Graphical abstract
Highlights
▸ Children with ASD and typical peers played a game involving social exclusion. ▸ Groups reported comparable ostracism-related distress. ▸ Event-related potentials revealed distinct temporal dynamics. ▸ Children with ASD rely on atypical neural circuitry to process social exclusion.
Keywords: ERP, EEG, Autism spectrum disorder, Social exclusion, Social neuroscience
Abstract
Despite significant social difficulties, children with autism spectrum disorder (ASD) are vulnerable to the effects of social exclusion. We recorded EEG while children with ASD and typical peers played a computerized game involving peer rejection. Children with ASD reported ostracism-related distress comparable to typically developing children. Event-related potentials (ERPs) indicated a distinct pattern of temporal processing of rejection events in children with ASD. While typically developing children showed enhanced response to rejection at a late slow wave indexing emotional arousal and regulation, those with autism showed attenuation at an early component, suggesting reduced engagement of attentional resources in the aversive social context. Results emphasize the importance of studying the time course of social information processing in ASD; they suggest distinct mechanisms subserving similar overt behavior and yield insights relevant to development and implementation of targeted treatment approaches and objective measures of response to treatment.
1. Introduction
Individuals with autism spectrum disorder (ASD) experience significant deficits in social interaction, including reduced reciprocity and poor peer relationships. Social difficulties emerge early in life, exerting continued developmental effects as these individuals navigate interpersonal interactions and learn from atypical experiences. The transition from childhood to adolescence entails increased reliance on a peer network for development of adaptive social behavior (Spear, 2000). During this developmental period, children show greater sensitivity to peer rejection in evaluations of their “worthiness” or “unworthiness” as an individual (O’Brien and Bierman, 1988, Sebastian et al., 2010a). Because children with ASD are more likely than typical peers to be victimized by bullies and to experience ostracism (van Roekel et al., 2010), they are especially vulnerable during such developmental transitions.
Despite social struggles, children with higher functioning ASD demonstrate intact comprehension of loneliness in social scenarios (Bauminger et al., 2003). Even with a cognitive understanding of exclusion and experienced bullying, it has been suggested that, due to difficulties with social cognition and reduced drive for interpersonal interaction in some individuals with ASD, the distress associated with social exclusion might be reduced in this population (Chiu et al., 2008, Mundy, 2003, Dichter et al., 2009). Recently, this proposition was examined by Sebastian et al. (2009) using the virtual social exclusion paradigm, Cyberball. Cyberball elicits feelings of exclusion by making participants feel deliberately ignored by peers (Williams et al., 2000). Participants are led to believe that they are playing an online game of catch with two other players, who, in reality, are pre-programmed computer representations. The participant receives throws from both of the other players in the first phase of the game, i.e., “fair play”. In the “social rejection” phase, however, the other players cease throwing the ball to the participant, excluding him or her from play and inducing mild social distress (Williams and Jarvis, 2006). Sebastian et al. (2009) found that, in the context of the Cyberball social exclusion paradigm, children with ASD reported levels of ostracism distress comparable to typically developing peers.
Although little is known about the neural substrates of social exclusion in ASD, the brain bases of social rejection have been examined in typically developing children and adults. Studies employing the Cyberball paradigm demonstrate that the experience of social exclusion activates a brain circuit encompassing anterior cingulate cortex (ACC) and ventrolateral prefrontal cortex (VLPFC) (Eisenberger et al., 2003, Onoda et al., 2009, Onoda et al., 2010, Sebastian et al., 2010a). Associations between dorsal ACC activation and affective responses to ostracism (Burklund et al., 2007) suggest that the ACC, also involved in detecting violations of expectations (Weissman et al., 2003), reflects experienced social distress. In contrast, the right VLPFC is presumed to represent mechanisms affecting cognitive regulation of this experienced pain (Eisenberger and Lieberman, 2004).
More recently, event-related potentials have been employed to examine the time course of neural processing of rejection events during an ongoing exclusion experience in Cyberball (Crowley et al., 2009, Crowley et al., 2010). In a study of middle childhood, Crowley et al. (2010) observed a differential response to rejection at short latency; children differentiated the experience of rejection rapidly, in less than one half-second. Slow-wave activity at longer latencies revealed enhanced response to rejection events over medial frontal scalp that was significantly correlated with self-reported ostracism-related distress. Neural sources of this activity were estimated to originate in cortical regions consistent with functional imaging studies, including subgenual cingulate cortex, ventral anterior cingulate cortex and insula.
Despite similarities in self-reported distress, it is not known whether comparable or distinct neural mechanisms underlie the experience of social exclusion in children with ASD. Furthermore, children with ASD may experience distress for entirely different reasons than typical counterparts (Bolling et al., 2011). While a growing body of literature suggests that individuals with ASD process socially relevant stimuli differently at early stages of perception (McPartland et al., 2004), it is unclear whether this is true for the processing of information relevant in terms of characteristics of the social context, such as Cyberball, rather than overt visual content. To unpack neural responses to social exclusion at the level of rejection events, the current study used event-related potentials (ERPs) to investigate temporal dynamics of peer exclusion and its relation to ostracism distress in children with ASD. The temporal acuity of the current methods complement the spatial information offered by prior work using functional magnetic resonance imaging by providing information about group differences at individual stages of processing (Banaschewski and Brandeis, 2007). Based on prior work in typical development revealing distinct responses to social exclusion at both early and late electrophysiological components, putatively representing visual attentional engagement and emotional arousal, respectively, we sought to evaluate several alternative hypotheses. The first, that children with ASD are insensitive to social exclusion, would be consistent with the observation of a failure to differentiate exclusion at both short and long latencies. Second, distinct response to exclusion at shorter latencies but not longer latencies would suggest preserved sensitivity to exclusion in terms of visual attention to exclusion-related cues despite attenuate emotional response; the converse was a third possible hypothesis. Finally, given prior findings of comparable self-reported distress during social exclusion (Sebastian et al., 2009), we investigated the possibility that individuals with ASD display patterns of brain response during social exclusion that are comparable to typically developing counterparts.
2. Materials and methods
2.1. Participants
Participants included 20 individuals with ASD and 34 medically and neuropsychiatrically healthy typically developing children. Exclusionary criteria for participants with ASD included seizures, neurological disease, history of serious head injury, sensory or motor impairment that would impede completion of the study protocol, active psychiatric disorder [other than ASD; screened with the Child Symptom Inventory: Fourth Edition (Gadow and Sprafkin, 1994)], or antiseizure medication known to affect brain electrophysiology (alprazolam, clonazepam, diazepam, lorazepam, phenobarbital, or primidone). Additional exclusionary criteria for typical participants included the above plus learning/language disability or family history of ASD in first-degree relatives. From an existing pool of subjects involved in on-going research at the Yale Child Study Center, participants were selected based on having a Full Scale IQ [Differential Ability Scales: Second Edition (Elliott, 2007) or Wechsler Abbreviated Scale of Intelligence (Psychological Corporation, 1999)] in the average range or higher (Standard Score of 80 or above). All individuals with ASD had a pre-existing diagnosis, and all participants met criteria for ASD on the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994), the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and according to DSM-IV-TR (APA, 2000) criteria as evaluated by an expert clinician. Typical participants were matched to the ASD sample in terms of chronological age [Typical: Range = 8–15, M(SD) = 11.0(1.69); ASD: Range = 5–15, M(SD) = 10.2(2.94)] and Full Scale IQ (Typical: Range = 91–122, M(SD) = 108.8(8.91); ASD: Range = 80–132, M(SD) = 102.4(15.33)]. Twenty of the ASD participants and sixteen of the typically developing participants were male. The final sample of individuals with autism contained four individuals prescribed medications for attentional difficulties (guanfacine, methylphenidate, clonidine) or mood symptoms (escitalopram); waveforms of these individuals were visually inspected to confirm comparability to the broader sample. All procedures were approved by the Human Investigation Committee at Yale School of Medicine and were carried out in accordance with the Declaration of Helsinki (1975/1983). All participants were debriefed about the deception involved in the experimental paradigm at the conclusion of study participation.
2.2. EEG procedures
2.2.1. Experimental paradigm
The experimental paradigm and administration were identical to that described in Crowley et al. (2010). Participants were informed that they would play an online game with two other children located in different rooms. To increase realism and personal investment in the game, participants selected a glove from six pictured choice images of gloves prior to the game; during the game, “opponents” were displayed as photographs of other children matched for age, sex, and ethnicity and displayed above their gloves. A pre-recorded female voice provided instructions to the participants, and the instructions were also displayed on screen. The Cyberball paradigm consisted of two blocks, a fair play block (108 trials) followed by an exclusion block (47 trials). During the fair play block, the participant threw the ball to the other players in 36 trials. The virtual players threw to the participant in 36 trials, which were recorded as inclusion events, and to each other in 36 trials, which were recorded as “not my turn” events. Whether or not the virtual players included the participant on any given trial was dictated by a pseudorandom list, which ensured that the participant waited 0, 1, or 2 trials before receiving the ball once more. Prior to a throw, the glove of the player “holding” the ball became outlined in yellow to cue attention to the relevant event. After a 500 ms delay, the ball appeared alongside the glove oriented towards the other player's glove in yellow, indicating that the ball would not come to the participant, or oriented towards the participant's glove in orange, indicating that the ball would come to the participant (see Fig. 1 in Crowley et al. (2010) for schematic diagram). When it was the player's turn to throw, the same sequence of events took place, but the ball did not change color. EEG data were time-locked to this color change. Participants were not instructed in advance regarding the relationship between ball color and throw outcome. Without pause, the fair play block transitioned into the 47-trial exclusion block, during which the participant was excluded for 44 trials (the player received the ball three times to maintain engagement with the game). Thirty-six of the trials in the exclusion block were considered exclusion events for ERP analysis. The first five trials of the exclusion block, as well as the three trials in which the participant received the ball and the three throws back from the participant to the computer players were omitted from analysis due to the possibility that participants would not experience them as rejection events.
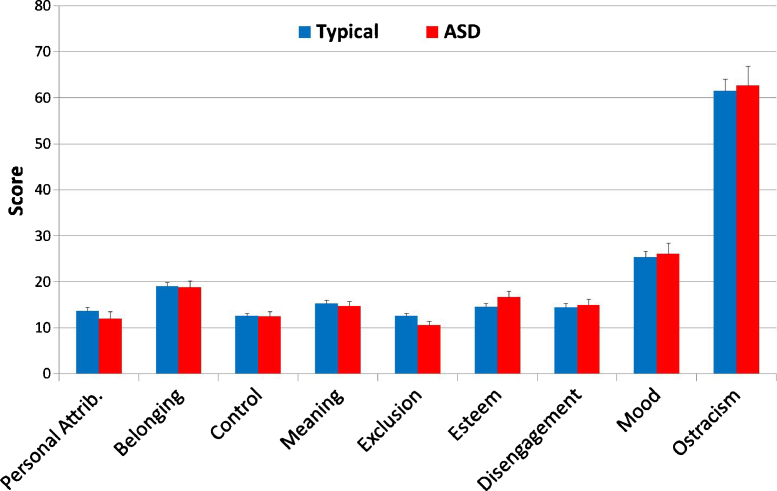
Fig. 1.
Self-reported distress subsequent to exclusion in a Cyberball game. Reported distress was comparable in children with ASD and typical peers in all domains.
2.2.2. Data collection
The paradigm was presented with E-prime 1.2 (Psychology Software Tools, Pittsburgh, Pennsylvania, USA) on a 17-in. CRT monitor from which participants sat 60 cm in a sound attenuated, dimly lit room. EEG was recorded with Netstation 4.4 software from 128 Ag/AgCl electrodes using Geodesic Sensor Nets [Electrical Geodesics, Inc. (EGI), Eugene, Oregon, USA] at 250 Hz (0.1 Hz high pass, 100 Hz low pass). Cz served as the reference point for all electrodes for recording, and impedances were kept at or below 40 kΩ.
2.2.3. Data editing and reduction
EEG data were low-pass filtered at 30 Hz prior to segmentation. Filtered data were first hand edited for channels featuring excessive noise or drift. Next, data were edited using the Net Station artifact detection routine with the following settings: bad channels (200 μV), eye movements (100 μV), and eye blinks (140 μV). The EEG for each trial was corrected for eye artifacts and participant movement (Gratton et al., 1983). Data from electrodes yielding poor signal quality on 50% of trials were removed and replaced with spherical spline interpolation. EEG data in which more than 10 channels were interpolated were excluded from analyses. Averaged data were baseline-corrected for 100 ms pre-stimulus and re-referenced to the average reference. Trial-by-trial data were subsequently averaged for each of the 128 electrodes individually for each outcome category: “my turn”, i.e., trials on which the participant received the ball; “not my turn”, i.e., trials on which the participant did not receive the ball during inclusion; and “rejection”, i.e., trials on which the participant did not receive the ball during exclusion. Participants with less than 25% (9) good trials for any condition were excluded from analyses; 6 of an initial 26 participants with ASD and 0 typically developing participants were excluded due to insufficient good data. Mean (SD) number of trials was 27.0 (7.8) for the “not-my-turn” condition and 25.7 (8.0) for the rejection condition. Based on Crowley et al. (2010), peak amplitude for an early positive component (P2; 160–280 ms) and mean amplitude for a late slow wave (LSW; 500–900 ms) were extracted in Net Station for each participant in each condition across a medial–frontal electrode cluster (Electrodes #4, 5, 6, 9, 10, 12, 14, 15, 16, 18, 19, 20, 24, 118, 124; displayed in Fig. 2). Temporal windows corresponding the components described in prior work were determined based on examination of the grand average and subsequently verified in individual subject averages.
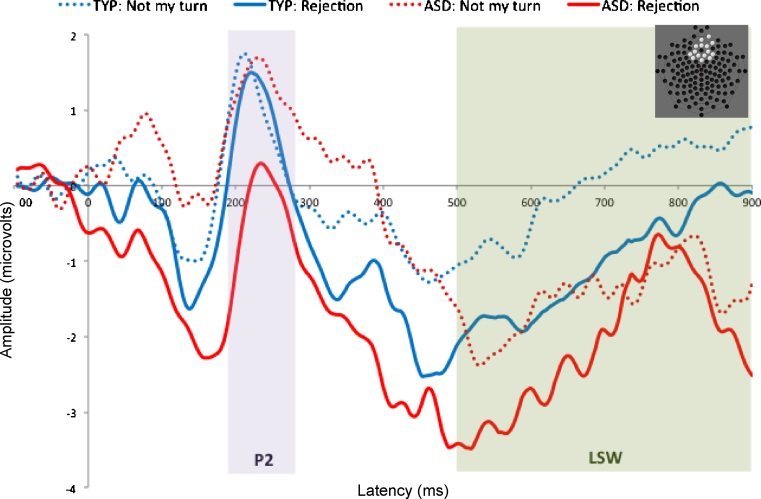
Fig. 2.
Waveforms elicited by “not my turn” and rejection events in children with ASD and typical controls. Highlighted portions of waveforms indicate P2 and LSW components.
2.3. Behavioral procedures
As per Crowley et al. (2010), immediately after the end of the paradigm participants completed a computerized version of the Need Threat Scale, a self-report measure of ostracism with robust psychometric properties (Van Beest and Williams, 2006, Sebastian et al., 2009). The Need Threat Scale gauges levels of distress according to a 5-point scale from “Not at all” to “Extremely.” It assesses dimensions of belonging (e.g., “I feel rejected”; possible range = 6–30), self-esteem (e.g., “I feel liked”; possible range = 5–25), meaningful existence (e.g., “I felt I did not matter”; possible range = 5–25), control (e.g., “I felt powerful”; possible range = 5–25), and mood (e.g., “I felt good”; possible range = 8–40). Scales assessing belonging, meaningful existence, control, and self-esteem are summed to calculate a total ostracism score (possible range = 21–105). In addition to the Need Threat Scale, we administered several novel complementary scales developed by our group (Crowley, 2010). These scales tapped personal attributions about other players (e.g., “I felt the other players were mean”; possible range = 4–20), experienced exclusion (e.g., “I wondered why aren't they throwing to me?”; possible range = 4–20), disengagement from the task (e.g., “I thought, ‘This game is dumb”’; possible range = 5–25). Behavioral scale scores were calculated such that lower scores were negatively valenced and higher scores were positively valenced (maximum total score = 220, minimum total score = 44).
3. Results and discussion
3.1. Ostracism-related distress
Self-report questionnaire data were analyzed with independent samples t-tests for each questionnaire subscale and for total score. No significant differences were detected between groups (all ps > .05), indicating that children with ASD and their typical counterparts reported comparable ostracism-related distress across domains assessing interpersonal attributions, belonging, control, meaningful existence, preoccupation with exclusion, self-esteem, disengagement from task, and mood (see Fig. 1).
3.2. ERP amplitude
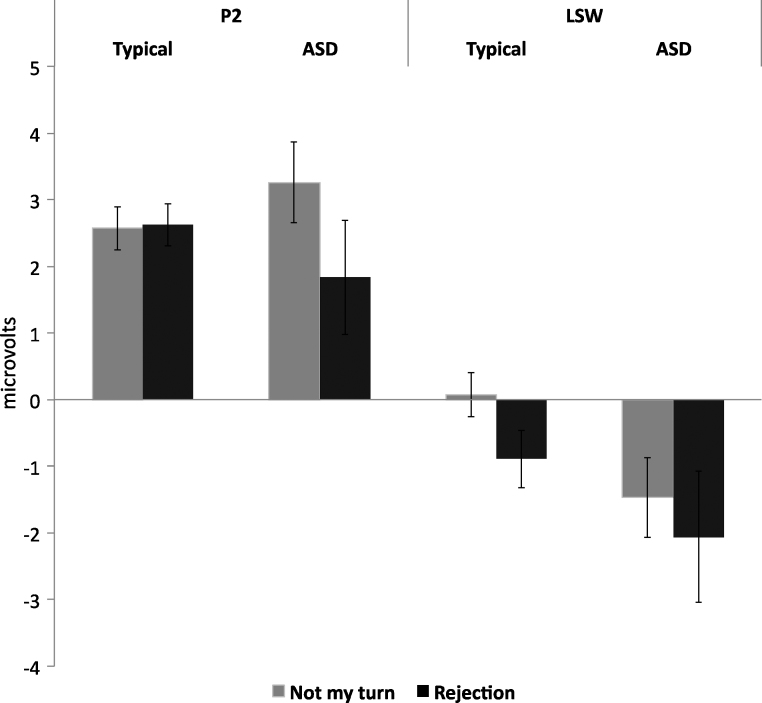
Waveforms for “not my turn” and rejection conditions are displayed in Fig. 2 with time windows encompassing the P2 and the LSW highlighted; mean amplitudes for each group and for each condition are displayed in Fig. 3. To confirm replication of prior findings in typically developing participants, we first contrasted the LSW elicited by rejection events with that evoked by “not my turn” events. For this and subsequent ANOVAs, effect size is presented as partial eta-squared , where .01 represents a small effect size, .06 represents a medium effect size, and .14 represents a large effect size (Kittler et al., 2007). Consistent with Crowley et al. (2010), in the typically developing sample, amplitude of the LSW was significantly more negative to rejection events [F(1,33) = 7.44, p = .01, ]; this effect was not evident in children with ASD [F(1,19) = 0.24, p = .63, ]. We next examined between-group differences with separate univariate repeated measures ANOVAs for P2 and LSW amplitude with condition (“not my turn”/rejection) as a within-subjects factor and group (ASD/TD) as a between-subjects factor. A main effect of condition [F(1,52) = 4.55, p = .04, ] indicated that, irrespective of group, P2 amplitude was larger to “not my turn” events than rejection events. Analysis of P2 amplitude revealed a condition by group interaction at the P2 [F(1,52) = 4.30, p = .04, ]; children with ASD responded comparably to typically developing children on “not my turn” trials but displayed an attenuated peak amplitude in the early positivity elicited by rejection. A main effect of group at the LSW [F(1,52) = 4.769, p = .03, ] indicated that, irrespective of condition, typically developing children showed greater amplitude. We conducted follow-up analyses to examine potential relationships between age and IQ and ERP parameters in the sample. Correlations between age, IQ, and P2 and LSW amplitude were not significant (all ps > .10).
Fig. 3.
Mean ERP amplitude at P2 and LSW for children with ASD and typical counterparts for “not my turn” and rejection conditions.
3.3. ERPs and reported distress
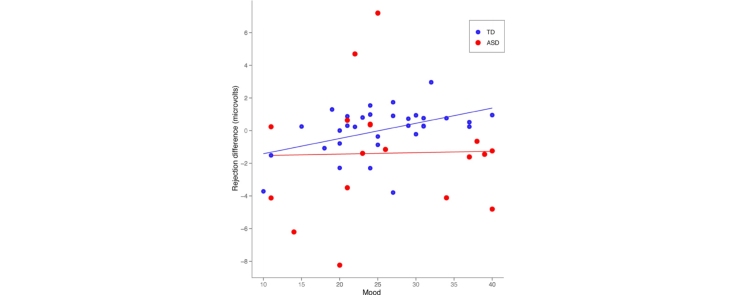
Pearson product-moment correlations were applied to investigate the relationship between ERP amplitude and the total ostracism and mood subscales of the self-report questionnaire. To control for individual differences in overall ERP amplitude, correlations were computed using the difference in amplitude between “not my turn” and rejection events for each ERP component (“not my turn” was subtracted from rejection). For the typical group, difference in P2 amplitude associated with rejection was significantly and positively correlated (medium to large effect size) with the mood subscale [r = .45, p = .01, medium to large effect size], such that greater amplitude to rejection versus “not my turn’ was associated with higher scores on the mood subscale (see Fig. 4). Also in the typical group, at the LSW, the difference between rejection and “not my turn” events was significantly and positively correlated with the mood subscale score [r = .50, p < .01, large effect size] and total ostracism score [r = .36, p = .04, medium effect size]. Children with ASD did not display significant correlations between ERP responses to rejection versus “not my turn” for either mood or total ostracism score; to explore whether other subscales might be capturing the experience of children with ASD, we examined correlations among individual subscales, despite a lack of a priori hypotheses. There were no significant correlations detected for any scores.
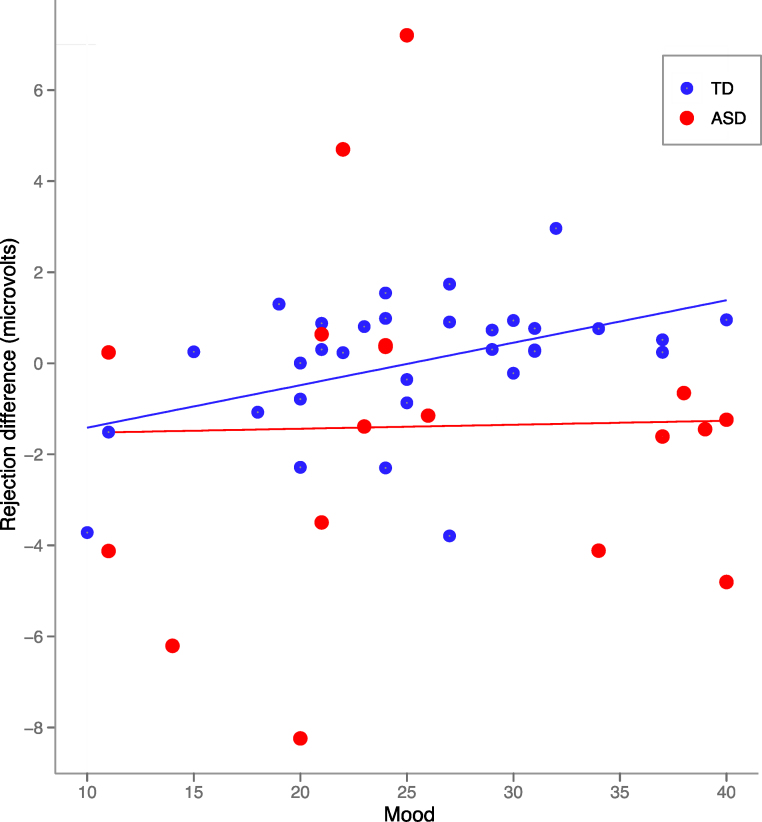
Fig. 4.
Relationship between mood and differential P2 amplitude elicited by rejection and “not my turn” events. Typically developing children displayed a positive correlation between differential amplitude and mood; children with ASD showed no relationship.
4. Discussion
We employed an ERP version of the Cyberball paradigm to measure brain activity in children with ASD during simulated social interactions. These are the first data to explore the temporal dynamics of brain activity associated with social exclusion in ASD, revealing a dissociation between reported distress and neural response in children with ASD and a difference in the temporal course of brain responses between children with ASD and typical peers. Both groups reported similar levels of distress in response to social exclusion. These results are generally consistent with those obtained in a prior study of ASD that examined self-reported experience alone (Sebastian et al., 2009); however, we did not observe the slight decrease in mood evident solely in typical individuals in the prior study. Because we did not administer questionnaires prior to the Cyberball game, we cannot determine whether the comparable mood results between groups in the present study reflect a difference at baseline. Alternatively, modifications to the paradigm to enhance realism (i.e., matching players to participants’ ethnicity, gender, and age, using digitized photos and names, choosing a personal glove for play) may have increased investment on the part of participants with ASD, influencing self-reported mood. To examine this possibility, we created a standardized metric to compare scores by dividing mood scores for each group in each study (Current TD = 25.44, Current ASD = 26.11; Prior TD 4.8, Prior ASD 5.4) by the maximum possible score on the mood scale (Current study = 40; Prior study = 7), essentially creating a score representing proportion of the possible maximum. According to this metric, the typical group mood score was similar in the current study (.64) to the prior study (.69), while the ASD group's mood score was lower in the current study (.65) than in the prior study (.77). The differential impact on mood in the present study suggests that our paradigm's visual realism and “in-group” matching may be critical factors in the study of mood modulation by social exclusion. Future research on social cognition in ASD would benefit from specific investigation of such factors.
Despite similarity in self-reported experience, ERPs revealed distinct patterns of neural activity underlying reactions to rejection events during social exclusion for each of the groups. Consistent with earlier work from our group (Crowley et al., 2010), the neural response of typically developing children was characterized by enhanced negativity, evident in a late slow wave over medial–frontal scalp electrodes, to rejection events versus “not my turn” events. Also in keeping with prior findings, differential amplitude associated with rejection events correlated with self-reported effects on mood and overall ostracism distress. Children with ASD showed a fundamentally distinct pattern of results. The late slow wave did not differentiate rejection from “not my turn” in the ASD group, nor were correlations observed among neural response and the reported experience of distress. In contrast to their typical peers, the children with ASD showed differentiation of rejection at an earlier frontal P2 component, although this response also did not associate with their reported experience of rejection. The absence of differential response to exclusion versus “not my turn” at the LSW suggests that children with ASD might be failing to make critical distinctions based on social context (i.e., the same outcome has a different connotation depending on the context in which it occurs, “not my turn” versus exclusion) at these late processing stages.
Results suggest that children with ASD are processing the experience of rejection differently than typical children. The temporal course of the early positivity (P2) indicates a role in more basic cognitive processing, such as visual attention. The frontal P2 usually appears in visual tasks and is related to selective visual attention (Key et al., 2005). We posit that reduced amplitude at the P2 for rejection events suggests attenuated engagement of attentional resources during the experience of exclusion in individuals with ASD. This may reflects the more dramatic impact of social exclusion on an already weakened social motivational system (Dawson et al., 2005), i.e., an accelerated acquisition of a sense of learned helplessness. It is understood that children with ASD display reduced orienting to social cues (Dawson et al., 2004); these results suggest this failure to discriminate meaningful social information is also evident when social meaning is conveyed by context alone. Furthermore, the selective impact in the context of exclusion implies this vulnerability is exacerbated by negative social experiences; the social attentional mechanisms of children with ASD may be most likely to dysfunction in the very contexts in which they are most vital. In contrast, typically developing children demonstrated preserved attentional engagement during rejection. Given the positive relationship between P2 amplitude during rejection and mood, this appeared to represent a protective factor. We propose that, in this paradigm, P2 represents adaptive engagement of attentional mechanisms to decipher cues relevant to determining social context and initiating the emotional processing indexed by subsequent components. The current results emphasize the importance of temporal dynamics in revealing processing strategies in typical and atypical development. In this instance, our measures of brain function revealed important group differences undetectable with behavioral methods alone. The current work highlights the need to understand the interplay of the forces of social drive and discouragement in ASD. Although it is often presumed that children with ASD possess reduced drive for social interaction (Dawson et al., 2005), it has long been acknowledged that some children with ASD posses preserved social drive despite insufficient social agility to successfully navigate social interactions (Wing and Gould, 1979). Current results emphasize that, rather than being invulnerable to social exclusion, children with ASD are also at risk for emotional and psychological consequences of ostracism. Our future work aims to examine the influence of social motivation on the experience of exclusion and to understand development of social motivation in the context of learning from aversive social experiences.
These findings are relevant to understanding emotion regulation in cognitive-behavioral therapy for children with ASD. Our results suggest under-responsiveness associated with brain mechanisms subserving regulation of emotional responses. In our study, children with ASD failed to display differentiation of rejection in the portion of their brain response hypothesized to reflect emotion regulation mechanisms, potentially reflecting ACC activity. Such late components have been posited to reflect facilitated attention to emotional stimuli (Cuthbert et al., 2000, Schupp et al., 2000) and emotional arousal (Schupp et al., 2000, Hajcak and Dennis, 2009, Hajcak et al., 2010) and have been shown to be reduced under conditions of voluntary reappraisal of negative emotion (Moser et al., 2006). In considering therapeutic approaches, these findings suggest the potential applicability of interventions addressing both cognitive reappraisal (corresponding to the functions indexed by the P2) and physiological down-regulation (corresponding to the LSW). For example, children with ASD might be taught strategies to reframe exclusion events (e.g., instead of thinking “They will never throw to me”, thinking “I am sure they will throw to me soon” or “I have other friends if they won't play”). Emotional arousal might be directly addressed through strategies aimed at physiological responses, such as progressive relaxation or taking deep breaths. Work in progress is applying the current experimental paradigm as a tool to apply such targeted treatment approaches and provide an objective indicator of response to treatment. We predict that cognitive reappraisal techniques would differentially target early activity and that more physiologically oriented methods would be reflected in reduced emotional arousal as indexed by the later component.
A limitation of the current study is its reliance on self-report for the measurement of emotional experience during social exclusion. A fundamental weakness of this methodology is that it inextricably relies on personal estimation of the magnitude of experience and presumes comparability of this metric between individuals. A problem with this assumption is that one individuals’ rating on a Likert scale does not necessarily reflect the same experience in another person; in other words, the ends of the scale may fall at different points in an experiential continuum for different individuals (Bartoshuk et al., 2005). This difficulty may be circumvented by assessing direct, objective measures of emotional arousal, such as electrodermal response or startle, as has been used in other social rejection studies (Downey et al., 2004). The use of self-report measures is especially problematic in ASD, given possible difficulties with introspection. A second limitation of this study is that, though we speculate that children with ASD experience distress for different reasons, our behavioral questionnaire solely focused on distress associated with the social elements of the task. Given the absence of correlations between ERP measures and self-reported distress in the ASD group, it is possible that a distinct constellation of psychological contributors to distress may be at play in children with ASD. Although there are a number of factors other than social exclusion that could have influenced our results, such as differential rates of learning about probabilistic experiences, we speculate that the different response in ASD may reflect, in part, response to perceived violation of an implicit rule (Bolling et al., 2011). These possibilities speak not only to experience during the course of the experiment but to broader social experience in ASD. The comments offered by participants during the exclusion portion of the experiment (e.g., “You're annoying guys”, “I wish I could talk to them”, “I hate being left out”) suggest that irrespective of the cognitive source of ostracism-related distress (e.g., social exclusion, per se, deviation from equivalent probability of outcome, or rule violation), the encounter was subjectively experienced in social terms. A third limitation of the current study is that because our sample included a limited number of individuals prescribed psychoactive medication, we lacked adequate statistical power to determine differential response to social exclusion in children receiving pharmacotherapy. This is a critical area for future research, as many children on the spectrum are prescribed medications specifically to address mood symptoms that might be affected by and might influence the experience of social exclusion. Finally, as is the case for most prior studies using the Cyberball paradigm, we cannot rule out the possibility that order of block administration may have influenced our results (discussed by Sebastian et al., 2010b). Future research could avert this confound by administering multiple blocks of exclusion, including a control group receiving two blocks of the same type, or by adding a throw type unrelated to exclusion that occurs in both blocks.
We see these findings as valuable, proximally, in terms of specifying the temporal dynamics involved in the neural mechanisms of perceiving and regulating emotional responses to social exclusion in ASD. In the longer term, an understanding of these mechanisms will be necessary for the development and implementation of therapies targeted to specific processes. For example, in the context of exclusion, cognitive-behavioral strategies might differentially address cognitive reappraisal of the perceived slight versus down-regulation of one's emotional response to the experience; understanding brain function at each stage of this process is a needed step in advancing such interventions for children with ASD. More broadly, from a social neuroscience perspective, because brain regions activated during Cyberball (e.g., ACC, VLPFC, insula) are also implicated in the neuropathology of ASD (Barnea-Goraly et al., 2004, Di Martino et al., 2009), examination of their functional integrity is of direct relevance to understanding the neuropathology of ASD and in meaningfully defining subgroups within a heterogeneous phenotype.
Acknowledgements
This work was supported by NIMH K23MH086785 (JM), NARSAD Young Investigator Awards (JM, MC), the NIH 2T32MH018268-26 (AN) Bial Foundation (MC), NIMH MH071284 (KP), NIDA K05DA020091 (LCM), and CTSA Grant number UL1 RR024139 (JM, LCM) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research (USA). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. [Google Scholar]
- Banaschewski T., Brandeis D. Annotation: what electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. J. Child Psychol. Psychiatry. 2007;48(5):415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Kwon H., Menon V., Eliez S., Lotspeich L., Reiss A.L. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol. Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Bartoshuk L., Fast K., Snyder D. Differences in our sensory worlds: invalid comparisons with labeled scales. Curr. Dir. Psychol. Sci. 2005;14:122–125. [Google Scholar]
- Bauminger N., Schulman C., Agam G. Peer interaction and loneliness in high-functioning children with autism. J. Autism Dev. Disord. 2003;33(5):489–507. doi: 10.1023/a:1025827427901. [DOI] [PubMed] [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., McPartland J.C., Mayes L.C., Pelphrey K.A. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund L.J., Eisenberger N.I., Lieberman M.D. The face of rejection: rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Soc. Neurosci. 2007;2:238–253. doi: 10.1080/17470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P.H., Kayali M.A., Kishida K.T., Tomlin D., Klinger L.G., Klinger M.R., Montague P.R. Self-responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57:463–473. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M.J. Yale Child Study Center; 2010. Scales for assessing personal attributions, preoccupation with exclusion and task disengagement in Cyberball. [Google Scholar]
- Crowley M.J., Wu J., Molfese P.J., Mayes L. Social exclusion in middle childhood: rejection events, slow-wave neural activity, and ostracism distress. Soc. Neurosci. 2010;5:483–495. doi: 10.1080/17470919.2010.500169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M.J., Wu J., McCarty E.R., David D.H., Bailey C.A., Mayes L.C. Exclusion and micro-rejection: ERP response predicts mitigated distress. NeuroReport. 2009;20:1518–1522. doi: 10.1097/WNR.0b013e328330377a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dawson G., Toth K., Abbott R., Osterling J., Munson J., Estes A., Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev. Psychol. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G., Webb S.J., McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Felder J.N., Bodfish J.W. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. SCAN. 2009;4:215–226. doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Ross K., Uddin L.Q., Sklar A.B., Castellanos F.X., Milham M.P. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol. Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G., Mougios V., Ayduk O., London B.E., Shoda Y. Rejection sensitivity and the defensive motivational system: insights from the startle response to rejection cues. Psychol. Sci. 2004;15:668–673. doi: 10.1111/j.0956-7976.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn. Sci. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Elliott C. 2nd ed. Harcourt Assessment; San Antonio, TX: 2007. The Differential Ability Scales. [Google Scholar]
- Gadow K., Sprafkin J. Checkmate Plus; Stony Brook, NY: 1994. Child Symptom Inventories Manual. [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Dennis T.A. Brain potentials during affective processing in children. Biol. Psychol. 2009;80(3):333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., MacNamara A., Olvet D.M. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev. Neuropsychol. 2010;35(2):129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Key A.P., Dove G.O., Maguire M.J. Linking brainwaves to the brain: an ERP primer. Dev. Neuropsychol. 2005;27:183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Kittler J.E., Menard W., Phillips K. Weight concerns in individuals with body dysmorphic disorder. Eat. Behav. 2007;8(1):115–120. doi: 10.1016/j.eatbeh.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Leventhal B., DiLavore P., Pickles A., Rutter M. The Autism Diagnostic Observation Schedule – Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- McPartland J., Dawson G., Webb S.J., Panagiotides H., Carver L.J. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J. Child Psychol. Psychiatry. 2004;45(7):1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Hajcak G., Bukay E., Simons R.F. Intentional modulation of emotional responding to unpleasantpictures: an ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Mundy P. Annotation: the neural basis of social impairments in autism: the role of the dorsal medial–frontal and anterior cingulate system. J. Child Psychol. Psychiatry. 2003;44(6):793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- O’Brien S.F., Bierman K.L. Conceptions and perceived influence of peer groups: interviews with preadolescents and adolescents. Child Dev. 1988;59(5):1360–1365. doi: 10.1111/j.1467-8624.1988.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Onoda K., Okamoto Y., Nakashima K., Nittono H., Ura M., Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc. Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Onoda K., Okamoto Y., Nakashima K., Nittono H., Yoshimura S., Yamawaki S., Yamaguchi S., Ura M. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. SCAN. 2010;5(4):385–391. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation . Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. [Google Scholar]
- Schupp H.T., Cuthbert B.N., Bradley M.M., Cacioppo J.T., Ito T., Lang P.J. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sebastian C., Blakemore S.J., Charman T. Reactions to ostracism in adolescents with autism spectrum conditions. J. Autism Dev. Discord. 2009;39:1122–1130. doi: 10.1007/s10803-009-0725-4. [DOI] [PubMed] [Google Scholar]
- Sebastian, C.L., Tan, G.C.Y., Roiser, J.P., Viding, E., Dumontheil, I., Blakemore, S.J., 2010a. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. NeuroImage, doi:10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed]
- Sebastian C., Viding E., Williams K.D., Blakemore S.J. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Van Beest I., Williams K.D. When inclusion costs and ostracism pays, ostracism still hurts. J. Pers. Soc. Psychol. 2006;91(5):918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- van Roekel E., Scholte R., Didden D. Bullying among adolescents with autism spectrum disorders: prevalence and perception. J. Autism Dev. Disord. 2010;40(1):63–73. doi: 10.1007/s10803-009-0832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D.H., Giesbrecht B., Song A.W., Mangun G.R., Woldroff M.G. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. NeuroImage. 2003;19:1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Williams K.D., Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav. Res. Methods. 2006;38(1):174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- Williams K.D., Cheung C.K.T., Choi W. Cyberostracism: effects of being ignored over the Internet. J. Pers. Soc. Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Wing L., Gould J. Severe impairments of social interaction and associated abnormalities. J. Autism Dev. Disord. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]