease transmission and must be addressed in any successful control strategy.[17,20,21] Interventions which further impoverish small landowners may not be adopted or may create unintended consequences. For example, confiscation of infected pigs without compensation encourages bypass of official slaughterhouses, illicit sale of infected animals, and clandestine introduction of contaminated pork into the marketplace.[22] Illicit sale allows pig raisers to recoup some value from their infected animals, although typically only 25–50% from that of a clean pig.[22,23] Pig corralling to keep swine from consuming sewage is resisted, as it reduces profit margin by requiring owners to purchase feed for their animals. Similarly, while vaccines can break transmission to pigs, cost and acceptability of repeated immunization may limit uptake by villagers.[24–26] Vaccines against highly virulent classical swine fever are widely available but infrequently used in domestically raised pigs.[27]
TARGETING INTESTINAL TAPEWORMS
Adult intestinal tapeworms are the immediate source of both human and pig cysticercosis, thus treatment of intestinal tapeworm carriers is a key to control. However, identification is complicated by the typical asymptomatic clinical course, poor sensitivity of the widely available light microscopy, as well as the cost and limited accessibility of high-performance screening tests. Multiple screening tools are available which can be applied relative to local operating conditions [Table 1].
Table 1.
Screening methods and reported performance for Taenia solium adult intestinal infection
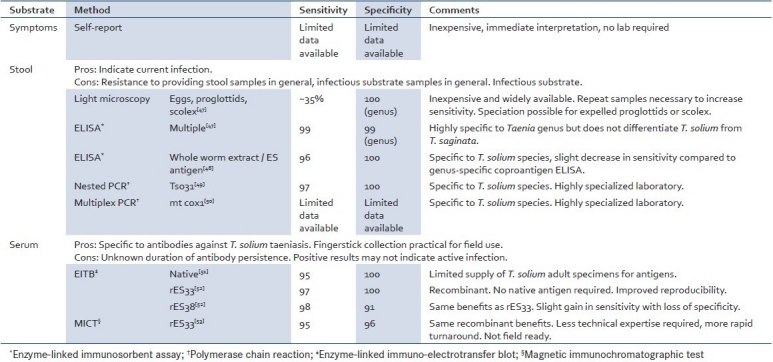
Single dose oral praziquantel and niclosamide are reported to be 90–95% efficacious for intestinal tapeworm treatment,[28] and have been used in mass treatment campaigns.[29–32] Niclosamide has the advantage that it is minimally absorbed from the intestinal tract and has no activity against T. solium cysts. Inadvertent damage to cysts during treatment with praziquantel can precipitate neurologic symptoms in people with undiagnosed NCC.[33,34]
MASS SCREENING AND CHEMOTHERAPY
Taken together, the low community prevalence and asymptomatic nature of adult tapeworm infection suggest a population-based strategy for screening and treatment. Mass human chemotherapy with niclosamide and praziquantel has been attempted in multiple countries.[30–32] While initial success is noted in decreased porcine cysticercosis and human taeniasis, these results are temporary if not sustained. Combined human and pig mass chemotherapy in Peru showed a return to baseline within 1—2 years.[32] Persistence of underlying conditions for transmission, decreased herd immunity among pigs, and migration of adult tapeworm carriers all contribute to endemic stability.[35] A longitudinal program is therefore critical to sustain control benefits.
FOCI-CENTERED INTERVENTIONS
Alternatively, intervention may be directed towards identified foci where risk of adult tapeworm infection is increased. One potential strategy involves screening or presumptive treatment for intestinal tapeworms within geographic vicinity of infected pigs. Risk concentration around infected pigs has been demonstrated in Peru and Tanzania, although this pattern was not seen in a study in Mexico.[36–38] Using infected pigs to identify at-risk foci has potential operational advantages in that 1) heavily infected pigs can be identified by tongue examination, and 2) the short lifespan of pigs raised for slaughter indicates relatively recent exposure to tapeworm eggs.
Attempts to identify at-risk foci around human cysticercosis may be neither efficacious nor practical. Epilepsy does not appear to cluster around intestinal tapeworm carriers, likely due to long latency between exposure and symptom onset in neurocysticercosis.[39] And although human seroprevalence shows both familial and geographic clustering around intestinal tapeworm carriers, antibodies against cysts may indicate remote exposure.[40]
INCREASING LOCAL AWARENESS
Community education campaigns, alone or combined with other interventions, can contribute to control effectiveness.[41,42] In addition to basic information about transmission routes, hygiene and methods for raising healthy pigs, educational messages should focus on immediate and tangible benefits of changed behavior, such as the economic benefits of raising healthy pigs. Provision of tangible incentives and infrastructure improvement may facilitate behavior modification.[41]
MONITORING PROGRAM EFFECTIVENESS
Several tools are available to estimate short-tem trends in community transmission of T. solium infection, including serologic antibody and antigen assays, radio-imaging, meat inspection and tongue examination of swine. All have inherent limitations in practicality or performance, and a combination may be necessary for accurate assessment. Measurements in pigs may be the most time-sensitive, as rapid turnover creates successive cohorts of at-risk pigs, which can be monitored serially. Pig seroprevalence has been used to trend community T. solium transmission following mass treatment campaigns.[31,32] Monitoring of sentinel pigs can also indicate areas of persistent risk or re-introduction of intestinal tapeworms into previously cleared areas.[43] Surveillance for infected pigs followed by treatment with oxfendazole could have the additional benefit of reducing the flow of contaminated pork into the marketplace.[44–46] However, further research into the safety and acceptability of oxfendazole-treated pork is needed.
CONCLUSIONS
While widespread development of sanitary infrastructure and animal husbandry practices could ultimately eradicate T. solium, this is not a short-term reality in most rural endemic areas. In the meantime, effective, affordable and sustainable interventions on local and regional levels are urgently needed to reduce immediate burden of disease. Regional elimination remains an important objective, as disease transmission rapidly returns to baseline if control interventions lapse. Progress in regional coordination is evident through formation of regional Cysticercosis Working Groups and advances in diagnostics, modeling and treatment are expected. However, technical advances from all sources must be continually translated into operating control projects to address ongoing harm. The Cysticercosis Working Group in Peru provides an excellent model, as it continues to expand field operations and progress towards elimination in Northern Peru.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Roman G, Sotelo J, Del Brutto O, Flisser A, Dumas M, Wadia N, et al. A proposal to declare neurocysticercosis an international reportable disease. Bull World Health Organ. 2000;78:399–406. [PMC free article] [PubMed] [Google Scholar]
- 2.Bern C, Garcia HH, Evans C, Gonzalez AE, Verastegui M, Tsang VC, et al. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis. 1999;29:1203–9. doi: 10.1086/313470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recommendations of the International Task Force for Disease Eradication. MMWR Recomm Rep. 1993;42:1–38. [PubMed] [Google Scholar]
- 4.Sorvillo FJ, Waterman SH, Richards FO, Schantz PM. Cysticercosis surveillance: locally acquired and travel-related infections and detection of intestinal tapeworm carriers in Los Angeles County. Am J Trop Med Hyg. 1992;47:365–71. doi: 10.4269/ajtmh.1992.47.365. [DOI] [PubMed] [Google Scholar]
- 5.Townes JM, Hoffmann CJ, Kohn MA. Neurocysticercosis in Oregon, 1995-2000. Emerg Infect Dis. 2004;10:508–10. doi: 10.3201/eid1003.030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del la Garza Y, Graviss EA, Daver NG, Gambarin KJ, Shandera WX, Schantz PM, et al. Epidemiology of neurocysticercosis in Houston, Texas. Am J Trop Med Hyg. 2005;73:766–70. [PubMed] [Google Scholar]
- 7.Wallin MT, Kurtzke JF. Neurocysticercosis in the United States: review of an important emerging infection. Neurology. 2004;63:1559–64. doi: 10.1212/01.wnl.0000142979.98182.ff. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Control of neurocysticercosis. Report of the Secretariat, Fifty-sixth World Health Assembly. World Health Organization. 2003 [Google Scholar]
- 9.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Brutto OH, Santibanez R, Idrovo L, Rodrìguez S, Díaz-Calderón E, Navas C, et al. Epilepsy and neurocysticercosis in Atahualpa: A door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–7. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 11.Medina MT, Duron RM, Martinez L, Osorio JR, Estrada AL, Zúniga C, et al. Prevalence, incidence, and etiology of epilepsies in rural Honduras: The Salama Study. Epilepsia. 2005;46:124–31. doi: 10.1111/j.0013-9580.2005.11704.x. [DOI] [PubMed] [Google Scholar]
- 12.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, et al. Neurocysticercosis: Association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–33. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 13.Cruz ME, Schantz PM, Cruz I, Espinosa P, Preux PM, Cruz A, et al. Epilepsy and neurocysticercosis in an Andean community. Int J Epidemiol. 1999;28:799–803. doi: 10.1093/ije/28.4.799. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez AL, Lindback J, Schantz PM, Sone M, Sakai H, Medina MT, et al. A population-based, case-control study of Taenia solium taeniasis and cysticercosis. Ann Trop Med Parasitol. 1999;93:247–58. [PubMed] [Google Scholar]
- 15.Garcia-Noval J, Moreno E, de Mata F, Soto de Alfaro H, Fletes C, Craig PS, et al. An epidemiological study of epilepsy and epileptic seizures in two rural Guatemalan communities. Ann Trop Med Parasitol. 2001;95:167–75. doi: 10.1080/00034980120050260. [DOI] [PubMed] [Google Scholar]
- 16.Del Brutto OH, Santibanez R, Noboa CA, Aguirre R, Diaz E, Alarcon TA. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology. 1992;42:389–92. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 17.Willingham AL, 3rd, Engels D. Control of Taenia solium cysticercosis/taeniosis. Adv Parasitol. 2006;61:509–66. doi: 10.1016/S0065-308X(05)61012-3. [DOI] [PubMed] [Google Scholar]
- 18.Rajkotia Y, Lescano AG, Gilman RH, Cornejo C, Garcia HH. for The Cysticercosis Working Group of Peru. Economic burden of neurocysticercosis: Results from Peru. Trans R Soc Trop Med Hyg. 2007;101:840–6. doi: 10.1016/j.trstmh.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Ito A, Urbani C, Jiamin Q, Vuitton DA, Dongchuan Q, Heath DD, et al. Control of echinococcosis and cysticercosis: A public health challenge to international cooperation in China. Acta Trop. 2003;86:3–17. doi: 10.1016/s0001-706x(02)00269-3. [DOI] [PubMed] [Google Scholar]
- 20.Pawlowski Z, Allan J, Sarti E. Control of Taenia solium taeniasis/cysticercosis: From research towards implementation. Int J Parasitol. 2005;35:1221–32. doi: 10.1016/j.ijpara.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez AE, Garcia HH, Gilman RH, Tsang VC. Cysticercosis Working Group in Peru. Control of Taenia solium. Acta Trop. 2003;87:103–9. doi: 10.1016/s0001-706x(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 22.The Cysticercosis Working Group Peru. The marketing of cysticercotic pigs in the Sierra of Peru. The Cysticercosis Working Group in Peru. Bull WHO. 1993;71:223–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Zoli A, Shey-Njila O, Assana E, Nguekam JP, Dorny P, Brandt J, et al. Regional status, epidemiology and impact of Taenia solium cysticercosis in Western and Central Africa. Acta Trop. 2003;87:35–42. doi: 10.1016/s0001-706x(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez AE, Gauci CG, Barber D, Gilman RH, Tsang VC, Garcia HH, et al. Vaccination of pigs to control human neurocysticercosis. Am J Trop Med Hyg. 2005;72:837–9. [PubMed] [Google Scholar]
- 25.Flisser A, Gauci CG, Zoli A, Martinez-Ocaña J, Garza-Rodriguez A, Dominguez-Alpizar JL, et al. Induction of protection against porcine cysticercosis by vaccination with recombinant oncospheres antigens. Infect Immun. 2004;72:5292–7. doi: 10.1128/IAI.72.9.5292-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assana E, Kyngdon CT, Gauci CG, Geerts S, Dorny P, De Deken R, et al. Elimination of Taenia solium transmission to pigs in a field trial of the TSOL18 vaccine in Cameroon. Int J Parasitol. 2010;40:515–9. doi: 10.1016/j.ijpara.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia HH, Gonzalez AE, Del Brutto OH, Tsang VC, Llanos-Zavalaga F, Gonzalvez G, et al. Strategies for the elimination of taeniasis/cysticercosis. J Neurol Sci. 2007;262:153–7. doi: 10.1016/j.jns.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 28.Schantz PM. Tapeworms (cestodiasis) Gastroenterol Clin North Am. 1996;25:637–53. doi: 10.1016/s0889-8553(05)70267-3. [DOI] [PubMed] [Google Scholar]
- 29.Cruz M, Davis A, Dixon H, Pawlowski ZS, Proano J. Operational studies on the control of Taenia solium taeniasis/cysticercosis in Ecuador. Bull WHO. 1989;67:401–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Sarti E, Schantz PM, Avila G, Ambrosio J, Medina-Santillan R, Flisser A. Mass treatment against human taeniasis for the control of cysticercosis: A population-based intervention study. Trans R Soc Trop Med Hyg. 2000;94:85–9. doi: 10.1016/s0035-9203(00)90451-6. [DOI] [PubMed] [Google Scholar]
- 31.Allan JC, Velasquez-Tohom M, Fletes C, Torres-Alvarez R, Lopez-Virula G, Yurrita P, et al. Mass chemotherapy for intestinal Taenia solium infection: Effect on prevalence in humans and pigs. Trans R Soc Trop Med Hyg. 1997;91:595–8. doi: 10.1016/s0035-9203(97)90042-0. [DOI] [PubMed] [Google Scholar]
- 32.Garcia HH, Gonzalez AE, Gilman RH, Moulton LH, Verastegui M, Rodriguez S, et al. Combined human and porcine mass chemotherapy for the control of T.solium. Am J Trop Med Hyg. 2006;74:850–5. [PubMed] [Google Scholar]
- 33.Flisser A, Madrazo I, Plancarte A, Schantz P, Allan J, Craig P, et al. Neurological symptoms in occult neurocysticercosis after single taeniacidal dose of praziquantel. Lancet. 1993;342:748. doi: 10.1016/0140-6736(93)91743-6. [DOI] [PubMed] [Google Scholar]
- 34.Hewagama SS, Darby JD, Sheorey H, Daffy JR. Seizures related to praziquantel therapy in neurocysticercosis. Med J Aust. 2010;193:246–7. doi: 10.5694/j.1326-5377.2010.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez AE, Lopez-Urbina T, Tsang B, Gavidia C, Garcia HH, Silva ME, et al. Transmission dynamics of Taenia solium and potential for pig-to-pig transmission. Parasitol Int. 2006;55:S131–5. doi: 10.1016/j.parint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Lescano AG, Garcia HH, Gilman RH, Guezala MC, Tsang VC, Gavidia CM, et al. Swine cysticercosis hotspots surrounding Taenia solium tapeworm carriers. Am J Trop Med Hyg. 2007;76:376–83. [PubMed] [Google Scholar]
- 37.Ngowi HA, Kassuku AA, Carabin H, Mlangwa JE, Mlozi MR, Mbilinyi BP, et al. Spatial clustering of porcine cysticercosis in Mbulu district, northern Tanzania. PLoS Negl Trop Dis. 2010;4:e652. doi: 10.1371/journal.pntd.0000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales J, Martínez JJ, Rosetti M, Fleury A, Maza V, Hernandez M, et al. Spatial distribution of Taenia solium porcine cysticercosis within a rural area of Mexico. PLoS Negl Trop Dis. 2008;2:e284. doi: 10.1371/journal.pntd.0000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Rodriguez S, et al. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: Clustering on human seroprevalence but not on seizures. PLoS Negl Trop Dis. 2009;3:e371. doi: 10.1371/journal.pntd.0000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia HH, Gilman RH, Gonzalez AE, Verastegui M, Rodriguez S, Gavidia C, et al. Hyperendemic human and porcine Taenia solium infection in Peru. Am J Trop Med Hyg. 2003;68:268–75. [PubMed] [Google Scholar]
- 41.Sarti E, Flisser A, Schantz PM, Gleizer M, Loya M, Plancarte A, et al. Development and evaluation of a health education intervention against Taenia solium in a rural community in Mexico. Am J Trop Med Hyg. 1997;56:127–32. doi: 10.4269/ajtmh.1997.56.127. [DOI] [PubMed] [Google Scholar]
- 42.Ngowi HA, Carabin H, Kassuku AA, Mlozi MR, Mlangwa JE, Willingham AL., 3rd A health-education intervention trial to reduce porcine cysticercosis in Mbulu District, Tanzania. Prev Vet Med. 2008;85:52–67. doi: 10.1016/j.prevetmed.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez AE, Gilman R, Garcia HH, McDonald J, Kacena K, Tsang VC, et al. Use of sentinel pigs to monitor environmental Taenia solium contamination. The Cysticercosis Working Group in Peru (CWG) Am J Trop Med Hyg. 1994;51:847–50. doi: 10.4269/ajtmh.1994.51.847. [DOI] [PubMed] [Google Scholar]
- 44.Gonzales AE, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Bernal T, et al. Effective, single-dose treatment or porcine cysticercosis with oxfendazole. Am J Trop Med Hyg. 1996;54:391–4. doi: 10.4269/ajtmh.1996.54.391. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez AE, Falcon N, Gavidia C, Garcia HH, Tsang VC, Bernal T, et al. Treatment of porcine cysticercosis with oxfendazole: A dose-response trial. Vet Rec. 1997;141:420–2. doi: 10.1136/vr.141.16.420. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez AE, Falcon N, Gavidia C, Garcia HH, Tsang VC, Bernal T, et al. Time-response curve of oxfendazole in the treatment of swine cysticercosis. Am J Trop Med Hyg. 1998;59:832–6. doi: 10.4269/ajtmh.1998.59.832. [DOI] [PubMed] [Google Scholar]
- 47.Allan JC, Craig PS. Coproantigens in taeniasis and echinococcosis. Parasitol Int. 2006;55:S75–80. doi: 10.1016/j.parint.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Guezala MC, Rodriguez S, Zamora H, Garcia HH, Gonzalez AE, Tembo A, et al. Development of a species-specific coproantigen ELISA for human Taenia solium taeniasis. Am J Trop Med Hyg. 2009;81:433–7. [PubMed] [Google Scholar]
- 49.Mayta H, Gilman RH, Prendergast E, Castillo JP, Tinoco YO, Garcia HH, et al. Sterling CR; Cysticercosis Working Group in Peru. Nested PCR for specific diagnosis of Taenia solium taeniasis. J Clin Microbiol. 2008;46:286–9. doi: 10.1128/JCM.01172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, et al. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol. 2004;42:548–53. doi: 10.1128/JCM.42.2.548-553.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkins PP, Allan JC, Verastegui M, Acosta M, Eason AG, Garcia HH, et al. Development of a serologic assay to detect Taenia solium taeniasis. Am J Trop Med Hyg. 1999;60:199–204. doi: 10.4269/ajtmh.1999.60.199. [DOI] [PubMed] [Google Scholar]
- 52.Levine MZ, Lewis MM, Rodriquez S, Jimenez JA, Khan A, Lin S, et al. Development of an enzyme-linked immunoelectrotransfer blot (EITB) assay using two baculovirus expressed recombinant antigens for diagnosis of Taenia solium taeniasis. J Parasitol. 2007;93:409–17. doi: 10.1645/GE-938R.1. [DOI] [PubMed] [Google Scholar]
- 53.Handali S, Klarman M, Gaspard AN, Dong XF, Laborde R, Noh J, et al. Development and evaluation of a magnetic immunochromatographic test to detect Taenia solium, which causes taeniasis and neurocysticercosis in humans. Clin Vaccine Immunol. 2010;17:631–7. doi: 10.1128/CVI.00511-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


