Abstract
Soil-transmitted helminths (STHs) form one of the most important groups of infectious agents and are the cause of serious global health problems. The most important STHs are roundworms (Ascaris lumbricoides), whipworms (Trichuris trichiura) and hookworms (Necator americanus or Ancylostoma duodenale); on a global level, more than a billion people have been infected by at least one species of this group of pathogens. This review explores the general concepts of transmission dynamics and the environment and intensity of infection and morbidity of STHs. The global strategy for the control of soil-transmitted helminthiasis is based on (i) regular anthelminthic treatment, (ii) health education, (iii) sanitation and personal hygiene and (iv) other means of prevention with vaccines and remote sensoring. The reasons for the development of a control strategy based on population intervention rather than on individual treatment are discussed, as well as the costs of the prevention of STHs, although these cannot always be calculated because interventions in health education are difficult to measure. An efficient sanitation infrastructure can reduce the morbidity of STHs and eliminates the underlying cause of most poverty-related diseases and thus supports the economic development of a country.
Keywords: Helminth infection, Prevention, Soil-transmitted Helminths
INTRODUCTION
Soil-transmitted helminths (STHs) form one of the most important groups of infectious agents and are the cause of serious global health problems; more than a billion people have been infected by at least one species of this group of pathogens.[1] At a global level, the most important STHs are roundworms (Ascaris lumbricoides), whipworms (Trichuris trichiura) and hookworms (Necator americanus or Ancylostoma duodenale) and are estimated to have infected 807 million, 604 million and 576 million people, respectively.[1,2] The greatest numbers of STH infections occur in Sub-Saharan Africa (SSA), East Asia, China, India and South America [Table 1].[3]
Table 1.
Global estimates of number of soiltransmitted helminth infections by region (millions of cases)[2]
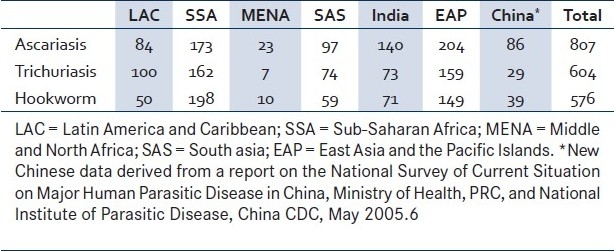
Geohelminths are more prevalent among children living in conditions of poor sanitation, and their impact on morbidity and mortality is more severe in malnourished populations.[4] As adult worms, the soil-transmitted helminths live for years in the human gastrointestinal tract. Most studies suggest that approximately 70% of the worm population is hosted by 15% of the host population. These few seriously infected individuals are at a higher risk of disease and are also the prime source of environmental contamination.[5] Inadequate hygiene and poor health care systems and facilities, as well as social indifference, make this situation worse, although STH control is often neglected, even in worm-infested countries. In the developing world, inadequate water supply and sanitation, as well as crowded living conditions, combined with lack of access to health care and low levels of education, make the poor particularly susceptible to infection and disease, including STHs.[3] In the last decade, an increasing number of international initiatives have established the aim to either reduce or to eliminate the disease burden caused by STHs and other helminthic parasites prevalent in the resource-poor regions of the world.[6–8]
STHs : TRANSMISSION DYNAMICS AND THE ENVIRONMENT
The A. lumbricoides is a roundworm that infests the entire small intestine; the adult hookworm of the Necator and Ancylostoma genera parasite the upper part of the human small intestine; and the adult T. trichiura (whipworms) lives in the large intestine, especially in the cecum. The STHs vary greatly in size, and female worms are larger than males.[9] STHs do not reproduce within the host, and this is a crucial feature of the epidemiology of these parasites.[10] To predict the global distribution of STH infections, an understanding of their biology, ecology and transmission dynamics is essential. The STH infection life cycle follows a general pattern; the parasites in adult stages inhabit part of the host intestine (A. lumbricoides and hookworm inhabit the small intestine; T. trichiura, the colon), reproduce sexually and produce eggs, which are passed in human feces and deposited in the external environment. Adult worms survive for several years and produce large numbers of eggs. Eggs can remain viable in the soil for several months (A. lumbricoides and T. trichiura); and larvae can survive for several weeks (hookworms), depending on the prevailing environmental conditions. Hookworm (A. duodenale and N. americanus) larvae can undergo hypobiosis (arrested development at a specific point in the nematode life cycle) in the human body under certain environmental conditions for several months. Infection occurs through accidental ingestion of eggs (A. lumbricoides and T. trichiura) or penetration of the skin (by hookworm larvae).[4]
The dynamic processes involved in STH transmission (free-living infective stages of development and survival) depend on the prevailing environmental conditions; climate is an important determinant of transmission of STH infections, with adequate moisture and warm temperature essential for larval development in the soil.[4] Soil moisture and relative atmospheric humidity are also known to influence the development and survival of eggs and larvae — where higher humidity is associated with faster development of ova; whereas at low humidity, the ova of A. lumbricoides and T. trichiura do not form embryos.[11] Field studies show that the abundance of hookworm larvae is related to atmospheric humidity.[12] These differing rates of development and survival will influence parasite establishment in the human host and, hence, infection levels. Although seasonal dynamics in transmission may occur, such fluctuations may be of little significance to the overall parasite equilibrium within communities.[13]
STHs : INTENSITY OF INFECTION AND MORBIDITY
There is evidence that individuals with many helminth infections have even worse infections with STHs.[14] Most studies suggest that approximately 70% of the worm population is hosted by 15% of the human host population. These few heavily infected individuals are at a higher risk of disease and are also the prime source of environmental contamination.[10] Under such conditions, the STH species are commonly co-endemic. Generally only the STH infections of moderate and high intensity in the gastrointestinal tract produce clinical manifestations, with the highest-intensity infections has been mostly common in children.[15] The numerical threshold at which worms cause disease in children has not been established, because this depends on the underlying nutritional status of the host. Each of the STHs produces characteristic disease syndromes. Since morbidity from these infections and the rate of transmission are directly related to the number of worms harbored in the host, the intensity of infection is the main epidemiological index used to describe soil-transmitted helminth infection.[13]
Intensity of infection is measured by the number of eggs per gram of feces, generally by the Kato-Katz fecal thick-smear technique.[16] For A. lumbricoides and T. trichiura, the most intense infections are found in children aged 5 to 15 years, with a decline in intensity and frequency in adulthood. Whether such age dependency indicates changes in exposure, acquired immunity or a combination of both remains controversial.[17] Although heavy hookworm infections also occur in childhood, frequency and intensity commonly remain high in adulthood, even in elderly people.[18] The STH infections are often referred to as being “overdispersed” in endemic communities, such that most worms are harbored by a few individuals in an endemic area.[15] There is also evidence of familial and household aggregation of infection, with the relative contribution of genetics and common household environment debated.[10]
The STHs are more frequently found among children living in conditions of poor sanitation, and their impact on morbidity and mortality is more severe in malnourished populations.[4] Lower estimates indicate that most hookworm cases do not result in severe anemia or pronounced protein loss in the host, whereas the higher estimates show the long-term results of infection, such as malnutrition and delayed cognitive development, especially in children.[18]
STHs : INTERVENTION FOR INFECTION CONTROL
A high prevalence of STHs, when combined with poor hygiene and malnutrition, is an indicator of a country's future problems, indicating that priority be given to eradicating STHs worldwide.[5] STHs are considered together since it is common for an individual, especially a child living in a less developed country, to be chronically infected with all three worms. Such children experience malnutrition, stunted growth, mental retardation, as well as cognitive and learning deficiencies.[1]
Large-scale environmental sanitation programs are complex, making interventions directly aimed at the transmission of STHs challenging to implement.[19] These interventions directly affect the transmission of several diseases in both the public and private domains.[20] Several factors need to be operative for an intervention to be successful. Amongst these are public investment in sewage networks and a collective will on the part of individual households to invest in a toilet and connect it to this network.[19]
The world health organization (WHO)[1] has recommended three interventions to control morbidity due to STH infections: regular drug treatment of high-risk groups for reduction of the worm burden over time, health education and sanitation supported by personal hygiene aimed at reducing soil contamination.
Anthelminthic treatment and massive treatment
Regular drug treatment represents the main approach for infection control in areas where infections are intensely transmitted, where resources for disease control are limited and where funding for sanitation is insufficient. Drug treatment can be administered in the community using alternative approaches — the treatment is offered to the entire community, irrespective of age, sex, infection status and any other social characteristics (universal treatment); the treatment is targeted at population groups, which may be defined by age, sex or other social characteristics, irrespective of the infection status (targeted treatment); and selective treatment representing individual-level administration of anthelminthic drugs, where selection is based on diagnosis to detect the most heavily-infected people who will be most at risk of serious morbidity and mortality.[21] The selection of the delivery strategy and the frequency of treatment are based on the analysis of available epidemiological data.
In accordance with theWHO[22], the recommended drugs for for use in public health interventions to control STH infections are:
Albendazole (400mg) tablets given in a single dose, reduced to 200mg for children between 12 and 24 months;
Levamisole (40mg) tablets given in a single dose by weight (2.5mg/kg). The drug Levamisole at a dose of 80mg has been successfully used in primary school–age children[23];
Mebendazole (500mg) tablets given in a single dose;
Pyrantel pamoate (250mg) tablets given in a single dose by weight (10mg/kg). A combined preparation of pyrantel-oxantel has been proved to be more effective than pyrantel alone in treating T. trichiura infection.[24]
Evidence suggests that mass delivery of deworming is preferable on efficacy, economic and equity grounds for approaches that require diagnostic screening.[25] School-based deworming also offers major advantages for untreated children and the whole community by reducing disease transmission in the community as a whole.[26]
Frequency of regular treatment should vary according to the intensity of transmission and rates of re-infection. These factors must be considered in relation to the resources available and the cost involved in drug purchase and distribution.[21] The STH infections can be classified as being of light, moderate or heavy intensity according to the thresholds established by the WHO,[27] based on the number of STH eggs per gram of feces. Helminths in different areas of the world have different levels of egg output,[28] so the thresholds proposed by the WHO are not rigid and should be adjusted for the local situation.
The World Health Assembly in 2001 endorsed a strategy for the prevention and control of schistosomiasis and soil-transmitted helminthiasis in high-transmission areas. In the short term, morbidity will be reduced by access to drugs (praziquantel and broad-spectrum anthelminthics) and good case management in all health services; regular treatment of at least 75% of school-age children by 2010; targeting other high-risk groups (young children, women of child bearing age and occupational groups) through existing public health programs and channels.
For long-term sustainability, environmental health will be required improving access to safe water and sanitation and improved hygienic behavior through health education.[29]
Health education
Health education aims to improve health and increase hygiene awareness and to change health-related behavior in the population. For diseases related to poverty, such as STH infections, the suggested solution might not be available or might be too expensive to adopt. Deprived communities understand the importance of the safe disposal of fecal matter and of wearing shoes, but poverty often hinders the construction of latrines and the purchase of shoes. The prevalence of STHs in the community can be used as an indicator of the conditions of living, environmental sanitation, level of education and the socioeconomic status of the community.
Providing information on the disease and the possible adoption of preventive measures frequently results in an increase in knowledge but not necessarily in behavioral change.[30]
Educational materials (posters, leaflets, radio and video messages) have been traditionally used to transmit and disseminate health-related messages, but strategies imported from the private sector are increasingly being advocated for their potential value in crafting and disseminating health-related messages.[31,32]
Reduction in the fecal contamination of soil can be achieved by recommending the use of latrines, developing self-protection from re-infection, and promoting personal/ family hygiene measures such as washing hands and proper food preparation. The knowledge of, and motivation for, behavioral change must be sustained by making available proper facilities for excreta disposal. Frequently, in STH-endemic areas, latrines are not available or are not in sufficient numbers to meet the needs of the population.
Romotion of latrine maintenance and use, washing of hands and proper food handling have benefits that go beyond the control of STH infections. From this perspective, it is reasonable to include health education in all STH-control programs, wherein the health education message can be provided in a simple and inexpensive way. Health education messages can be delivered by teachers in schools, thereby fostering changes in health-related behavior in children, which in turn involves their parents and guardians.[21,34,35] The marketing of health education in order to create increased health awareness and changes in habits of defecation are important when aiming to reduce STH infections.
Sanitation and personal hygiene
Human STHs are fecal-borne infections, and transmission occurs either directly (hand-to-mouth) or indirectly (through food and water). Sanitation in the context of economic development is the only definitive intervention that eliminates these infections.[21] STH infections are never a public health problem where hygiene and sanitation standards are appropriate. Improvement of sanitation standards always has a repercussion on infection and re- infection levels.[19,36,37]
Agglomeration and the type of excreta-disposal facility were the only significant predictors of re-infection in studies conducted in the West Indies, showing that the prevalence of STH infections was significantly lower in areas with better sanitation.[38] Similar results were obtained in the plantation sector of Sri Lanka,[39] in urban slums of Bangladesh[40] and in a study in Salvador, Brazil,[41] suggesting that sewerage and drainage can have a significant effect on STH infections, reducing transmission occurring in the public domain.
An extensive program of environmental sanitation was conducted in the Brazilian city of Salvador, Bahia, aimed at expanding the city's sanitation network from 26% to 80%. A significant and marked reduction in the rate of childhood diarrhea and in the prevalence and incidence of geohelminths infections has already been reported to occur since the introduction of the sanitation intervention program called “Blue Bay”.[19,42]
In Zimbabwe, despite the marked increase in the number of latrines, no relationship was found between hookworm re-infection intensities and the availability of latrines in individual farms.[43] A study in the Senegal[44] demonstrated that, despite high coverage of the program of provision of latrines, the majority of the children in a village, interviewed with a questionnaire, claimed to defecate elsewhere.
Sanitation is inadequate in most cities in developing countries, with major effects on STH infections. In this situation, piped sewers are an appropriate solution, and it is questionable as to whether efforts should focus on systems based on onsite solutions, such as latrines. In a meta-analysis study,[45] data suggested that sewerage typically has a positive effect on enteric infectious disease burden.
A systematic review and meta-analysis[46] suggested that water, sanitation, and hygiene interventions as well as their combination, are effective at reducing diarrheal illnesses and STH infections. This review identifies many research questions that need more attention: the role of community versus household connections for water supply interventions, the role of sanitation interventions in the reduction of diarrheal illnesses, and the longevity of the health-related effects of individual interventions. In another review,[47] consistent findings 30 studies of intervention and 24 observational studies during a 20-year period support the conclusion that hygiene interventions other than infrastructure implementation are important for preventing infections, particularly the STH infections. While these results may not be surprising or “new,” they are nevertheless impressive and important because they demonstrate that even in an era of unprecedented “cleanliness” and improved public health infrastructure, there is a continued, measurable, positive effect of personal and community hygiene.
Environmental factors such as water supply for domestic and personal hygiene, sanitation and housing conditions; and other factors such as socioeconomic, demographic and health related behavior are known to influence this infection. Two principal factors in maintaining endemicity of these helminths are favorable qualities of the soil and the frequent contamination of the environment by human feces. Their transmission within the community is predominantly related to human habits with regard to eating, defecation, personal hygiene and cleanliness.
Sanitation factors such as the reliability of water supply, frequency of rubbish collection and proximity to overflowing or visible sewage are not under the control of individual households. These do not reflect personal hygiene, and their significance suggests that the impact of environmental sanitation on health could have been greater if the governmental systems had been properly operated and maintained. Improved disposal of excreta offers a more sustainable method of control, among many other benefits.[48] Since domestic risk factors assume greater importance after public domain transmission is controlled, the environmental sanitation creates opportunities for synergy with other inputs, such as hygiene promotion, which are aimed at such domestic risk factors.[41]
The effect of improved sanitation is slow to development and may take decades to achieve a measurable impact. Often, the high costs involved prevent the provision of sanitation to the communities most in need, and sanitation does not become effective until it covers a high percentage of the population.[49,50]
Other ways of prevention and control
Vaccines for STH
Vaccine development has driven the field of immunology since it incorporates the selection and presentation of benign antigens or attenuated pathogens to stimulate an acquired protective response. Vaccination has proven to be the most cost-effective and efficient procedure for disease management. The need to control chronic and emerging diseases and bio-security concerns stimulate demand for new vaccines.[51]
Helminths are exquisitely adapted to evading and modulating the mammalian immune response; and interestingly, similar evasion mechanisms can be shared among distantly related species. This begs the obvious question of whether this ability can ever be exploited for therapeutic purposes.[52] Various authors have reported epidemiological and experimental data to suggest that a reduction in helminth infection is linked to rising rates of autoimmunity and atopy.[53–56]
The association between intestinal parasites and atopy is not new. This association has been studied since the 1970s, when the concept of “hygiene hypothesis”[57] was introduced based on the observation that there was an inverse correlation between household size and allergic rhinitis. Various results indicate an inverse relationship where in individuals infected with STHs are less susceptible to allergic disorders, as a result of immunological mechanisms that remain unknown.[58–60]
Immunological studies of the mechanisms by which early exposure to geohelminth infections affects immune polarization to inhalant allergens are likely to provide important insights into the early regulation of the immune response and may lead to the design of novel interventions for the prevention of STH infections.[54] One prediction of the hygiene hypothesis is that the rising rate of inflammatory disorders is due specifically to a paucity of infection during infancy, which in turn tunes the immune response in subsequent adulthood to a less pathogenic modality. This being the case, therapeutic dosing of a helminth (or products thereof) to relieve fulminant inflammatory disease in an adult may be relatively ineffective. The patient's immune repertoire, both adaptive and innate, has already been shaped by the absence of parasite antigens and is subject only to relatively minor perturbations.[52]
Early enteric exposures to STH infections in infancy may provide important maturational and regulatory signals for the developing immune response that allows it to control allergic inflammation directed against both parasitic and environmental aeroallergens. Typically, STH infections are chronic in endemic areas, and, as with other helminth parasites, it is likely that geohelminths have developed ways of modulating the host immune response to permit adult development and survival. Likewise, the human host may have developed mechanisms to limit the pathology associated with the long-term presence of these highly allergenic parasites.[54]
Many aspects of vaccine design and implementation are driven by advancing molecular technology and the basic information of host/ pathogen interactions that target pathogen vulnerability and reduced host pathology. Experimental vaccine development under controlled conditions in the laboratory requires field testing to isolate important modulating factors. An underlying parasitic infection is a profound, albeit reversible, modifier of vaccine efficacy.[51] It is critical to develop vaccination and challenge studies on the relevant host species and to extend the work to field trials in order to ensure the success of vaccination through an integrated strategy for the control of STH disease.
Remote sensoring
Studies have investigated spatial patterns of STH infections[18,61,62] and other helminths. These studies have focused on the use of RS data to identify ecological correlates of infection and develop statistical models of disease risk.
Geographical distributions are continually updated as new epidemiological data are collected, and as intervention reduces the prevalence of infection. Analysis of the cost-effectiveness of the tools, which is germane to their long-term and sustainable use, is currently underway. Experiences in Uganda demonstrate the usefulness of remote sensoring (GIS or RS) as geographic decision-making tools for implementing helminth control on both national and local scales.[63]
An important emerging trend is that national governments are beginning to use this approach for designing and developing sustainable national programs. GIS/ RS has been employed by governments to plan and conduct nationwide rapid epidemiological assessments of STHs and schistosomiasis in Chad[64] and Eritrea,[65] and to design and implement national parasite-control programs, in both cases as part of national development programs with World Bank assistance. The results from the survey helped the government plan the country's school-based control program, and resulted in significant cost savings for the program since it identified the need to target far fewer schools than had first been anticipated. The sampling methodology proved to be substantially less expensive and more practical than traditional approaches developed without the benefit of GIS/ RS. The national survey revealed that infection was highly focal and that deworming interventions could be precisely targeted, with significant savings in financial and technical resources.
STH: COSTS OF PREVENTION
The cost-benefits of the control measures for morbidity due to STH infections are influenced by the ecological and environmental situation, by the availability of local anthelminthic drug production facilities and by the presence of infrastructure and facilities that can be used to reach the high-risk groups: school-age children and adults with heavy infection.[21]
The infrastructure for the delivery of such a package of health care to millions of poor people already exists in many endemic areas through primary health care provision, public and private schools, faith-based organizations and social institutions. In deprived communities, where sanitation is practically nonexistent and the prevalence and intensity of infection are high, a suitable infrastructure (such as the school system or a national immunization day) should be used to distribute at least regular treatment to the groups at risk. The cost of adding this intervention is normally marginal.[21,24] Fenwick and colleagues[66] estimated that a package of interventions could be provided at a cost of US$0.40 per person per year. About 1.3 million preschool children were dewormed during the vitamin A distribution campaign in Nepal, with the cost of the intervention estimated at US$1.7 million.[67] Thirty countries now conduct combined deworming and school feeding programs. The average cost per child per year is 70 US cents: 4 cents for mebendazole; 25 cents for praziquantel; 30 cents for training, monitoring and educational materials; and the remaining 11 cents for delivering both drugs.[68]
In Cambodian schools, deworming is promoted by means of a school kit, which contains deworming tablets, health education posters and pamphlets for teachers, games and attractive pictures giving simple messages on how to prevent infection. The coverage of primary school–age children was 84% in 2003, and the biannual deworming campaign from 2004 onward is estimated to cost US$0.04 per child treated.[69]
The advantage of regular deworming lies in its simplicity (one tablet per child), cheap delivery (by teachers through schools), and safety record (the benefits of treatment far outweigh the risk of minor side effects). Many organizations, including NGOs, could include an STH control package in their routine activities and, even with limited budgets, relieve the burden of STHs in the population covered.[21,23]
The cost-benefit of health education, however, should not be measured merely in terms of cost-effectiveness alone. Health education in community health has the same role as medical information and counseling given by the physician to the patient in clinical medicine. The effects of establishing a good relationship between the health system and the community are not always directly measurable with regard to the success of the control measures. The effect of health education in community health includes improvement in loyalty and trust between the educators and the community. When such a relationship is established, the community is no longer a simple recipient of the medical intervention but becomes one of the partners in the process of dissemination of health education.[21]
The cost of sanitation is always higher when compared to other measures. The magnitude of the problem of providing sewerage is a big challenge in large urban centers in developing countries. The STH control in Viet Nam, based on regular deworming, latrine construction and health education, has shown that the cost per child for each latrine has been estimated at US$7.9. The construction of new latrines was considered important as a good example for the schoolchildren and a way of providing essential sanitation at least in schools.[70]
The installation costs of modern sewerage, similar to the type found in developed countries, for the poor population of Lagos, Nigeria, could amount to a billion US dollars or more. Progress has been made in developing a variety of latrines for rural communities, but these may not be appropriate for slums and squatter settlements with a shortage of land for dwellings and at sea level.[71] The resources needed to improve hygienic standards can be huge, but the collaboration of different initiatives dealing with hygiene and prevention of diseases related to poor hygiene will help create the synergy needed to reduce both disease and poverty. A reliable evaluation of the advantage of investments in sanitation must include the consequences for other health services and for economic development.
An efficient sanitation infrastructure removes the underlying cause of most poverty-related communicable diseases and thus supports the economic development of a country.[21]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Deworming for health and development. Reports on the third global meeting of the partners for parasite control. Geneva: WHO; 2005. World Health organization. [Google Scholar]
- 2.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–21. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Silva NR, Brooker S, Hotez PJ, Montressor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trend Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Brooker S, Clements A, Bundy DAP. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:223–65. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundy DAP, de Silva NR. Can we deworm this wormy world? Br Med Bull. 1998;54:421–32. doi: 10.1093/oxfordjournals.bmb.a011698. [DOI] [PubMed] [Google Scholar]
- 6.Chai JY, Lee SH. Hayzshi S, editor. The successful implementation of the nationwide control programme of ascariasis. Collected Papers on the Control of Soil-Transmitted Helminthiases. 2001:267–71. [Google Scholar]
- 7.Hara T. Hayashi S, editor. Large scale control against intestinal helminthic infections in Japan, with special reference to the activies of Japan Association of Parasite Control. Collected Papers on the Control of Soil-Transmitted Helminthiases. 2001:267–71. [Google Scholar]
- 8.Molyneux DH, Bradley M, Hoerauf A, Kyelem D, Taylor MJ. Mass drug administration for lymphatic filariasis and onchocerciasis. Trends Parasitol. 2003;19:516–22. doi: 10.1016/j.pt.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Despommier D, Gwadz RW, Hotez PJ, Knirsch CA. Parasitic diseases. 5th ed. New York: Apple Tree Production; 2005. [Google Scholar]
- 10.Betohony J, Brooker S, Albonico M, Geiger S, Loukas A, Dimert D, et al. Soil-transmitted helminth infections: Ascaridiasis. trichuriasis and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 11.Lorcan PO, Holland CV. The Public health importance of Ascaris lumbricoides. Parasitol. 2000;121:S51–71. doi: 10.1017/s0031182000006442. [DOI] [PubMed] [Google Scholar]
- 12.Crompton DW. Ascaris and ascariasis. Adv Parasitol. 2001;48:285–375. doi: 10.1016/s0065-308x(01)48008-0. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RM, May RM. Infectious Diseases of Humans:Dynamics and Control. 1st ed. Oxford: Oxford University Press; 1991. [Google Scholar]
- 14.Raso G, Luginbuhl A, Adjoua CA, Tian-Bi NT, Silué KD, Matthys B, et al. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Cote d’Ivoire. Int J Epidemiol. 2004;33:1092–102. doi: 10.1093/ije/dyh241. [DOI] [PubMed] [Google Scholar]
- 15.Chan MS, Medley GF, Jamison D, Bundy DA. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitol. 1994;109:373–87. doi: 10.1017/s0031182000078410. [DOI] [PubMed] [Google Scholar]
- 16.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 17.Galvani AP. Age-dependent epidemiological patterns and strain diversity in helminth parasites. J Parasitol. 2005;91:24–30. doi: 10.1645/GE-191R1. [DOI] [PubMed] [Google Scholar]
- 18.Brooker S, Kabatereine NB, Tukahebwa EM, Kazibwe F. Spatial analysis of the distribution of intestinal nematode infections in Uganda. Epidemiol Infect. 2004;132:1065–71. doi: 10.1017/s0950268804003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barreto M, Genser B, Strina A, Teixeira MG, Assis AMO, Rego RF, et al. Effect of city-wide sanitation programme on reduction in rate of chilhood diarrhoea in northeast Brazil: assessment by two cohort studies. Lancet. 2007;370:1622–8. doi: 10.1016/S0140-6736(07)61638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Trop Med Int Health. 1996;1:27–34. doi: 10.1046/j.1365-3156.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 21.Albonico M, Montresor A, Crompton DW, Savioli L. Intervention for the control of soil-transmitted helminthiasis in the community. Adv Parasitol. 2006;61:312–48. doi: 10.1016/S0065-308X(05)61008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Model Formulary. Based on the 13th Model List of Essential Medicines 2003. Geneva: WHO; 2004. World Health Organization; pp. 82–5. [Google Scholar]
- 23.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Heath Organ. 2003;81:343–52. [PMC free article] [PubMed] [Google Scholar]
- 24.Albonico M, Bickle Q, Haji HJ, Ramsan M, Khatib JK, Savioli L, et al. Evaluation of the efficacy of pyrantel-oxantel for the treatment of soil-transmitted nematode infections. Trans R Soc Trop Med Hyg. 2002;96:685–90. doi: 10.1016/s0035-9203(02)90352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren KS, Bundy DA, Anderson RM, Davis AR, Henderson DA, Jamison DT, et al. Helminth infections. In: Jamison DT, Mosley WH, Measham AR, Bobadilla JL, editors. Disease Control Priorities in Developing Countries 2001. Oxford: Oxford University Press; 1993. pp. 131–60. [Google Scholar]
- 26.Bundy DA, Wong MS, Lewis LL, Horton J. Control of geohelminths by delivery of targeted chemotherapy through schools. Trans R Soc Trop Med Hyg. 1990;81:115–20. doi: 10.1016/0035-9203(90)90399-y. [DOI] [PubMed] [Google Scholar]
- 27.Prevention and Control of Schistosomiasis and Soil Transmitted Helminthiasis. Report of a WHO Expert Committee. Geneva: WHO Technical Report Series 912; 2002. World Health Organization. [PubMed] [Google Scholar]
- 28.Hall A, Holland C. Geographical variation in Ascaris lumbricoides fecundity and its implications for helminth control. Parasitol Today. 2000;16:540–4. doi: 10.1016/s0169-4758(00)01779-8. [DOI] [PubMed] [Google Scholar]
- 29.Report of the 54th World Health Assembly. Control of Schistosomiasis and Soil-Transmitted Helminth Infections. Geneva: WHO; 2001. World Health Organization. [Google Scholar]
- 30.O’Cathain A, Walters SJ, Nicholl JP, Thomas KJ, Kirkham M. Use of evidence based leaflets to promote informed choice in maternity care: randomised controlled trial in everyday practice. Br Med J. 2002;324:643. doi: 10.1136/bmj.324.7338.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bull FC, Holt CL, Kreuter MW, Clark EM, Scharff D. Understanding the effects of printed health education materials: Which features lead to which outcomes? J Health Commun. 2001;6:265–79. doi: 10.1080/108107301752384442. [DOI] [PubMed] [Google Scholar]
- 32.Walsh DC, Rudd RE, Moeykens BA, Moloney TW. Social marketing for public health. Health Aff. 1993;12:104–19. doi: 10.1377/hlthaff.12.2.104. [DOI] [PubMed] [Google Scholar]
- 33.Cairncross S. Sanitation in the developing world: current status and future solutions. Int J Environ Health Res. 2003;13:S123–31. doi: 10.1080/0960312031000102886. [DOI] [PubMed] [Google Scholar]
- 34.Guanghan H, Dandan L, Shaoji Z, Xiaojun Z, Zenghua K, Guojun C. The role of health education for schistosomiasis control in heavy endemic area of Poyang Lake region, People's Republic of China. Southeast Asian J Trop Med Public Health. 2000;31:467–72. [PubMed] [Google Scholar]
- 35.Lansdown R, Ledward A, Hall A, Issae W, Yona E, Matulu J, et al. Schistosomiasis, helminth infection and health education in Tanzania: achieving behaviour change in primary schools. Health Educ Res. 2000;17:425–33. doi: 10.1093/her/17.4.425. [DOI] [PubMed] [Google Scholar]
- 36.Hong SH, Misek DE, Wang H, Puravs E, Hinderer R, Giordano TJ, et al. Identification of a Specific Vimentin Isoform That Induces an Antibody Response in Pancreatic Cancer. Biomark Insights. 2006;1:175–83. [PMC free article] [PubMed] [Google Scholar]
- 37.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: A review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;24:2–300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry FJ. Re-infection with Ascaris lumbricoides after chemotherapy: A comparative study in three villages with varying sanitation. Trans R Soc Trop Med Hyg. 1988;82:460–4. doi: 10.1016/0035-9203(88)90162-9. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen E, Ismail M, Amarasinghe DK, Hettiarachchi I, Dassenaieke CT. The effect of the availability of latrines on soil-transmitted nematode infections in the plantation sector in SriLanka. Am J Trop Med Hyg. 1994;51:36–9. doi: 10.4269/ajtmh.1994.51.36. [DOI] [PubMed] [Google Scholar]
- 40.Henry FJ, Huttly SR, Ahmed MU, Alam A. Effect of chemotherapy in slums and villages in Bangladesh. Southeast Asian J Trop Med Public Health. 1993;24:307–12. [PubMed] [Google Scholar]
- 41.Moraes LR, Cancio JA, Cairncross S. Impact of drainage and sewerage on intestinal nematode infections in poor urban areas in Salvador, Brazil. Trans R Soc Trop Med Hyg. 2004;98:197–204. doi: 10.1016/s0035-9203(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 42.Mascarini-Serra LM, Telles CA, Prado MS, Mattos SA, Strina A, Alcantara-Neves N, et al. Reductions in the prevalence and Incidence of geohleminth infections following a city-wide sanitation program in a Brazilian Urban Centre. PLoS Negl Trop Dis. 2010;4:e-588. doi: 10.1371/journal.pntd.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradley M, Chandiwana SK, Bundy DA. The epidemiology and control of hookworm infection in the Burma Valley area of Zimbabwe. Trans R Soc Trop Med Hyg. 1993;87:145–7. doi: 10.1016/0035-9203(93)90463-z. [DOI] [PubMed] [Google Scholar]
- 44.Sow S, de Vlas SJ, Polman K, Gryseels B. Hygiene practices and contamination risks of surface waters by schistosome eggs: The case of an infested village in Northern Senegal. Bull Soc Pathol Ex. 2004;7:12–4. [PubMed] [Google Scholar]
- 45.Norman G, Pedlley S, Bahi T. Effects of sewerage on diarrhea and enteric infections: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10:536–44. doi: 10.1016/S1473-3099(10)70123-7. [DOI] [PubMed] [Google Scholar]
- 46.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: A systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 47.Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: A meta-analysis. Am J Public Health. 2008;98:1372–81. doi: 10.2105/AJPH.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.New York: 1999. Unicef. Sanitation and Hygiene; a Right for Every Child. [Google Scholar]
- 49.Asaolu SO, Ofoezie IE. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003;86:283–94. doi: 10.1016/s0001-706x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 50.Esrey SA, Potash JB, Roberts L, Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ. 1991;69:609–21. [PMC free article] [PubMed] [Google Scholar]
- 51.Joseph F, Urban J, Nina R, Gloria I, Solano-Aguilara HD, Dawsona OI, et al. Infection with parasitic nematodes confounds vaccination efficacy. Vet Parasitol. 2007;148:14–20. doi: 10.1016/j.vetpar.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol. 2006;28:515–23. doi: 10.1111/j.1365-3024.2006.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003;111:995–1000. doi: 10.1067/mai.2003.1348. [DOI] [PubMed] [Google Scholar]
- 54.Cooper PJ. The Potential impact of early exposures to geohelminth infections on the development of atopy. Clin Rev Allergy Immunol. 2006;26:5–14. doi: 10.1385/CRIAI:26:1:5. [DOI] [PubMed] [Google Scholar]
- 55.Coper PJ, Chico ME, Vaca MG, Moncayo AL, Bland JM, Mafla E, et al. Effect of albendazole treatments on the prevalence of atopy in children living in communities endemic for geohelminth parasites: a clusterrandomised trial. Lancet. 2006;367:1598–603. doi: 10.1016/S0140-6736(06)68697-2. [DOI] [PubMed] [Google Scholar]
- 56.Wilson RA. From genomes to vaccines via the proteome. Mem Inst Oswaldo Cruz. 2004;99:45–50. doi: 10.1590/s0074-02762004000900008. [DOI] [PubMed] [Google Scholar]
- 57.Strachan DP. Hay fever, hygiene and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feary J, Britton J, Leonardi-Bee J. Atopy and current intestinal parasite infection: A systematic review and meta-analysis. Allergy. 2010 doi: 10.1111/j.1398-9995.2010.02512.x. [In press] [DOI] [PubMed] [Google Scholar]
- 59.Carvalho EM, Bastos LS, Araujo MI. Worms and allergy. Parasite Immunol. 2006;28:525–34. doi: 10.1111/j.1365-3024.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim AB, Drake-Lee AB. Infection, allergy and the hygiene hypothesis: historical perspective. J Laryngol Otol. 2003;117:946–50. doi: 10.1258/002221503322683812. [DOI] [PubMed] [Google Scholar]
- 61.Brooker S, Pratap S, Waikagul J, Suvanee S, Kojima S, Takeuchi T, et al. Mapping soil-transmitted helminth infections in Southeast Asia and implications for parasite control. Southeast Asian J Trop Med Public Health. 2003;34:24–36. [PubMed] [Google Scholar]
- 62.Saathoff E, Olsen A, Sharp B, Kvalsvig JD, Appleton CC, Kleinschmidt A. Ecologic covariates of hookworm infection and reinfection in rural KwaZulu-Natal/South Africa: A geographic information system-based study. Am J Trop Med Hyg. 2005;72:384–91. [PubMed] [Google Scholar]
- 63.Kabatereine NB, Tukahebwa EM, Kazibwe F, Namwangye H, Zaramba S, Brooker S, et al. Progress towards country-wide controlof schistosomiasis and soil-transmitted helminthiasis in Uganda. Trans R Soc Trop Med Hyg. 2006;100:208–15. doi: 10.1016/j.trstmh.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Brooker S, Beasley NM, Ndinaromtan M, Madjiouroum EM, Baboguel M, Djenguinabe E, et al. Use of remote sensing and a geographical information system in a national helminth control programme in Chad. Bull World Health Organ. 2002;80:783–9. [PMC free article] [PubMed] [Google Scholar]
- 65.London, PCD: Imperial College; 2003. Partnership for Child Development. A Situation Analysis of the Health of School Children in Eritrea. [Google Scholar]
- 66.Fenwick A, Molyneux DH, Nautulya V. Achieving the millennium development goals. Lancet. 2005;365:1029–30. doi: 10.1016/S0140-6736(05)71134-X. [DOI] [PubMed] [Google Scholar]
- 67.Fiedler JL. The nepal national vitamin a Program: prototype to emulate or donor enclave? Health Policy Plan. 2000;15:145–56. doi: 10.1093/heapol/15.2.145. [DOI] [PubMed] [Google Scholar]
- 68.Geneva: WHO; 2003. [Last accessed on 2011 Jan 15]. World health organization. Action against worms; p. 2. Available from: http://www.who.int/wormcontrol/newsletter/en/ [Google Scholar]
- 69.Sinoun M, Tsuyuoka R, Socheat D, Montresor A, Palmer K. Financial costs of deworming children in all primary schools in Cambodia. Trans R Soc Trop Med Hyg. 2005;99:664–8. doi: 10.1016/j.trstmh.2004.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haller L, Hutton G, Bartram J. Estimating the costs and health benefits of water and sanitation improvements at global level. J Water Health. 2007;5:467–80. doi: 10.2166/wh.2007.008. [DOI] [PubMed] [Google Scholar]
- 71.Crompton DW, Savioli L. Intestinal parasitic infections and urbanization. Bull World Health Organ. 1993;71:1–7. [PMC free article] [PubMed] [Google Scholar]


