Abstract
Congenital heart malformations remain the leading cause of death related to birth defects. Recent advances in developmental and regenerative cardiology have shed light on a mechanistic understanding of heart development that is controlled by a transcriptional network of genetic and epigenetic factors. This article reviews the roles of chromatin remodelling factors important for cardiac development with the current knowledge of cardiac morphogenesis, regeneration, and direct cardiac differentiation. In the last 5 years, critical roles of epigenetic factors have been revealed in the cardiac research field.
Keywords: Epigenetics, Chromatin remodelling factors, Baf60c, Cardiac transcription factors, Cardiac cell fate, Cardiac reprogramming
1. Introduction: cardiac morphogenesis and diseases
The heart is the first functional organ to form in developing embryos, and cardiogenesis takes place in a highly conserved manner from insects to vertebrates. After the formation of three germ layers (ectoderm, endoderm, and mesoderm), pre-cardiac mesodermal cells arise bilaterally from the nascent mesoderm. These cells migrate into the midline and differentiate, giving rise to the contractile heart.
In mammals and birds, the bilateral cardiogenenic mesodermal cells migrate and merge at the anterior midline to generate the cardiac crescent, a crescent-shaped heart-forming region at the cranial border of the embryonic disc.1–3 Upon folding of the embryonic disc, the cardiac crescent positions towards a developing neck area of the embryo and the edges of the cardiac crescent migrate to fuse and form the primitive heart tube.4 The resulting heart tube undergoes the process of looping and chamber formation, accompanied by the activation of specific cardiac gene expression programmes required for the differentiation and maturation of pre-cardiac cells to generate the myocardium of the atrial and ventricular chambers, as well as the inflow tract, atrioventricular canal, and outflow tract.5,6
Two distinct pools of pre-cardiac fields were identified, both of which contribute to heart formation.7 The first heart field (FHF) cells are derived from the cardiac crescent and give rise to the formation of the left ventricle and a part of the atria.8 Cells from the second heart field (SHF), described by Kelly et al.,9 are located in the pharyngeal mesoderm dorsal to the heart tube, giving rise to the outflow tract and right ventricle myocardium at the arterial pole of the heart.9–11
The induction, expansion, and differentiation of pre-cardiac cells are controlled by various signalling molecules, including bone morphogenetic proteins (BMPs),12 fibroblast growth factors (FGFs),8 and wingless-related MMTV integration site (Wnt) proteins.13 BMP and FGF signals are important for the induction and differentiation of cardiogenesis.12,14,15 BMPs play a key role in the specification of FHF cells by activating the expression of cardiac transcription factors such as Nkx2–5, Gata4, and Tbx5.16,17 SHF progenitors, on the other hand, require Wnt/β-catenin signalling for their proper development.13,18,19 Wnt/β-catenin signals positively regulate the expansion of SHF progenitors and affect the expression of Islet1 (Isl1), a marker for multi-potent cardiac progenitor cells (CPCs).18,20,21
The mammalian heart consists of various cell types, including atrial and ventricular cardiomyocytes, fibroblasts, endocardial cells, epicardial cells, cells from the conduction system (sinoatrial node, atrioventricular node, purkinje fibers), smooth muscle cells making up the aorta and (coronary) arteries, and cells from the autonomous nervous system. Formation of the functional heart requires proper development of these cardiac cells through tight transcriptional regulation of cardiac genes. The fact that congenital cardiac anomalies occur at a high frequency [∼1–2% of human live births suffer from a form of congenital heart defects (CHDs)] and mutations in numerous transcription factors can cause CHDs indicates the complexity of cardiac development.22–24 At the epigenetic level, transcription factors are regulated by the assembly of DNA in higher-order chromatin structures (Figure 1). In this review, we will focus on epigenetic chromatin remodelling factors that are important for cardiac development, and discuss how these factors can be exploited to regulate the directed differentiation of non-cardiac cells towards fully functional cardiomyocytes in the search for new therapies against human CHDs.
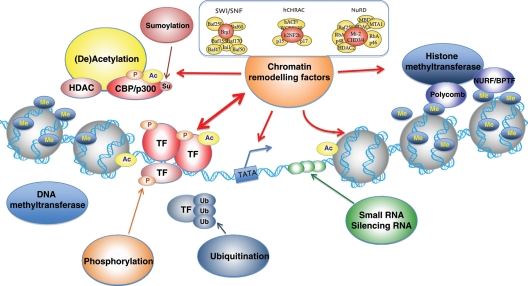
Figure 1.
Chromatin structure and remodelling factors. DNA is organized in chromatin composed of condensed nucleosomes: units of 146 bp of DNA wrapped twice around an octamer of two copies of each histone protein H2A, H2B, H3, and H4. The flexible amino-terminal tails of the histones, protruding outward from the nucleosome, allow for post-translational modifications through (de)acethylation, phosporylation, ubiquitination, methylation, and sumoylation. Such covalent modifications alter DNA–histone interactions, affecting accessibility of transcription factors.
2. Epigenetic factors and their roles in cardiac development
2.1. Epigenetic regulation: chromatin remodelling and DNA methylation
Eukaryotic development requires epigenetic mechanisms to control gene transcription for cell specification and differentiation. Chromatin remodelling is one of the essential epigenetic mechanisms for gene regulation (Figure 1). Chromatin is a multifaceted complex that serves to efficiently pack the large amount of DNA in the 5 μm cell nucleus and to regulate gene transcription.25–27 It consists of nucleosomes that are formed by wrapping of DNA around a core of histones.28 Condensation of nucleosomes enables the packing of all the genomic molecules into the relatively small nucleus.26 This compact, higher-order organization of chromatin requires regulatory mechanisms to allow the access of transcription factors to the DNA.29–32 The chromatin state often determines gene activation and repression. ‘Open chromatin’ (euchromatin) refers to a lightly packed form of DNA that allows active gene transcription, whereas ‘closed chromatin’ (heterochromatin) is a tightly packed form of DNA in which transcription is repressed.28,33,34
The state of chromatin structure can be regulated by ATP-dependent chromatin remodelling complexes or modifications of histone tails.32,35 The ATP-dependent chromatin remodelling complexes use the energy of ATP hydrolysis to modify chromatin structure. They can be classified into the complexes of SWI/SNF, ISWI, nucleosome remodelling and deacetylase complex (NuRD), and INO80 on the basis of their catalytic ATPase subunits.31,35–38 Modification of histone tails is often enzymatically reversible39–41 and results in an alteration of the interaction between chromatin and DNA. These modifications include acetylation,42 methylation,43 phosphorylation,44 sumoylation,45 and ubiquitination.46
Another epigenetic mechanism that regulates gene transcription, besides histone modification, is DNA methylation.47,48 DNA methylation typically occurs at CpG sites that contain cytosine-guanine nucleotides in a linear sequence. CpG-rich islands, short stretches of DNA with a relatively high frequency of CpG sites,48 are often found at promoters of mammalian genes, and the extent of methylation at these sites is well correlated with the transcription status of corresponding genes. DNA methylation functions to stably silence gene transcription.47
2.2. Chromatin remodellers for cardiac development and CHDs
2.2.1. Brg1/Brm-associated factor complex
The Brg1/Brm-associated factor (BAF) chromatin remodelling complex is the mammalian SWI/SNF complex composed of at least 11 subunits, and their variable arrangements contribute to distinct functions during development.31,49
The ATPase subunit of the BAF complex is encoded either by homologous genes Brg1 (Brahma-related gene 1) or Brm, but Brg1 is the indispensable ATPase of the BAF complex.31,49,50 Brg1 acts in the BAF complex to increase promoter accessibility for transcription factors, but it can also directly bind to transcription factors such as Gata proteins to regulate gene transcription.51 Mice heterozygous for Brg1 deletion exhibit cardiac morphogenetic defects, suggesting haploinsufficiency of Brg1 in heart development.52 It turns out that the proper dosage of Brg1 is critical for normal heart development, as the disruption of the balance between Brg1 and CHD-causing cardiac transcription factors such as Tbx5, Tbx20, and Nkx2–5 leads to severe cardiac anomalies.52 In mouse embryos, Brg1 activates β-myosin heavy chain (β-MHC, expressed primarily in foetal myocytes) while repressing α-MHC expressed in adult myocytes.53 Although silenced in adult mice, Brg1 is reactivated upon cardiac stress in adult myocytes and induces an α-MHC to β-MHC shift, suggesting its role in maintaining myocytes in an embryonic state.53,54
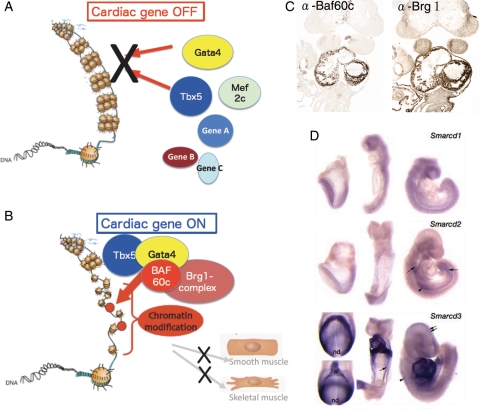
Baf60c is the cardiac-specific subunit of the BAF complex during early development and required for the ectopic induction of cardiomyocyte differentiation in combination with Gata4 and Tbx5.55 (Figure 2, Figure 3A and B). Baf60c is encoded by the gene Smarcd3, whose mRNA is initially restricted to the developing heart from mouse embryonic day (E) 7.5.50,56 Its subfamily members, Smarcd1 and Smarcd2, which encode Baf60a and Baf60b, respectively, are not expressed in the developing heart at these stages, indicating the tissue specificity of Baf60c in embryonic development (Figure 3C and D).50,57 Baf60c cooperates with Tbx5 to initiate their target gene activation for FHF formation.55,58,59 Baf60c deficiency leads to outflow tract shortening, hypoplastic right ventricles and atria, and lack of atrioventricular canal.56,57 Baf60a plays a role in linking the glucocorticoid receptor to the BAF complex, and is involved in c-fos/c-jun-mediated transcriptional activity. The precise role of Baf60b is unclear, but it is specifically ubiquitinated by Unkempt, a RING finger protein partner of Rac GTPase. Although the significance of this ubiquitination is not completely understood, it is thought to be involved in maintaining the stoichiometry of the SWI/SNF complex.
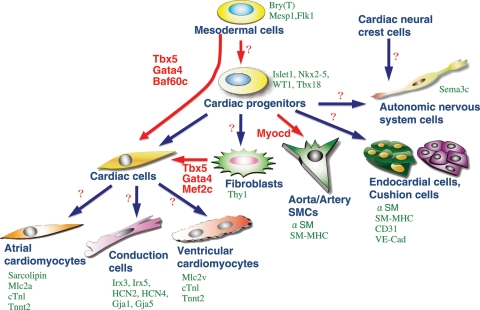
Figure 2.
Cardiac cell types derived from multipotent progenitors. Differentiated cardiac cell types are marked by indicated genes (green). Recently, several factors have been defined as master regulators for cardiomyogenesis (red). The combination of Tbx5, Gata4, and Baf60c induces direct differentiation of mesodermal cells into ectopic beating myocytes, bypassing the cardiac progenitor state.55 Tbx5, Gata4, and Mef2c together can also induce cardiomyocytes from fibroblasts.91 Factors for direct induction of other cardiac cell types are currently unknown (question marks).
Figure 3.
Chromatin remodelling factor-mediated regulation of cardiac transcription factors. (A) In the absence of Baf60c, the cardiac transcription factors Gata4, Mef2c, and Tbx5 may not have access to their target genes. The highly condensed euchromatin in which the DNA is tightly wrapped around histones makes transcription factors inaccessible to regulatory DNA, thereby represses transcription. (B) Chromatin remodelling factors modify chromatin organization by unwinding DNA from the histones, making target sequences accessible for transcription factors. Baf60c acts as a bridge to bring the Brg1 complex together with the transcription factors Tbx5 and Gata4 in a tissue-specific manner. (C) Heart-restricted expression of Baf60c and Brg1 shown by immunostaining. (D) Expression of Smarcd3 (encoding Baf60c) restricted to cardiogenic mesoderm and the developing heart shown by in situ hybridization (adapted from Lickert et al.56).
Polybromo (BAF180), the prominent subunit of the BAF-related PBAF complex, is also involved in cardiogenesis by potentiating transcriptional activation mediated by nuclear receptors, such as RXRα, VDR, and PPARγ.60 Deletion of Baf180 does not lead to early embryonic lethality, but, similar to RXRα deletion, results in a very thin cardiac wall with diminished trabeculae.61 BAF180 is expressed in the epicardium and holds a non-redundant function to that of Baf60c in the respect that it mediates late aspects of cardiac chamber maturation and coronary development.60 Ablating other subunits of the BAF complex (Baf47, Baf155, or Baf250) cause embryonic lethality at pre-implantation (Baf47, 155) or E6.5 (Baf250) in mice, indicating an essential role of the BAF complex for early embryonic development.31,62,63
2.2.2. NuRD and histone deacetylase complex
The NuRD complex contains histone deacetylases that function as transcriptional repressors.64 Similar to the BAF complex, the NuRD complex exhibits diverse functions as a result of variable assemblies. Their ATPase activity resides in the two Mi-2 proteins, CHD3 and/or CHD4.65 NuRD complexes mediate gene repression and regulate cell patterning and differentiation during early development.66,67 The NuRD complex associates with Whsc1 (Wolf–Hirschhorn syndrome candidate 1) methyltransferase68 and interacts with the Spalt-family zinc-finger transcription factor Sall4,69 which is involved in inter-ventricular septum development,70 suggesting that the complex may play a role in heart development.
2.2.3. Histone methyltransferase
Whsc1 is a histone methyltransferase that regulates activation of Nkx2–5, a homeobox protein critical for cardiac morphogenesis.68 Whsc1 physically associates with Nkx2–5 and is required for the negative regulation of Nkx2–5 and its target genes, possibly through histone H3 trimethylation at lysine 36 H3K6me3.68 Similar to Nkx2–5 mutations, Whsc1 mutations cause CHD, including atrial and ventricular septum defects in mice and human.68,71
Smyd1 (SET and MYND domain 1), a member of the lysine methyltransferase family, is specifically expressed in muscle tissue and acts as a transcriptional repressor by catalysing histone methylation through the SET domain.72,73 Smyd1-deleted mouse embryos exhibit severe cardiac defects, including loss of the right ventricle with disrupted cardiomyocyte maturation.72 In zebrafish, Smyd1 is essential for cardiac muscle contraction and myofiber maturation,74 suggesting a conserved role of Smyd1 for cardiac development.
2.2.4. High mobility group chromatin protein
The high mobility group (HMG) of nuclear proteins exerts its function by architectural remodelling of the chromatin structure and by forming multi-protein complexes with promoter/enhancer sites, leading to transcriptional activation of their target genes.75 The cardiac HMG member, HMGA2, was shown to play important roles for cardiac differentiation in vitro and in vivo. Overexpression or siRNA-mediated knockdown of HMGA2 enhances or blocks cardiomyocyte differentiation in vitro, respectively. In Xenopus embryos, normal heart formation is blocked upon morpholino-mediated knockdown of HMGA2.75 The fact that ‘HMGA2 is abundantly expressed during embryogenesis, whereas its expression is almost undetectable in adult tissues' further indicates its role for embryonic heart development.75 Furthermore, Nkx2–5 appears to be a target of HMGA2; in the presence of BMP, HMGA2 forms a protein complex with Smad1/4 and synergistically up-regulates promoter activity of Nkx2–5 in the presence of BMP stimulation through Smad- and HMGA2-binding elements. Moreover, promoter activity of Nkx2–5 requires a conserved HMGA2-binding site.75
3. Cell fate specification and epigenetic signalling
3.1. Transcription factors with instructive roles for cardiac differentiation
Cardiac transcription factors play critical roles in the early processes of cardiac cell specification and lineage determination. A number of gain-of-function experiments have been carried out to identify factors to induce cardiac differentiation. For instance, the ectopic overexpression of Gata5, a zinc-finger transcription factor essential for proper heart and endoderm development, induces the expression of several cardiac genes (Nkx2–5, Gata4, Gata6) in zebrafish.76 Gata4 possesses a similar potential in Xenopus.77 However, the observed ectopic heart tissues appear to be formed as a secondary effect, as the overexpression of Gata genes causes additional axis-formation along the rostro-caudal axis.76,77 Conditional deletion of β-catenin in the early endoderm layer leads to ectopic heart formation with Nkx2–5 expression, and this phenotype is attributed to blockage of the inhibitory role of the Wnt pathway on cardiac differentiation.78,79,80 In Xenopus, overexpression of myocardin was sufficient to induce ectopic expression of α-SMA, α-cardiac actin, and Nkx2–5. However, myocardin alone appears to be insufficient for establishing beating heart cells.81 One of the key regulators for early heart development is Tbx5, a T-box transcription factor.58,82,83 Tbx5 specifies the left-right identity of the cardiac chambers and the development of the ventricular septum.84 Mice heterozygous for Tbx5 exhibit malformed cardiac chambers with an abnormal inter-ventricular septum, and homozygous deletion of Tbx5 alleles results in the absence of the left ventricle.58,59 Similarly, human mutations in Tbx5 cause the Holt–Oram syndrome, which is characterized by atrial septal defects, upper limb defects, and anomalies of the digits.58,85,86 The importance of Tbx5 in heart development is also exemplified by the fact that its role is evolutionarily conserved among species.70,87 Although overexpression of Tbx5 affects cardiac septum morphogenesis, it is not enough to induce cell differentiation into cardiomyocytes. Given that no single transcription factor so far has been shown to sufficiently induce cardiomyocytes, the developmental programme of cardiogenesis might be activated through multiple factors.
3.2. Master regulators for cardiomyogenesis
‘Master regulators’ control multiple genes to direct cell differentiation and are sufficient to activate an entire developmental programme. In 1988, Davis and colleagues demonstrated that overexpression of MyoD, a basic-helix-loop-helix (bHLH) transcription factor, is sufficient to convert fibroblasts to skeletal muscle cells.88,89 Similarly, another bHLH-type transcription factor, myocardin, is sufficient to activate the developmental programme of smooth muscle differentiation.81,90 However, as described earlier, no single transcription factor is known to act as a master regulator for cardiomyogenesis.
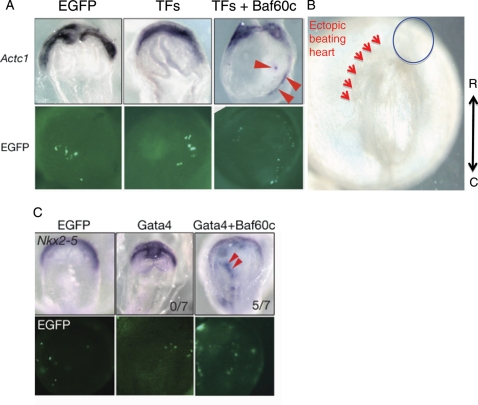
Recently, various combinations of cardiac transcription factors were used in an attempt at the directed transdifferentiation of non-cardiac cells into the cardiomyocyte lineage.55,57 In this study, developmentally critical cardiac transcription factors (Gata4, Nkx2–5, and Tbx5) were introduced into mesodermal cells of developing mouse embryos in different combinations. However, any combination of Gata4, Nkx2–5, and/or Tbx5 did not fully induce cardiomyocyte differentiation, suggesting that these transcription factors are not sufficient for cardiomyogenesis (Figure 4A, Table 1). Surprisingly, the addition of Baf60c, a cardiac-specific subunit of BAF chromatin remodelling complexes,56 led to ectopic differentiation of mesodermal cells into beating cardiomyocytes.55
Figure 4.
Ectopic induction of cardiomyogenesis by defined factors (Tbx5, Gata4, and Baf60c). (A) Ectopic induction of cardiac tissues by co-overexpression of TFs (Tbx5, Nkx2–5, and Gata4) and Baf60c. The early cardiomyocyte marker Actc1 was used to monitor the induction of cardiomyocytes. The chromatin remodelling component Baf60c is required for the induction. (B) Beating heart tissues (arrowheads) are observed in non-cardiogenic mesoderm upon overexpression of Tbx5, Gata4, and Baf60c. At this stage, the endogenous heart cells do not beat, indicating accelerated cardiac differentiation by the defined factors. (C) Whole-mount in situ hybridization showing that Gata4 requires Baf60c to induce ectopic expression of Nkx2–5. EGFP expression indicates transfected cells (adapted from Takeuchi and Bruneau55).
Table 1.
Combinatorial activation of beating heart by Gata4, Tbx5, and Baf60c +, transfection of DNA; O, cardiac marker induction or beating heart induction
| Actc1, Myl7 induction | Beating heart induction | Tbx5 | Gata4 | Gata1 | Nkx2–5 | Baf60c | Baf60b |
|---|---|---|---|---|---|---|---|
| × | × | + | − | − | − | − | |
| × | × | − | + | − | − | − | |
| × | × | − | − | + | − | − | |
| × | × | + | + | − | − | − | |
| × | × | + | − | + | − | − | |
| × | × | − | − | − | + | − | |
| × | × | + | − | − | + | − | |
| × | × | − | + | − | + | − | |
| × | × | + | + | − | + | − | |
| ○ | × | − | + | − | − | + | − |
| ○ | × | − | + | − | − | − | + |
| ○ | × | − | − | + | − | + | − |
| × | × | − | − | + | − | − | + |
| ○ | ○ | + | + | − | − | + | − |
| ○ | ○ | + | + | − | + | + | − |
Chromatin modification is a dynamic process required for the proper function of transcription factors, allowing them to have access to their target loci (Figure 3A and B). Genome-wide screening revealed the presence of cardiac-specific chromatin remodelling factors,56 indicating their potential involvement in directed transdifferentiation. Indeed, expression dosage of Baf60c allowed Gata4 to access its target genes by modifying their chromatin structures, leading to the ectopic expression of cardiac genes. Tbx5 overexpression promoted cardiomyogenesis by repressing the activation of non-cardiac mesodermal genes.55 Chromatin immunoprecipitation assays confirmed these findings by showing the presence of the heart-specific Baf60c-remodelled chromatin. Furthermore, the binding of Gata4 and Tbx5 to the cTnT promoter region required Baf60c-mediated chromatin remodelling, suggesting that the combination of Gata4, Tbx5, and Baf60c acts as a master regulator for cardiomyocyte differentiation from mesodermal cells (Figure 3).55
More recently, Ieda et al.91 demonstrated that combinatorial overexpression of developmentally critical transcription factors is sufficient to the direct reprogramming of cardiac fibroblasts into functional cardiomyocytes. Interestingly, Gata4 and Tbx5 were also required for the reprogramming, although Mef2c was used instead of Baf60c (Figure 2). The induced cardiomyocytes expressed the cardiac-specific markers Actc1, Myh6, Ryr2, and Connexin43, whereas Col1a2—a marker for fibroblasts—was markedly down-regulated. Strikingly, they exhibited a global gene expression profile similar to that of cardiomyocytes, with cardiomyocyte-like chromatin patterns on several genes, indicating epigenetic resetting. H3K27me3 (trimethylated histone H3 of lysine 27) and H3K4me3 (trimethylated histone H3 of lysine 4) mark transcriptionally inactive or active chromatin, respectively.92 Further methylation analyses of induced cardiomyocytes revealed decreased levels of H3K27me3 and increased levels of H3K4me3 in the promoters of cardiomyocyte genes.91
Curiously, Baf60c was not required for the reprogramming of cardiac fibroblasts. This is likely due to cell-type differences between embryonic mesodermal cells and fibroblasts. It is reasonable to speculate that cardiac or dermal fibroblasts share similar chromatin patterns with cardiogenic cells, so that overexpression of cardiac chromatin remodellers may not be necessary for the event. Also, Mef2c may regulate expression of chromatin remodelling factors required for cardiac reprogramming.
3.3. Approaches for cardiac regeneration
CHDs are the most common birth defects in humans, and heart disease remains the leading cause of human death worldwide. The high morbidity and mortality is largely attributed to the limited regenerative capacity of the heart. Recent research has focused on developing new strategies, especially cell-mediated therapies, to treat damaged hearts. One approach is to utilize pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) or induced PSCs (iPSCs). These cells are highly plastic and can expand and differentiate into most of existing cells, including functional cardiomyocytes.75,93,94 After birth, the heart itself is insufficient in its regenerative response upon damage, such as from infarcts, as most cardiomyocytes are terminally differentiated and do not proliferate. Therefore, ESCs or iPSCs may hold potential for treating cardiac defects. In addition, iPSC transplantation is advantageous over whole-organ transplantation in that these cells can be directly obtained from patients to avoid immune rejection.95 However, transplantation of undifferentiated iPSCs into the mouse heart has resulted in teratoma formation.96 Analysis of these teratomas revealed cell types from all three embryonic germ layers, indicating that existing cardiac cells do not guide iPSCs to differentiate into cardiac cells.96,97 As described earlier, cardiogenesis takes place in a highly coordinated fashion by interactions of multiple factors,12,98–101 and it will therefore be important to understand the cardiogenic mechanisms of these factors for iPSC-mediated cardiac therapy.
CPC-based therapy offers a better approach for heart regeneration. CPCs are committed pre-cardiac cells with a potential to differentiate into multiple cardiac cell types, including cardiomyocytes, smooth muscle cells, and endothelial cells.20,21,102 The identification of Isl1+ cells led to the discovery of the undifferentiated CPCs, which has advanced our knowledge of multi-potent CPCs.20,103 They are also marked by Nkx2-5 or Flk1 and can be isolated from early developing embryos or differentiating pluripotent cells.21,102,104 A recent trial of embryonic CPC transplantation in post-myocardial infarcted hearts of non-human primates showed successful engraftment with myocardial differentiation,105 suggesting that CPCs can be used as an effective source for heart regeneration. Understanding the mechanisms of lineage-specific differentiation of CPCs will accelerate the CPC-mediated cardiac therapeutics.
Hattori et al.106 recently introduced a novel approach to isolate cardiomyocytes. By use of tetramethylrhodamine ethyl ester perchlorate (a fluorescent dye specific to mitochondria), they successfully isolated embryonic and neonatal cardiomyocytes (>99% purity) by fluorescence-activated cell sorting. Moreover, transplantation of these purified cardiomyocytes did not induce teratoma formation, and their aggregation resulted in long-term survival of the transplanted myocytes in vivo.106 Further studies will be necessary to test their effects on damaged hearts and large animals. Induced cardiomyocytes from directed differentiation also have tremendous therapeutic potential to treat heart disease. However, the differentiation method will need to be optimized before a clinical trial. For example, the differentiation efficiency needs to be improved with quantitative studies and more rigorous functional assays should be carried out in vitro and in vivo. In addition, it will be important to test whether endogenous cells such as cardiac fibroblasts can be directly differentiated into cardiomyocytes in vivo.
4. Future perspectives
Numerous genetic and epigenetic factors regulating cardiac morphogenesis, differentiation, and maturation have been identified through decades of progress in developmental cardiology. The knowledge gained from the developmental studies led to the recent breakthrough discoveries of defined factors, whose co-overexpression is sufficient to instruct non-cardiac cells to convert into cardiomyocytes. The defined factors (Gata4 and Tbx5 with Baf60 or Mef2c), also essential for cardiac development, are transcription and chromatin remodelling factors that act cooperatively with others, highlighting the importance of a mechanistic understanding of transcriptional and chromatin regulation. It would be interesting to see whether direct differentiation of other cardiac lineages such as smooth muscle, endothelial, or conduction cells also occurs through defined factors. As illustrated in Figure 2, different types of cardiac cells express distinct gene products, but it is mostly unknown if these cell types can be directly obtained from multi-potent progenitors by defined factors. It would be important to identify factors that can activate the programmes for individual cardiac lineage determination.
Although cardiac transcription factors have been extensively studied for their roles and targets, the mechanisms by which chromatin remodellers modulate activation or repression of specific signalling and transcriptional networks are not well understood. Further investigation will be required to elucidate the roles of cardiac epigenetic factors for a better understanding of the process of cardiogenesis, as well as directed cardiac differentiation or reprogramming.
Given that the mammalian heart has limited regeneration capacity, direct reprogramming is emerging as a novel form of potential cardiac therapeutics along with CPC-mediated transplantation.105,106,107 We are rapidly entering into a new era of cardiac regenerative medicine that combines knowledge from the diverse fields of heart biology, including developmental, molecular and cellular cardiology, and cardiac physiology. This integrative approach and effort should accelerate novel discoveries for future cardiac therapeutics as well as preventive strategies for CHD.
Funding
This work was funded by JST PRESTO, JSPS(A), and HFSP CDA for J.K.T. H.V.W. is also supported by Sankyo Foundation of Life science, as well as the Faculty of Science from the University of Amsterdam, the Amsterdam University College Scholarship Fund and the Stichting Bekker-la Bastide Fonds. C.K. was supported by grants from AHA and NHLBI (R00HL092234)/NIH. K.K.T. was supported by a grant from NEXT program, JSPS.
Acknowledgements
We thank B.G. Bruneau and A.F. Moorman for helpful discussion about this manuscript, R. Ishihara for artwork in Figures 1 and 2, and N. Yokota for editorial assistance.
Conflict of interest: none declared.
References
- 1.Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. doi:10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. doi:10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 3.Garry DJ, Olson EN. A common progenitor at the heart of development. Cell. 2006;127:1101–1104. doi: 10.1016/j.cell.2006.11.031. doi:10.1016/j.cell.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Moorman AFM, Webb S, Brown NA, Lamers W, Anderson RH. Development of the heart: (1) formation of the cardiac chambers and arterial trunks. Heart. 2003;89:806–814. doi: 10.1136/heart.89.7.806. doi:10.1136/heart.89.7.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, et al. Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol. 2000;223:266–278. doi: 10.1006/dbio.2000.9753. doi:10.1006/dbio.2000.9753. [DOI] [PubMed] [Google Scholar]
- 6.Moorman AFM, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- 7.Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol. 2007;18:67–76. doi: 10.1016/j.semcdb.2007.01.001. doi:10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mima T, Ueno H, Fischman DA, Williams LT, Mikawa T. Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proc Natl Acad Sci USA. 1995;92:467–471. doi: 10.1073/pnas.92.2.467. doi:10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf1-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. doi:10.1016/S1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 10.Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, et al. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. doi:10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- 11.Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 12.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. doi: 10.1038/nbt1093. doi:10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 13.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;20:18531–18536. doi: 10.1073/pnas.0703113104. doi:10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsan BH, Schultheiss TM. Regulation of avian cardiogenesis by Fgf8 signaling. Development. 2002;129:1935–1943. doi: 10.1242/dev.129.8.1935. [DOI] [PubMed] [Google Scholar]
- 15.Lough J, Barron M, Brogley M, Sugi Y, Bolender DL, Zhu X. Combined BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev Biol. 1996;178:198–202. doi: 10.1006/dbio.1996.0211. doi:10.1006/dbio.1996.0211. [DOI] [PubMed] [Google Scholar]
- 16.Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, et al. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol Cell Biol. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. doi:10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, et al. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. doi:10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon C, Arnold J, Hsiao E, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator in mammalian cardiogenesis. Proc Natl Acad Sci USA. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. doi:10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. doi:10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. doi:10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. doi:10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. doi:10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman JI. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol. 1995;16:155–165. doi: 10.1007/BF00794186. [DOI] [PubMed] [Google Scholar]
- 25.Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–453. doi: 10.1038/nature01411. doi:10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- 26.Khorasanizadeh S. The nucleosome: from genomic organization to genomic regulation. Cell. 2004;116:259–272. doi: 10.1016/s0092-8674(04)00044-3. doi:10.1016/S0092-8674(04)00044-3. [DOI] [PubMed] [Google Scholar]
- 27.Woodcock CL, Dimitrov S. Higher-order structure of chromatin and chromosomes. Curr Opin Genet Dev. 2001;11:130–135. doi: 10.1016/s0959-437x(00)00169-6. doi:10.1016/S0959-437X(00)00169-6. [DOI] [PubMed] [Google Scholar]
- 28.Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184:868–871. doi: 10.1126/science.184.4139.868. doi:10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- 29.Luo RX, Dean DC. Chromatin remodeling and transcriptional regulation. J Natl Cancer Inst. 1999;91:1288–1294. doi: 10.1093/jnci/91.15.1288. doi:10.1093/jnci/91.15.1288. [DOI] [PubMed] [Google Scholar]
- 30.Delgado-Olguin P, Takeuchi JK, Bruneau BG. Chromatin modification and remodeling in heart development. ScientificWorldJournal. 2006;6:1851–1861. doi: 10.1100/tsw.2006.315. doi:10.1100/tsw.2006.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. doi:10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;12:32–42. doi: 10.1038/nrg2485. doi:10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136:200–206. doi: 10.1016/j.cell.2009.01.009. doi:10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin W, Dent SY. Functions of histone-modifying enzymes in development. Curr Opin Genet Dev. 2006;16:137–142. doi: 10.1016/j.gde.2006.02.002. doi:10.1016/j.gde.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Varga-Weisz P. ATP-dependent chromatin remodeling factors: nucleosome shufflers with many missions. Oncogene. 2001;20:3076–3085. doi: 10.1038/sj.onc.1204332. doi:10.1038/sj.onc.1204332. [DOI] [PubMed] [Google Scholar]
- 36.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. doi:10.1016/S0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 37.Vignali M, Hassan AH, Neely KE, Workman JL. ATP-dependent chromatin-remodeling complexes. Mol Cell Biol. 2000;20:1899–1910. doi: 10.1128/mcb.20.6.1899-1910.2000. doi:10.1128/MCB.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De La Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet. 2006;7:461–473. doi: 10.1038/nrg1882. doi:10.1038/nrg1882. [DOI] [PubMed] [Google Scholar]
- 39.Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev. 2005;15:163–176. doi: 10.1016/j.gde.2005.01.005. doi:10.1016/j.gde.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. doi: 10.1038/nsmb1337. doi:10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- 41.Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. doi:10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 42.Verdone L, Caserta M, Di Mauro E. Role of histone acetylation in the control of gene expression. Biochem Cell Biol. 2005;85:344–353. doi: 10.1139/o05-041. doi:10.1139/o05-041. [DOI] [PubMed] [Google Scholar]
- 43.Lee DY, Teyssier C, Strahl BD, Stallcup MR. Role of protein methylation in regulation of transcription. Endocr Rev. 2005;26:147–170. doi: 10.1210/er.2004-0008. doi:10.1210/er.2004-0008. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez RM, Hnilica LS. Tissue specificity of histone phosphorylation. Science. 1967;157:1324–1325. doi: 10.1126/science.157.3794.1324. doi:10.1126/science.157.3794.1324. [DOI] [PubMed] [Google Scholar]
- 45.Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci USA. 2003;100:13225–13230. doi: 10.1073/pnas.1735528100. doi:10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhananjayan SC, Ismail A, Nawaz Z. Ubiquitin and control of transcription. Essays Biochem. 2005;41:69–80. doi: 10.1042/EB0410069. doi:10.1042/EB0410069. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nature Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. doi:10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 48.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. doi:10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 49.Bruneau BG. Chromatin remodeling in heart development. Curr Opin Genet Dev. 2010;20:505–511. doi: 10.1016/j.gde.2010.06.008. doi:10.1016/j.gde.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Xue Y, Zhou S, Kuo A, Cairns BR, Crabtree GR. Diversity and specialization of mammalian SWI/SNF complexes. Genes Dev. 1996;10:2117–2130. doi: 10.1101/gad.10.17.2117. doi:10.1101/gad.10.17.2117. [DOI] [PubMed] [Google Scholar]
- 51.Kadam S, Emerson BM. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol Cell. 2003;11:377–389. doi: 10.1016/s1097-2765(03)00034-0. doi:10.1016/S1097-2765(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi JK, Lou X, Alexander JM, Sugizaki H, Delgado-Olguin P, Holloway AK, et al. Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat Commun. 2011;2:187. doi: 10.1038/ncomms1187. doi:10.1038/ncomms1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hang CT, Yang J, Han P, Cheng HL, Shang C, Ashley E, et al. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–67. doi: 10.1038/nature09130. doi:10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Côté J, Quinn J, Workman JL, Peterson CL. Stimulation of GAL4 derivative binding to nucleosomal DNA by the yeast SWI/SNF complex. Science. 1994;265:53–60. doi: 10.1126/science.8016655. doi:10.1126/science.8016655. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–712. doi: 10.1038/nature08039. doi:10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F, Adamson SL, et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432:107–112. doi: 10.1038/nature03071. doi:10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi JK, Lickert H, Bisgrove BW, Sun X, Yamamoto M, Chawengsaksophak K, et al. Baf60c is a nuclear Notch signaling component required for the establishment of left-right asymmetry. Proc Natl Acad Sci USA. 2007;104:846–851. doi: 10.1073/pnas.0608118104. doi:10.1073/pnas.0608118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. doi:10.1016/S0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 59.Mori AD, Zhu Y, Vahora I, Nieman B, Koshiba-Takeuchi K, Davidson L, et al. Tbx5-dependent rheostatic control of cardiac gene expression and morphogenesis. Dev Biol. 2006;297:566–586. doi: 10.1016/j.ydbio.2006.05.023. doi:10.1016/j.ydbio.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Zhai W, Richardson JA, Olson EN, Meneses JJ, Firpo MT, et al. Polybromo protein BAF180 functions in mammalian cardiac chamber maturation. Genes Dev. 2004;15:3106–3116. doi: 10.1101/gad.1238104. doi:10.1101/gad.1238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. doi:10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 62.Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, et al. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol. 2001;21:7787–7795. doi: 10.1128/MCB.21.22.7787-7795.2001. doi:10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klochendler-Yeivin A, Fiette L, Barra J, Muchardt C, Babinet C, Yaniv M. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Rep. 2000;1:500–506. doi: 10.1093/embo-reports/kvd129. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, LeRoy G, Seelig HP, Lane WS, Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. doi:10.1016/S0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]
- 65.Xue Y, Wong J, Moreno GT, Young MK, Côté J, Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. doi:10.1016/S1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- 66.Kaji K, Caballero IM, MacLeod R, Nichols J, Wilson VA, Hendrich B. The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nat Cell Biol. 2006;8:285–292. doi: 10.1038/ncb1372. doi:10.1038/ncb1372. [DOI] [PubMed] [Google Scholar]
- 67.Kaji K, Nichols J, Hendrich B. Mbd3, a component of the NuRD co-repressor complex, is required for development of pluripotent cells. Development. 2007;134:1123–1132. doi: 10.1242/dev.02802. doi:10.1242/dev.02802. [DOI] [PubMed] [Google Scholar]
- 68.Nimura K, Ura K, Shiratori H, Ikawa M, Okabe M, Schwartz RJ, et al. A histone H3 lysine 36 trimethyltransferase links Nkx-5 to Wolf-Hirschhorn syndrome. Nature. 2009;460:287–292. doi: 10.1038/nature08086. doi:10.1038/nature08086. [DOI] [PubMed] [Google Scholar]
- 69.Lu J, Jeong HW, Kong N, Yang Y, Carroll J, Luo HR, et al. Stem cell factor SALL4 represses the transcriptions of PTEN and SALL1 through an epigenetic repressor complex. PloS One. 2009;4:e5577. doi: 10.1371/journal.pone.0005577. doi:10.1371/journal.pone.0005577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koshiba-Takeuchi K, Takeuchi JK, Arruda EP, Kathiriya IS, Mo R, Hui CC, et al. Cooperative and antagonistic interactions between Sall4 and Tbx5 pattern the mouse limb and heart. Nat Genet. 2006;38:175–183. doi: 10.1038/ng1707. doi:10.1038/ng1707. [DOI] [PubMed] [Google Scholar]
- 71.Bergemann AD, Cole F, Hirschhorn K. The etiology of Wolf-Hirschhorn syndrome. Trends Genet. 2005;21:188–195. doi: 10.1016/j.tig.2005.01.008. doi:10.1016/j.tig.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Gottlieb PD, Pierce SA, Sims RJ, Yamagishi H, Weihe EK, Harriss JV, et al. Bop encodes a muscle-restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nat Genet. 2002;31:25–32. doi: 10.1038/ng866. [DOI] [PubMed] [Google Scholar]
- 73.Leinhart K, Brown M. SET/MYND lysine methyltransferase regulate gene transcription and protein activity. Genes. 2011;2:210–218. doi: 10.3390/genes2010210. doi:10.3390/genes2010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan X, Rotllant J, Li H, De Deyne P, Du SJ. SmyD1, a histone methyltransferase, is required for myofibril organization and muscle contraction in zebrafish embryos. Proc Natl Acad Sci USA. 2006;103:2713–2718. doi: 10.1073/pnas.0509503103. doi:10.1073/pnas.0509503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monzen K, Ito Y, Naito AT, Kasai H, Hiroi Y, Hayashi D, et al. A crucial role of a high mobility group protein HMGA2 in cardiogenesis. Nat Cell Biol. 2008;10:567–574. doi: 10.1038/ncb1719. doi:10.1038/ncb1719. [DOI] [PubMed] [Google Scholar]
- 76.Reiter JF, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, et al. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. doi:10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Latinkić BV, Kotecha S, Mohun TJ. Induction of cardiomyocytes by GATA4 in Xenopus ectodermal explants. Development. 2003;130:3865–3876. doi: 10.1242/dev.00599. doi:10.1242/dev.00599. [DOI] [PubMed] [Google Scholar]
- 78.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of β-catenin in the embryonic endoderm. Dev Cell. 2002;3:171–181. doi: 10.1016/s1534-5807(02)00206-x. doi:10.1016/S1534-5807(02)00206-X. [DOI] [PubMed] [Google Scholar]
- 79.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. doi:10.1016/S0012-1606(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 80.Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, et al. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. doi:10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Small EM, Warkman AS, Wang DZ, Sutherland LB, Olson EN, Krieg PA. Myocardin is sufficient and necessary for cardiac gene expression in Xenopus. Development. 2005;132:987–997. doi: 10.1242/dev.01684. doi:10.1242/dev.01684. [DOI] [PubMed] [Google Scholar]
- 82.Stennard FA, Harvey RP. T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development. 2005;132:4897–4910. doi: 10.1242/dev.02099. doi:10.1242/dev.02099. [DOI] [PubMed] [Google Scholar]
- 83.Hoogaars WM, Barnett P, Moorman AF, Christoffels VM. T-box factors determine cardiac design. Cell Mol Life Sci. 2007;64:646–660. doi: 10.1007/s00018-007-6518-z. doi:10.1007/s00018-007-6518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takeuchi JK, Ohgi M, Koshiba-Takeuchi K, Shiratori H, Sakaki I, Ogura K, et al. Tbx5 specifies the left/right ventricles and ventricular septum position during cardiogenesis. Development. 2003;130:5953–5964. doi: 10.1242/dev.00797. doi:10.1242/dev.00797. [DOI] [PubMed] [Google Scholar]
- 85.Li QY, Newbury-Ecob RA, Terrett JA, Wilson DI, Curtis AR, Yi CH, et al. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997;15:21–29. doi: 10.1038/ng0197-21. doi:10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- 86.Mori AD, Bruneau BG. TBX5 mutations and congenital heart disease: Holt-Oram syndrome revealed. Curr Opin Cardiol. 2004;19:211–215. doi: 10.1097/00001573-200405000-00004. doi:10.1097/00001573-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 87.Koshiba-Takeuchi K, Mori AD, Kaynak BL, Cebra-Thomas J, Sukonnik T, Georges RO, et al. Reptilian heart development and the molecular basis of cardiac chamber evolution. Nature. 2009;461:95–98. doi: 10.1038/nature08324. doi:10.1038/nature08324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. doi:10.1016/0092-8674(87)90585-X. [DOI] [PubMed] [Google Scholar]
- 89.Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. doi:10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 90.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci USA. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. doi:10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. doi:10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. doi:10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 93.Basson M. Singling out heart cells. Nat Med. 2007;13:33. doi: 10.1038/nm0107-33. doi:10.1038/nm0107-33. [DOI] [PubMed] [Google Scholar]
- 94.Kawai T, Takahashi T, Esaki M, Ushikoshi H, Nagano S, Fujiwara H, et al. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ J. 2004;68:691–702. doi: 10.1253/circj.68.691. doi:10.1253/circj.68.691. [DOI] [PubMed] [Google Scholar]
- 95.Gonzales C, Pedrazzini T. Progenitor cell therapy for heart disease. Exp Cell Res. 2009;315:3077–3085. doi: 10.1016/j.yexcr.2009.09.006. doi:10.1016/j.yexcr.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 96.Moretti A, Bellin M, Jung CB, Thies TM, Takashima Y, Bernshausen A, et al. Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors. FASEB J. 2010;24:700–711. doi: 10.1096/fj.09-139477. doi:10.1096/fj.09-139477. [DOI] [PubMed] [Google Scholar]
- 97.Nussbaum J, Minami E, Laflamme MA, Virag JAI, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. doi:10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 98.Kami D, Shiojima I, Makino H, Matsumoto K, Takahashi Y, Ishii R, et al. Gremlin enhances the determined path to cardiomyogenesis. PloS One. 2008;3:2407. doi: 10.1371/journal.pone.0002407. doi:10.1371/journal.pone.0002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wobus AM, Kaomei G, Shan J, Wellner MC, Rohwedel J, Guanju Ji, et al. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol. 1997;29:1525–1539. doi: 10.1006/jmcc.1997.0433. doi:10.1006/jmcc.1997.0433. [DOI] [PubMed] [Google Scholar]
- 100.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, García-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. doi:10.1016/S0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 101.Lim JY, Kim WH, Kim J, Park SI. Involvement of TGF-β1 signaling in cardiomyocyte differentiation from P19CL6 cells. Mol Cells. 2007;24:431–436. [PubMed] [Google Scholar]
- 102.Kattman SJ, Huber TL, Keller GM. Multipotent Flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. doi:10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 103.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells of the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. doi:10.1016/S1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. doi:10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 105.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. doi:10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hattori F, Chen H, Yamashita H, Tohyama S, Satoh YS, Yuasa S, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. doi:10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 107.Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A Regulatory pathway involving Notch/β-Catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. doi:10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]