Abstract
The Rho small GTPase has been implicated in many cellular processes, including actin cytoskeletal regulation and transcriptional activation. The molecular mechanisms underlying Rho function in many of these processes are not yet clear. Here we report that in Drosophila, reduction of maternal Rho1 compromises signaling pathways consistent with defects in membrane trafficking events. These mutants fail to maintain expression of the segment polarity genes engrailed (en), wingless (wg), and hedgehog (hh), contributing to a segmentation phenotype. Formation of the Wg protein gradient involves the internalization of Wg into vesicles. The number of these Wg-containing vesicles is reduced in maternal Rho1 mutants, suggesting a defect in endocytosis. Consistent with this, stripes of cytoplasmic β-catenin that accumulate in response to Wg signaling are narrower in these mutants relative to wildtype. Additionally, the amount of extracellular Wg protein is reduced in maternal Rho1 mutants, indicating a defect in secretion. Signaling pathways down-regulated by endocytosis, such as the EGFR and Torso pathways, are hyperactivated in maternal Rho1 mutants, consistent with a general role for Rho1 in regulating signaling events governing proper patterning during Drosophila development.
Keywords: Rho GTPase, signaling, endocytosis, secretion, membrane trafficking, EGFR, Torso, Wingless, Drosophila
Introduction
The specification of pattern during embryonic development requires the proper regulation of the various signaling pathways necessary to determine cell fate. In this context, overactive signaling can be as deleterious as a lack of signaling, necessitating mechanisms to control signaling activity. Downregulation of a number of signaling pathways has been known for some time to involve the endocytosis, and subsequent degradation, of receptor-ligand complexes (reviewed in: Di Fiore and Gill, 1999; Gonzalez-Gaitan, 2003). Recently, data has accumulated linking endocytosis not only to downregulation of signaling, but also to activation of signaling and dispersal of ligands (e.g. Parks et al., 2000; Piddini and Vincent, 2003). In Drosophila, endocytosis of receptor-ligand complexes plays a role in the regulation of signaling pathways important in patterning the early embryo, including the activity of signaling pathways downstream of the Epidermal Growth Factor Receptor (EGFR; Sturtevant et al., 1994) and Torso receptor tyrosine kinases (Lloyd et al., 2002). Failure to properly downregulate these pathways in response to signaling results in the ectopic accumulation of their targets (Lloyd et al., 2002).
While simple secretion of ligands into the extracellular space is a necessary component of their dispersal, recent data are highlighting the role played by active membrane trafficking processes, particularly in the formation of morphogen gradients. In the wing disc, the distribution of the morphogen Wingless (Wg), the Drosophila Wnt-1 homolog, has been suggested to involve its encapsulation within vesicular structures termed argosomes, which are then transported from cell to cell through the endocytic compartment (Greco et al., 2001). In the early Drosophila embryo, Wg protein can also be detected in punctate structures identified through EM studies as multi-vesicular bodies (MVBs; van den Heuvel et al., 1989) spreading out from the stripes of cells actively expressing Wg. Formation of these vesicles requires endocytosis, and they are thought to be important in the formation of the proper Wg protein gradient (Dierick and Bejsovec, 1998). Posterior to each Wg stripe, these vesicles are thought to influence the shape of the Wg protein gradient by targeting Wg to lysosomes and degradation (Dubois et al., 2001). Alternatively, these vesicles have also been suggested to regulate Wg protein distribution through transcytosis of Wg from one cell to another (Pfeiffer et al., 2002).
One class of proteins demonstrated to have a role in the regulation of membrane trafficking, particularly endocytosis, is the Rho family of small GTPases, including Rho and its relatives Rac and Cdc42. Expression of constitutively active (CA) Rho or Rac in mammalian tissue culture cells has been shown to inhibit transferrin-receptor-mediated endocytosis (Lamaze et al., 1996). Microinjection of the Rho-specific inhibitor C3 exoenzyme blocks constitutive endocytosis in Xenopus oocytes, whereas injection of CARho stimulates it (Schmalzing et al., 1995). Overexpression of mammalian RhoB, which localizes to endosomes, inhibits the trafficking of EGFR-positive vesicles in tissue culture cells (Adamson et al., 1992), and mammalian RhoD also localizes to early and recycling endosomes and is involved in their trafficking (Murphy et al., 1996; Murphy et al., 2001).
In addition to its roles in endocytosis, Rho has been linked to a wide variety of cellular functions, including cytoskeletal regulation, transcriptional activation, cell cycle progression, and others, though it is not clear in all cases how directly Rho is involved (reviewed in: Hall, 1998; Mackay and Hall, 1998). Our previous work identified a segmentation defect due to a failure to maintain segment polarity gene expression as one of the phenotypes associated with loss of maternal Rho1 (Magie et al., 1999). In this paper, we describe a general role for Rho1 in signaling pathways involving membrane trafficking during early development in Drosophila. Reduction of maternal Rho1 activity results in the misregulation of a number of signaling pathways, including those mediated by the EGFR and Torso receptor tyrosine kinases, as well as aberrant Wg protein localization that results in a failure to properly activate the Wg signaling pathway and maintain expression of the segment polarity genes En, Wg, and Hh. The segmentation phenotype of maternal Rho1 mutants suggests that the effects of Rho1 on segment polarity gene signaling are limiting in this context.
Results and Discussion
Maternal Rho1 mutants fail to maintain segment polarity gene expression and exhibit aberrant Wingless protein localization
To examine the phenotypes associated with loss of maternally-deposited Rho1 we utilized a change-of-function mutation in an RNA polymerase II subunit, wimp, to reduce expression of maternal Rho1 (Magie et al., 1999; Magie et al., 2002; Parkhurst and Ish-Horowicz, 1991). It is not possible to completely eliminate maternal Rho1 function, as germline clones of Rho1 cannot be generated due to cell inviability (Magie et al., 1999). The reduction in Rho1 expression is achieved by generating females doubly heterozygous for wimp and Rho11B, a strong Rho1 allele, then mating them to wildtype males (see Materials and Methods and Supplemental Fig. 1). The resulting embryos will hereafter be referred to as maternal Rho1 mutants.
Endocytic processes have been shown to be involved in the dispersal of some ligands (Entchev et al., 2000; Greco et al., 2001; Strigini and Cohen, 2000). This is particularly important in the context of morphogen gradient formation, both for morphogens that act long range such as Dpp, as well as short-range morphogens such as Hedgehog (Hh) and Wg. In the Drosophila embryo, proper regulation of segment polarity gene products, and in particular the distribution of Wg protein, is crucial to the proper patterning of the embryonic epidermis (Hays et al., 1997; O’Keefe et al., 1997).
Embryos with reduced maternal Rho1 exhibit a segmentation phenotype due in part to improper maintenance of segment polarity gene expression (74% penetrance; n=126; Magie et al., 1999). In particular, expression of the segment polarity genes en, wg, and hh, while initiated normally, are not maintained properly, leading to the fusion or absence of stripes (Fig. 1A–F). Because we cannot make clones due to the requirement of Rho1 for cell viability, we are not able to directly assess if the Wg- or En/Hh-expressing cell or both require Rho1 function for proper maintenance of segment polarity gene expression.
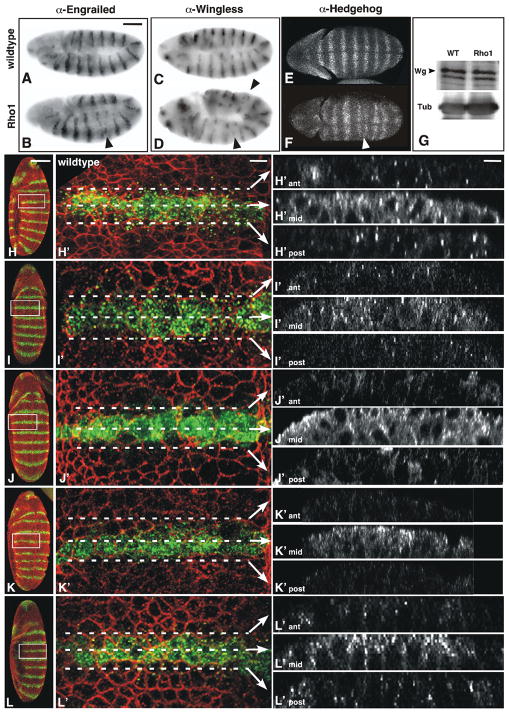
Figure 1. En and Wg expression is not maintained properly and Wingless protein distribution is aberrant in maternal Rho1 mutants.
Stage 9 wildtype (A, C, E) or maternal Rho1 mutant (B, D, F) embryos stained with anti-Engrailed (A–B) anti-Wingless (C–D) or anti-Hedgehog (E–F) antibodies. While En expression is initiated normally, as development proceeds some stripes become reduced, others disappear entirely (arrowhead in B). Likewise, early Wg and Hh expression in maternal Rho1 mutants looks normal, but in later stages Wg and Hh stripes also become reduced or eliminated (arrowheads in D, F). (G) Western analysis of Wg protein levels in wildtype and maternal Rho1 mutant embryo lysates. Tubulin levels shown as loading control. (H-L′) Z-series projections of wildtype (H, H′), wimp/+ (I, I′), shi (J, J′), maternal Rho1 mutant (K, K′), and maternal Rho1 rescue (L, L′) stage 9 embryos stained with antibodies against Wingless (Wg, green) and Discontinuous Actin Hexagon (Dah, red) to outline cell boundaries. The boxed regions of embryos in H, I, J, K, L are shown at higher magnification in H′, I′, J′, K′, L′. Z-series cross-sections noted by dashed lines in H′, I′, J′, K′, L′ are projected in ant, mid, post. Note the punctate accumulations of Wg protein spreading out from the Wg-expressing cells in wildtype and wimp/+ (H-I′), which are reduced in shi mutants (J-J′), which are defective in endocytosis, and maternal Rho1 mutants (K-K′). This phenotype is rescued by the presence of a Rho1 transgene (L-L′). The larger cells in J′ relative to H′ result from cytokinesis defects associated with shibire mutants (Bejsovec and Wieschaus, 1995). Scale bars: (A, H) 100μm; (H′) 10μm.
The segmentation phenotype associated with reduced maternal Rho1 could result from different biochemical mechanisms. One possibility is that, similar to the involvement of Ras in MAPK signaling, Rho1 acts directly in a signal transduction pathway leading to transcriptional activation. Alternatively, Rho1 could affect the Wg signal transduction pathway through its effects on a general cellular process such as regulation of the cytoskeleton or membrane trafficking. To distinguish between these possibilities, we examined Wg protein distribution in maternal Rho1 mutants relative to wildtype (Fig. 1H-H′) and wimp/+ controls (Fig. 1I-I′). In wildtype stage 9 embryos, Wg is expressed in a stripe 2-cells wide and can be detected in multi-vesicular bodies (MVBs) up to a distance of several cells away (Fig. 1H-H′; van den Heuvel et al., 1989). The formation of these vesicular structures has been shown to require endocytosis, as they are absent in embryos mutant for shibire (shi), which encodes the Drosophila homolog of Dynamin and cannot undergo endocytosis (Fig. 1J-J′; Bejsovec and Wieschaus, 1995). Similar to shi mutants, maternal Rho1 mutants also exhibit fewer Wg positive vesicles than wildtype embryos (Fig. 1K-K′), though the total amount of Wg protein present in these mutants is the same as wildtype (Fig. 1G). This effect is rescued by the presence of a Rho1 transgene expressed under the control of its own promoter (Fig. 1L-L′; see Materials and Methods). The presence of Wg protein in vesicular structures spreading out from cells that actively express it indicates the importance of membrane trafficking in the formation of the Wg protein gradient.
Maternal Rho1 mutants exhibit defective secretion of Wg
Actin cytoskeletal regulation has been linked to regulation of both the endocytic and secretory pathways (reviewed in: Stamnes, 2002). To determine if defective secretion/exocytosis in maternal Rho1 mutants also contributes to the lack of Wg-containing vesicles, we examined the amount of extracellular Wg in wildtype, wimp/+, shibire, and maternal Rho1 mutants by incubating embryos in primary antibody prior to permeabilization (Fig. 2; see Materials and Methods). In wildtype and wimp/+ control embryos, extracellular Wg can be seen surrounding the cells that produce it (Fig. 2A-B′; Pfeiffer et al., 2002). In shibire mutants, the amount of extracellular Wg is increased relative to wildtype, as expected for a mutant that can exocytose, but not properly internalize, Wg (Fig. 2C-C′). In maternal Rho1 mutants the amount of extracellular Wg is reduced relative to wildtype, indicating an additional role for Rho1 in secretion of Wg (Fig. 2D-D′), and suggesting an underlying role for Rho1 in multiple actin-based processes. As with conventional Wg staining (Fig. 1L-L′), this phenotype is rescued by the presence of a Rho1 transgene (Fig. 2E-E′).
Figure 2. Maternal Rho1 mutants exhibit defective secretion of Wg.
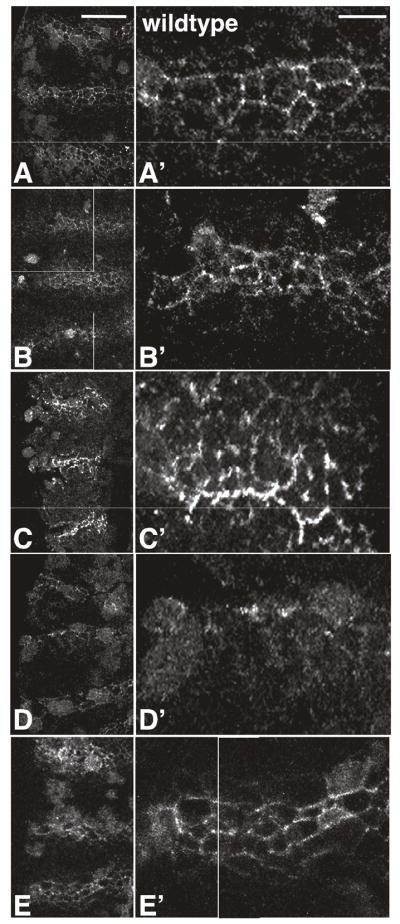
Extracellular Wg staining in wildtype (A, A′), wimp/+ (B, B′), shi (C, C′), maternal Rho1 mutants (D, D′), and maternal Rho1 rescue (E, E′) embryos. (A, B, C, D, E) show a ventral view of 3 stripes, (A′, B′, C′, D′, E′) are higher magnification views of one of the stripes in A, B, C, D, or E, respectively. Scale bars: (A) 50μm; (A′) 25μm.
S2R+ cells treated with Rho1 dsRNA cannot respond properly to Wg signaling
In many systems, Wg signaling results in the cytoplasmic accumulation of β-catenin, which then enters the nucleus, binds transcription factors of the TCF/LEF family and activates transcription from target genes (reviewed in: Barker et al., 2000; Novak and Dedhar, 1999). S2R+ cells express the Wg receptor, D-Frizzled2 (Dfz2; Yanagawa et al., 1998). Treatment of these cells with conditioned medium containing Wg results in the cytoplasmic accumulation of Armadillo (Arm), the Drosophila homolog of β-catenin (compare Fig. 3A and B; Yanagawa et al., 1998).
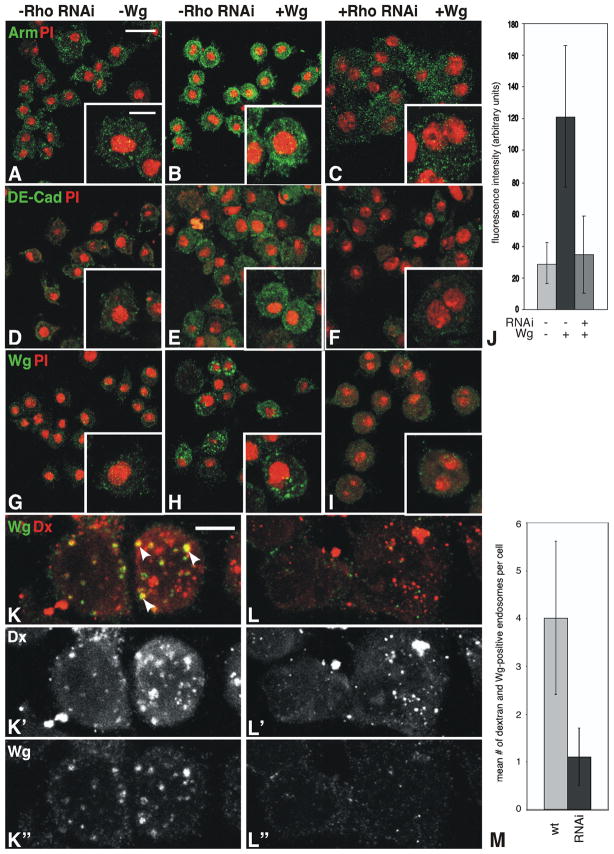
Figure 3. S2R+ cells treated with Rho1 dsRNA do not respond properly to Wg signaling.
Wildtype (A–B, D–E, G–H) and Rho1 dsRNA treated (C, F, I) S2R+ cells without (A, D, G) or with (B–C, E–F, H–I) Wg treatment. Accumulation of Arm (A–C) and DE-Cad (D–F) in response to Wg signaling is lost with Rho1 dsRNA treatment. Upon treatment with Wg-conditioned medium, Wg-positive punctate structures can be seen in wildtype cells (H), but not those treated with Rho1 dsRNA (I). (J) Quantitation of Arm fluorescence relative to the nonspecific cytoplasmic protein Cdc2 indicates Arm accumulation in response to Wg following dsRNA treatment is reduced by 72%. Wildtype (K-K”) and Rho1 dsRNA-treated (L-L”) cells that have internalized Texas-Red-conjugated 70kD dextran molecules (Dx; red) stained with antibodies to Wg (green). The Dx (K′, L′) and Wg (K”, L”) channels are indicated separately below the merge (K, L). Note the punctate yellow accumulations (arrows in K), indicating co-localization of Wg and Dx in wildtype cells. Cells treated with Rho1 dsRNA fail to properly internalize Wg, (L-L”). (M) Quantitation of the number of dextran plus Wg-containing endosomes per cell in wildtype and Rho1 dsRNA-treated cells. Scale bars: (A) 10μm, inset 5μm; (K) 5μm.
Treatment of S2 cells with Rho1 dsRNA has been shown to result in a multinucleate phenotype due to defects in cytokinesis, mimicking defects in cellularization exhibited by maternal Rho1 mutants (Supplemental Fig. 2; Somma et al., 2002). Rho1 dsRNA treatment in S2R+ cells treated with Wg attenuates the Wg-induced accumulation of Arm and DE-Cad (Fig. 3C, F, J). Cells not exposed to Wg-conditioned medium show little Wg staining (Fig. 3G). Upon treatment with Wg, punctate staining could be seen in treated cells (Fig. 3H) due to the internalization of Wg. Treatment of cells with Rho1 dsRNA abolishes this punctate staining pattern (Fig. 3I), and Wg staining is more restricted to the cell surface than in wildtype cells, suggesting that similar to what we observe in embryos, Wg internalization is compromised.
To directly assess endocytic activity in these cells, we added fluorescently-labeled dextran (70kD) to the Wg-conditioned medium prior to treating the cells. Wildtype cells show an internalization of both Wg and dextran, with Wg-containing endosomes comprising a subset of those present within the cell (Fig. 3K-K”; yellow endosomes marked with arrows in Fig. 4K). These Wg-containing endosomes are reduced in number in cells treated with Rho1 dsRNA, as shown by the lack of yellow staining in Fig. 3L-L” (Fig. 3M; n=50, p<0.00001). While some endosomes form in Rho1 dsRNA-treated cells (Fig. 3L-L”), they are smaller and reduced in number relative to wildtype cells (n=50, p=0.026).
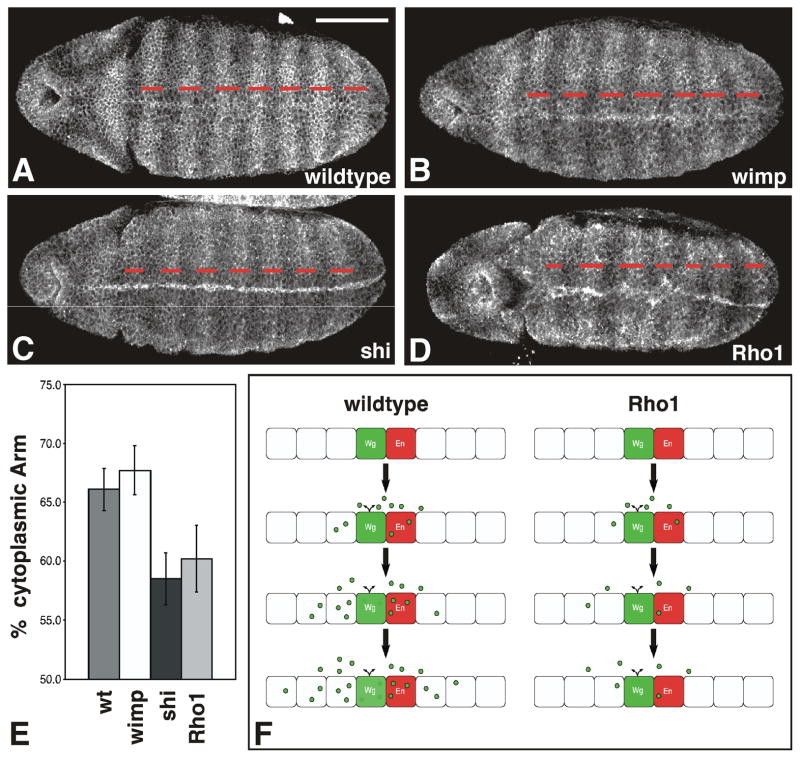
Figure 4. Maternal Rho1 mutants exhibit aberrant cytoplasmic Armadillo accumulation.
(A-D) Representative stage 9 wildtype (A), wimp (B), shibire (C), or maternal Rho1 mutant (D) embryos stained with antibodies to Arm. (E) Graph depicts the percentage of the embryo length between stripes 2–7 that consists of cells with elevated levels of cytoplasmic Arm (red dashes in A–D) for each of the genotypes listed above. shibire and maternal Rho1 mutants show decreased overall stripe width relative to wildtype or wimp controls (n=10, p<0.001). (F) Schematic depicting wildtype embryos (left) and maternal Rho1 mutants (right). Wg (green) spreads outward from Wg-expressing cells as development proceeds (top to bottom). Wg is found in the extracellular space and vesicular structures within cells. In Rho1 mutants the number of vesicles is reduced, and the Wg protein gradient does not form properly. Scale bar: 100μm.
Cytoplasmic Arm accumulation is aberrant in maternal Rho1 mutants
As in S2R+ cells, activation of Wg signaling in the embryo also leads to accumulation of cytoplasmic Arm (Peifer et al., 1994). In stage 9–10 embryos stripes of cytoplasmic Arm accumulation can be seen in cells responding to Wg signaling (Fig. 4). Consistent with previously published results, these stripes are narrower in shibire mutants relative to wildtype or wimp controls, due to the failure to form a proper Wg protein gradient (compare Fig. 4A,B with Fig. 4C; Bejsovec and Wieschaus, 1995). Similarly, maternal Rho1 mutants also exhibit narrower overall stripe width (Fig. 4D–E; n=10, p<0.001 for shibire; n=10, p<0.001 for maternal Rho1). The similarity between the shibire and maternal Rho1 mutant phenotypes suggests that the aberrant Wg protein distribution in these embryos results in their failure to properly activate Wg signaling (Fig. 4F).
Maternal Rho1 mutants exhibit general endocytosis defects
Membrane trafficking is an important aspect of the regulation of many developmental signaling pathways (Gonzalez-Gaitan, 2003). Initial links between endocytosis and signal transduction were shown to involve the attenuation of signal by internalizing receptor-ligand complexes and targeting them for lysosomal degradation, as in the case of Epidermal Growth Factor Receptor (EGFR) signaling (Dickson et al., 1983). To determine whether maternal Rho1 mutants exhibit a general defect in membrane trafficking, we examined the activity of signaling pathways known to require endocytosis for their proper function (Fig. 5). EGFR signaling is under feedback control whereby activation of signaling leads to endocytosis of active receptor and attenuation of the signal (Sturtevant et al., 1994). The accumulation of dual-phosphorylated extracellular signal-related kinase (dpERK) in response to phosphorylation by EGFR has been used as a marker for EGFR signaling, and Drosophila mutants such as hepatocyte growth factor-related tyrosine kinase substrate (hrs) that are unable to properly target activated EGFR for degradation show higher levels of dpERK accumulation compared to wildtype (Lloyd et al., 2002). Similar to hrs mutants, maternal Rho1 mutants exhibit ectopic accumulation of dpERK relative to wildtype and wimp/+ controls (Fig. 5A–C). This effect is not simply due to patterning defects, as the segmentation mutant prd, while missing segments, exhibits dpERK staining levels similar to wildtype within each segment (Fig. 5D). To verify that this effect is due to the failure to attenuate EGFR signaling, we immunoprecipitated the Drosophila EGFR from wildtype, wimp/+, and maternal Rho1 mutant embryo lysates. If active receptor is not endocytosed and degraded properly, the phosphorylated form of EGFR accumulates to higher levels (Boni-Schnetzler and Pilch, 1987). In maternal Rho1 mutants we observe higher levels of phosphorylated EGFR relative to wildtype, indicating that the dpERK accumulation is likely due to overactive EGFR signaling (Fig. 5E).
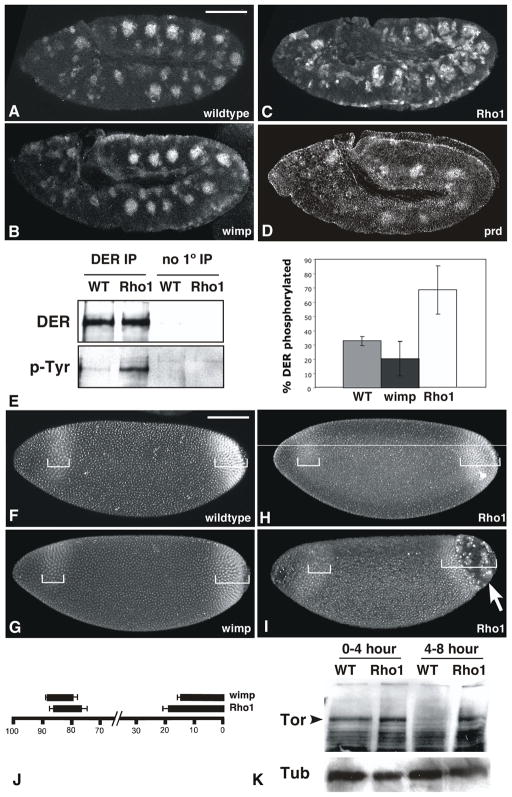
Figure 5. Maternal Rho1 mutants are generally defective in endocytosis.
(A–D) Wildtype (A), wimp/+ (B) maternal Rho1 mutant (C), and prd (D) mutant embryos labeled with antibodies to dpERK and visualized with immunofluorescence. Note the ectopic accumulation of dpERK in Rho1 mutants relative to wildtype, wimp/+, and prd controls. (E) Drosophila EGFR (DER) was immunoprecipitated from wildtype, wimp/+ and maternal Rho1 mutant embryo lysates. Western analysis was used to identify the overall amount of DER protein immunoprecipitated and its phosphorylation state. The graph indicates the amount of phosphotyrosine detected relative to the total amount of DER protein. (F–I) Wildtype (F), wimp/+ (G), and maternal Rho1 mutant (H, I) embryos labeled with antibodies against Tll. Brackets indicate the extent of the anterior and posterior expression domains. Note the posterior cellularization defect indicated by the arrow in I. (J) Quantitation of the extent of Tll expression domains, expressed as % embryo length. (K) Western analysis of Torso protein levels in 0–4 and 4–8 hour wildtype and maternal Rho1 embryo lysates. 50μg of total protein loaded per lane, Tubulin levels shown as loading control. Scale bars: 100μm.
Another signal transduction pathway whose activity is regulated by endocytosis is that of the receptor tyrosine kinase Torso, which is involved in the specification of terminal pattern in Drosophila (Sprenger and Nusslein-Volhard, 1993). Accumulation of the transcription factor tailless (tll), a downstream target of Torso signaling, reflects the level of Torso activity (Ghiglione et al., 1999). In wildtype embryos Tll is expressed at the posterior pole of the embryo, and in a stripe near the anterior pole (Fig. 5F). In the membrane trafficking mutant hrs, the anterior Tll stripe is shifted posteriorly 50% and the posterior domain is expanded by 25% (Lloyd et al., 2002). The effect on Tll expression we observe in maternal Rho1 mutants is of similar magnitude as that reported for hrs, with a posterior shift of the anterior stripe by 27%, and an expansion of the posterior domain by 25% relative to wimp/+ controls (Fig. 5F–I; bottom bars in Fig. 5J; n=10, p < 0.002 for the anterior stripe, n=10, p < 0.0001 for the posterior domain). Additionally, maternal Rho1 mutants exhibit terminal cellularization defects similar to those resulting from endocytosis defects in hrs mutants (Lloyd et al., 2002) and mutants affecting Torso signaling such as pole hole (arrow in Fig. 5I; Perrimon et al., 1986). To verify that the expansion of Tll expression is due to a failure to properly downregulate Torso protein levels, we performed Western analysis of 0–4 and 4–8 hour wildtype and maternal Rho1 embryo lysates (Fig. 5K). As expected, Torso protein is present in 0–4 hour wildtype lysates, but absent from 4–8 hour wildtype lysates. In maternal Rho1 mutant lysates, however, Torso protein persists in 4–8 hour lysates, indicating that the expansion of Tll expression is due to the failure to downregulate Torso signaling. Taken together, the misregulation of EGFR and Torso signaling pathways suggests that maternal Rho1 mutants are generally compromised in endocytosis.
Our data indicate that a number of signaling pathways important during early development in Drosophila are compromised in maternal Rho1 mutants. Our observation that secretion of Wg protein is aberrant in these mutants together with the endocytosis defects we observe in S2R+ cells treated with Rho1 dsRNA and in maternal Rho1 embryos indicates that Rho1 plays a general role in membrane trafficking processes in the early embryo. The biochemical mechanisms through which Rho proteins affect membrane trafficking are currently unclear. One possibility is that the function of Rho1 in this process is a byproduct of its regulation of the actin cytoskeleton. In yeast there is evidence that the actin cytoskeleton is important in endocytosis, as mutations in actin and some actin-binding proteins inhibit endocytosis (Lanzetti et al., 2001; Munn, 2001). In addition, yeast Rho1 has been shown to be involved in endocytosis of the α-receptor (deHart et al., 2003). In mammalian cells, treatment with pharmacological agents that perturb actin structure can affect endocytosis in a cell type specific way. In polarized epithelial cells, for example, treatment with the actin-depolymerizing drug cytochalasin D inhibits endocytosis specifically at the apical, but not the basolateral surface (Gottlieb et al., 1993). RhoA has also been implicated in endocytosis in polarized epithelial cells (Leung et al., 1999). In Drosophila, Rho1 has clear roles in actin cytoskeletal regulation during oogenesis and embryogenesis, consistent with the notion that Rho1 may be acting primarily through its effects on the actin cytoskeleton (Magie et al., 1999; Magie et al., 2002; Johndrow et al., 2004).
Our observation that the segmentation phenotype in maternal Rho1 mutants is the result of general defects in membrane trafficking processes (both secretion and endocytosis) and not a primary effect on transcriptional activation has important implications for the interpretation of data linking Rho to disparate cellular processes. While current data cannot exclude the possibility that Rho directly acts in transcriptional activation or through many disparate mechanistic pathways, data is accumulating that suggests Rho may act primarily as a regulator of the actin cytoskeleton and other functions it has been linked to are indirect effects. For instance, the ability of Rho to influence transcriptional activation through the serum response factor (SRF; Geneste et al., 2002), as well as affect cell cycle progression (Roovers and Assoian, 2003), are due to its direct effects on actin cytoskeletal regulation. Identifying the molecular mechanisms underlying each of Rho’s activities will be crucial to determining whether Rho1 has direct effects on a number of pathways or has a small number of primary functions that indirectly affect other functions. Investigations of Rho GTPase function in genetically amenable model organisms are providing a diversity of developmental contexts in which to examine all aspects of Rho biology, and the ability to examine specific, loss-of-function phenotypes will continue to aid identification of the mechanisms underlying Rho function.
Materials and Methods
Fly stocks
Flies were cultured and crossed on yeast-cornmeal-molasses-malt extract medium at 25°C. The Rho1 allele used in this study is Rho11B/CyO, an imprecise P-element excision line that removes the Rho1 coding region C-terminal to amino acid 52 and produces no protein detectable by immunofluorescence (Supplemental Fig. 1). Other alleles used: shi1 (Poodry, 1990), wimp/TM3 (Parkhurst and Ish-Horowicz, 1991).
Construction of the Rho1 rescue construct and transformant lines
The Rho1 rescue construct was made by subcloning a 7kb HindIII-MluI fragment of genomic DNA containing the Rho1 locus was into the pCasper4 transformation vector lacking BamI and EcoRI sites in the polylinker. The 3.7 kb EcoRI-BamHI fragment within the coding region was excised and replaced with the corresponding 1.2 kb fragment from the Rho1 cDNA. This Rho1 rescue construct vector (500 μg/mL) was injected along with the pTURBO helper plasmid (100 μg/mL) (Mullins et al., 1989) into isogenic w1118 flies as described (Spradling, 1986). Transgenics were scored by eye color and the insertions were mapped and balanced using standard genetic methods. This transgene rescues the Rho11B allele to viability.
Cell culture
Drosophila cell lines used in this study: S2, S2R+ (S. Yanagawa), and S2hs-Wg (S. Cumberledge). Cells were grown at 25°C in Schnieder’s medium (Invitrogen) supplemented with 10% heat-inactivated FBS, 25mM glutamine, penicillin and streptomycin.
RNAi in cells
To generate Rho1 dsRNA, the Rho1 ORF was cloned into pBluescript in both orientations. Templates were produced by linearizing the constructs with KpnI or BamHI. ssRNA was transcribed from the T7 promoter of each template using the Megascript RNA production kit (Ambion), annealed, and dsRNA purified as per the kit protocol. Cells were washed 1X in Schneider’s medium without FBS, and resuspended in medium without FBS containing 45μg Rho dsRNA/mL (experimental) or no dsRNA (control). Cells were placed in 6-well culture dishes coated with gelatin and incubated at 25°C for 1hr. 2mL Schneider’s medium with FBS was added to each well and the cells were incubated at 25°C for 3 days.
To assess RNAi efficiency, cell lysates were made by resuspending 1 well of cells in 200μL L-buffer +protease inhibitors, sonicating 3x 10 sec and spinning out cellular debris. Lysates were separated by SDS-PAGE and analyzed by Western blot.
Wg treatment of S2R+ cells
Wg-conditioned medium was produced by culturing S2hs-Wg cells in Schneider’s medium +FBS, heat shocking them at 37°C for 30min and allowing them to recover at least 3 hrs. Following this recovery period, the S2hs-Wg cells were spun down, and the medium added to S2R+ cells that were then incubated at least 3hrs at 25°C. The biochemical responses of these cells to Wg exposure were assessed by making lysates and analyzing them by SDS-PAGE and Western blots, or by fixing and staining cells (see below).
Immunofluorescence
Cells
Cells were grown in 6-well culture dishes coated with gelatin and containing a coverslip. Following experimental treatment (RNAi +Wg exposure), the coverslips were removed and the cells fixed in 4% formaldehyde in PBS for 15min. They were washed 3X in PBS +0.1% Tween, then incubated in 1º antibody for 1hr. They were again washed 3X in PBS +0.1% Tween, then incubated in 2º antibody +propidium iodide as described (Magie et al., 2002) for 1hr. They were then washed 3X in PBS +0.1% Tween, visualized on a Leica TCS confocal microscope and processed with Adobe Photoshop.
Experiments examining dextran uptake were conducted by adding 0.25ug/mL Texas-Red-conjugated Dextran-70kDa (Molecular Probes) to the Wg-conditioned medium used for Wg-treatment. Following Wg-treatment, cells were washed 3X in PBS to remove non-internalized dextran, then fixed as described.
Embryos
Embryos were prepared and detection of proteins was performed as described (Parkhurst et al., 1990). Propidium iodide staining was done as described (Magie et al., 2002).
1º antisera used: anti-DE-cadherin (H. Oda; 1:100); anti-Dah (T. Hsieh; 1:50); anti-DER (N. Baker; 1:500); anti-Wg and anti-Arm (Developmental Studies Hybridoma Bank; 1:100 and 1:50); anti-phosphotyrosine (4G10; Upstate Biotechnology; 1:1000); anti-Rho1 P1D9 (Magie et al., 2002); anti-Hh (I. Guerrero; 1:600) and anti-dpERK (Cell Signaling Technologies; 1:500).
2º antisera used: anti-mouse or anti-rat Alexa 488, or anti-rabbit Alexa 568 (Molecular Probes; 1:2000).
Quantitation of Arm stripe widths was performed by independently identifying the limits of stripe and interstripe regions with dotted lines, splitting the difference when these did not overlap exactly. The percentage of the total distance between stripes 2 and 7 that was covered by Arm expression was determined for each embryo scored, and these results averaged for each genotype.
Extracellular Wg staining
Embryos were dechorionated by hand, lined up on double stick tape and covered with 4% formaldehyde in PBS without detergent. The vitelline envelope was punctured with a glass needle, and the embryos fixed for 10′. They were then devitellinized by hand, incubated in 1º anti-Wg (1:50) in PBS for 1hr, washed 3X in PBS +0.1% Tween, incubated in 2º anti-mouse Alexa 488 (Molecular Probes; 1:2000) in PBS +0.1% Tween 30 minutes, washed 3X in PBS +0.1% Tween and mounted for visualization by confocal microscopy. Control experiments utilizing a primary antibody that recognizes α-Spectrin, a cytoplasmic protein, indicate that this technique specifically labels extracellular proteins (data not shown).
Supplementary Material
Acknowledgments
We thank Jon Cooper, James Johndrow, Darren Kamikura, Alicia Rosales, Valeri Vasioukhin, and Sarah Woolner for their advice during the course of this work and their comments on the manuscript. We are grateful to N. Baker, S. Cumberledge, I. Guerrero, T. Hsieh, H. Oda, B. Stramer, G. Struhl, S. Yanagawa, the Bloomington Fly Stock Center, and the Developmental Studies Hybridoma Bank for providing fly stocks, antibodies, and cell lines used in this study. This work was supported by NIH grant GM066847 (to S.M.P).
References
- Adamson P, Marshall CJ, Hall A, Tilbrook PA. Post-translational modifications of p21rho proteins. J Biol Chem. 1992;267:20033–8. [PubMed] [Google Scholar]
- Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Bejsovec A, Wieschaus E. Signaling activities of the Drosophila wingless gene are separately mutable and appear to be transduced at the cell surface. Genetics. 1995;139:309–20. doi: 10.1093/genetics/139.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni-Schnetzler M, Pilch PF. Mechanism of epidermal growth factor receptor autophosphorylation and high-affinity binding. Proc Natl Acad Sci U S A. 1987;84:7832–6. doi: 10.1073/pnas.84.22.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deHart AK, Schnell JD, Allen DA, Tsai JY, Hicke L. Receptor internalization in yeast requires the Tor2-Rho1 signaling pathway. Mol Biol Cell. 2003;14:4676–84. doi: 10.1091/mbc.E03-05-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore PP, Gill GN. Endocytosis and mitogenic signaling. Curr Opin Cell Biol. 1999;11:483–8. doi: 10.1016/s0955-0674(99)80069-6. [DOI] [PubMed] [Google Scholar]
- Dickson RB, Hanover JA, Willingham MC, Pastan I. Prelysosomal divergence of transferrin and epidermal growth factor during receptor-mediated endocytosis. Biochemistry. 1983;22:5667–74. doi: 10.1021/bi00293a033. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Bejsovec A. Functional analysis of Wingless reveals a link between intercellular ligand transport and dorsal-cell-specific signaling. Development. 1998;125:4729–38. doi: 10.1242/dev.125.23.4729. [DOI] [PubMed] [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–24. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Schwabedissen A, Gonzalez-Gaitan M. Gradient formation of the TGF-beta homolog Dpp. Cell. 2000;103:981–91. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Geneste O, Copeland JW, Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. J Cell Biol. 2002;157:831–8. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglione C, Perrimon N, Perkins LA. Quantitative variations in the level of MAPK activity control patterning of the embryonic termini in Drosophila. Dev Biol. 1999;205:181–93. doi: 10.1006/dbio.1998.9102. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M. Signal dispersal and transduction through the endocytic pathway. Nat Rev Mol Cell Biol. 2003;4:213–24. doi: 10.1038/nrm1053. [DOI] [PubMed] [Google Scholar]
- Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Hannus M, Eaton S. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–45. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the Actin Cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hays R, Gibori GB, Bejsovec A. Wingless signaling generates pattern through two distinct mechanisms. Development. 1997;124:3727–36. doi: 10.1242/dev.124.19.3727. [DOI] [PubMed] [Google Scholar]
- Johndrow JE, Magie CR, Parkhurst SM. Rho GTPase Function in Flies: Insights from a Developmental and Organismal Perspective. Biochem Cell Biol. 2004 doi: 10.1139/o04-118. in press. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–9. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Lanzetti L, Di Fiore PP, Scita G. Pathways linking endocytosis and actin cytoskeleton in mammalian cells. Exp Cell Res. 2001;271:45–56. doi: 10.1006/excr.2001.5369. [DOI] [PubMed] [Google Scholar]
- Leung SM, Rojas R, Maples C, Flynn C, Ruiz WG, Jou TS, Apodaca G. Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA. Mol Biol Cell. 1999;10:4369–84. doi: 10.1091/mbc.10.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–9. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Mackay DJ, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–8. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- Magie CR, Meyer MR, Gorsuch MS, Parkhurst SM. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development. 1999;126:5353–64. doi: 10.1242/dev.126.23.5353. [DOI] [PubMed] [Google Scholar]
- Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–82. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Rio DC, Rubin GM. cis-acting DNA sequence requirements for P-element transposition. Genes Dev. 1989;3:729–38. doi: 10.1101/gad.3.5.729. [DOI] [PubMed] [Google Scholar]
- Munn AL. Molecular requirements for the internalisation step of endocytosis: insights from yeast. Biochim Biophys Acta. 2001;1535:236–57. doi: 10.1016/s0925-4439(01)00028-x. [DOI] [PubMed] [Google Scholar]
- Murphy C, Saffrich R, Grummt M, Gournier H, Rybin V, Rubino M, Auvinen P, Lutcke A, Parton RG, Zerial M. Endosome dynamics regulated by a Rho protein. Nature. 1996;384:427–32. doi: 10.1038/384427a0. [DOI] [PubMed] [Google Scholar]
- Murphy C, Saffrich R, Olivo-Marin JC, Giner A, Ansorge W, Fotsis T, Zerial M. Dual function of rhoD in vesicular movement and cell motility. Eur J Cell Biol. 2001;80:391–8. doi: 10.1078/0171-9335-00173. [DOI] [PubMed] [Google Scholar]
- Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–37. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe L, Dougan ST, Gabay L, Raz E, Shilo BZ, DiNardo S. Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development. 1997;124:4837–45. doi: 10.1242/dev.124.23.4837. [DOI] [PubMed] [Google Scholar]
- Parkhurst SM, Bopp D, Ish-Horowicz D. X:A ratio, the primary sex-determining signal in Drosophila, is transduced by helix-loop-helix proteins. Cell. 1990;63:1179–91. doi: 10.1016/0092-8674(90)90414-a. [DOI] [PubMed] [Google Scholar]
- Parkhurst SM, Ish-Horowicz D. wimp, a dominant maternal-effect mutation, reduces transcription of a specific subset of segmentation genes in Drosophila. Genes Dev. 1991;5:341–57. doi: 10.1101/gad.5.3.341. [DOI] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–85. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development. 1994;120:369–80. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Mohler D, Engstrom L, Mahowald AP. X-linked female-sterile loci in Drosophila melanogaster. Genetics. 1986;113:695–712. doi: 10.1093/genetics/113.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr Biol. 2002;12:957–62. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Piddini E, Vincent JP. Modulation of developmental signals by endocytosis: different means and many ends. Curr Opin Cell Biol. 2003;15:474–81. doi: 10.1016/s0955-0674(03)00072-3. [DOI] [PubMed] [Google Scholar]
- Poodry CA. shibire, a neurogenic mutant of Drosophila. Dev Biol. 1990;138:464–72. doi: 10.1016/0012-1606(90)90212-2. [DOI] [PubMed] [Google Scholar]
- Roovers K, Assoian RK. Effects of rho kinase and actin stress fibers on sustained extracellular signal-regulated kinase activity and activation of G(1) phase cyclin-dependent kinases. Mol Cell Biol. 2003;23:4283–94. doi: 10.1128/MCB.23.12.4283-4294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schmalzing G, Richter HP, Hansen A, Schwarz W, Just I, Aktories K. Involvement of the GTP binding protein Rho in constitutive endocytosis in Xenopus laevis oocytes. J Cell Biol. 1995;130:1319–32. doi: 10.1083/jcb.130.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma MP, Fasulo B, Cenci G, Cundari E, Gatti M. Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Mol Biol Cell. 2002;13:2448–60. doi: 10.1091/mbc.01-12-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC. P element-mediated transformation. In: Roberts DB, editor. Drosophila, a Practical Approach. IRL Press; Oxford: 1986. pp. 175–97. [Google Scholar]
- Sprenger F, Nusslein-Volhard C. The Terminal System of Axis Determination in the Drosophila Embryo. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Vol. 1. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 365–86. [Google Scholar]
- Stamnes M. Regulating the actin cytoskeleton during vesicular transport. Curr Opin Cell Biol. 2002;14:428–33. doi: 10.1016/s0955-0674(02)00349-6. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Sturtevant MA, O’Neill JW, Bier E. Down-regulation of Drosophila Egf-r mRNA levels following hyperactivated receptor signaling. Development. 1994;120:2593–600. doi: 10.1242/dev.120.9.2593. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Nusse R, Johnston P, Lawrence PA. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989;59:739–49. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]
- Yanagawa S, Lee JS, Ishimoto A. Identification and characterization of a novel line of Drosophila Schneider S2 cells that respond to wingless signaling. J Biol Chem. 1998;273:32353–9. doi: 10.1074/jbc.273.48.32353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.