Abstract
Oncolytic viruses are being developed as anticancer drugs. They propagate selectively in tumor tissue and destroy it without causing excessive damage to normal non-cancerous tissues. When used as drugs, they must meet stringent criteria for safety and efficacy and be amenable to pharmacological study in human subjects. Specificity for neoplastic tissue is the key to safety, and this goal can be achieved through a variety of ingenious virus-engineering strategies. Antiviral immunity remains a significant barrier to the clinical efficacy of oncolytic viruses but this is being addressed by using novel immune-evasive delivery strategies and immunosuppressive drugs. Noninvasive pharmacokinetic monitoring is facilitated by engineering marker genes into the viral genome. Clinical data on the pharmacokinetics of oncolytic viruses will be the key to accelerating their development and approval as effective anticancer drugs. This review introduces concepts relevant to the use of viruses as anticancer drugs, emphasizing targeting mechanisms as well as safety and efficacy issues that are currently limiting their clinical success.
Introduction
Viruses are nature’s nanoparticles – a vast untapped bioresource. More than 2400 viral species are known, with extraordinarily diverse morphologies and biochemical compositions [1,2]. Their diameters range from 20 to 500 nm, and their genomes from 3000 to 375 000 nucleotides. They have single- or double-stranded RNA or DNA genomes packaged into icosahedral or helical protein shells, which are sometimes wrapped in a lipid envelope. The particle protects the viral genome, carries it from cell to cell in the infected host organism and transmits it from infected to uninfected hosts. Once delivered into a susceptible target cell, the viral genome usurps the cellular biosynthetic machinery to manufacture progeny viruses that spread to adjacent cells, leading to a characteristic pattern of tissue destruction. This provokes innate and adaptive immune responses (cellular and humoral), which combat the infection and protect the host from future exposures to the same virus. From one pharmacological perspective, the viral genome can be seen as a new class of tissue-destructive drug, and the viral particle as a nanosized nucleic acid delivery vehicle.
An oncolytic virus is one that propagates selectively in tumor tissue and destroys it without causing excessive damage to normal non-cancerous tissues [3]. Interest in this approach has fluctuated widely during the past century, reaching fever pitch in the 1950s, followed by near abandonment in the 1970s and a resurgence of interest in the 1990s [4]. The first marketing approval for an oncolytic virus was granted by Chinese regulators in 2005. The virus was the H101 type 5 adenovirus, which carries E1B-55KD and partial E3 gene deletions. Approval was granted based on superior response rates in head and neck cancer patients treated with combined intratumoral H101 plus chemotherapy compared with those treated with chemotherapy alone. Durability of these responses was not determined and prolongation of survival was not shown [5]. Numerous additional oncolytic viruses are currently in Phase I and II clinical testing in many different countries. However, for viruses to be more widely approved and used as anticancer drugs, they will have to meet stringent criteria for safety and efficacy, and should be amenable to pharmacological study in human subjects. These three issues are the subject of this review, exemplified where possible by reference to oncolytic measles viruses because these are the agents that are most intensively studied in our laboratories.
Safety
For safety, oncolytic viruses must be highly cancer specific, causing minimal damage and destruction to normal tissues. Consideration should also be given to the possibility that an oncolytic virus could evolve into a pathogen as it propagates in the patient, and to the possibility of person-to-person transmission, either of the original oncolytic virus or of a pathogenic derivative [6,7]. All possible steps should be taken to minimize these risks, including the development of a ‘worst-case scenario’ contingency plan.
Specificity
The specificity problem has been effectively addressed and we are currently blessed with a diverse armamentarium of oncolytic viruses with proven ability to propagate selectively in tumor tissue (Table 1). A few are naturally oncolytic [8] or have serendipitously evolved during tissue culture passage to become oncolytic [9], but most have been engineered in some way to enhance their tumor specificity. Targeting mechanisms that have been exploited to date can be classified into the four broad categories of transcriptional, translational, transductional and pro-apoptotic. Each of these mechanisms is further explained below in relation to the viral replication cycle (Box 1). Each of the targeting mechanisms is shown in Figure 1.
Table 1.
Selected oncolytic viruses with different mechanisms of tumor selectivitya
| Family | Genome (kb) | Structure | Examples | Mechanismb | Refs |
|---|---|---|---|---|---|
| Adenoviridae | dsDNA | Non-enveloped | Onyx-015 | 3 (ΔE1B) | [68] |
| 36–38 | Icosahedral | HB101 | 3 (ΔE1B, ΔE3) | [5] | |
| 70–90 nm | Ad-hTERT | 1,3 (hTERT-E1) | [69] | ||
| CG7870 | 1,3(PSA-E1B, probasin-E1A) | [70] | |||
| Δ-24 RGD (E1A deleted) | 3,4 (ΔE1B, avb3) | [71] | |||
| Herpesviridae | dsDNA | Enveloped | NV1020 | 2 (Δγ34.5) | [72] |
| 120–200 | 150–200 | G207 | 2 (Δγ34.5, ΔICP6) | [73–75] | |
| OncoVexGMCSF | 2 (Δγ34.5, ΔICP47) | [76,77] | |||
| KeM34.5 | 1,2 (Musashi1-γ34.5) | [78] | |||
| Parvoviridae | ssDNA | Non-enveloped | H1 | 1 (P4 promoter) | [79] |
| 5 | Icosahedral | ||||
| 18–26 | |||||
| Poxviridae | dsDNA | Enveloped | Vaccinia JX594-GM-CSF | 2 (ΔTK) | [80] |
| 130–280 | 200 × 400 | Vaccinia vSP | 3 (ΔSP1, ΔSP2) | [81] | |
| Myxoma virus | 2 (Akt, ERK) | [82,83] | |||
| Coronaviridae | +ssRNA | Enveloped | Murine hepatitis virus | 4 (pseudoreceptor) | [84] |
| 16–21 | 80–160 | Feline infectious peritonitis virus | 4 (EGFR adaptor) | [85] | |
| Orthomyxoviridae | −ssRNA | Enveloped | Influenza A delNS1 virus | 2 (ΔNS1) | [86] |
| 13.6 | 90–120 | ||||
| Paramyxoviridae | −ssRNA | Enveloped | Mumps virus | 2 (IFN defects) | [87,88] |
| 16–20 | Helical | MV-CEA | 4 (CD46) | [57,60] | |
| 150–300 | MV-NIS | 4 (CD46) | [59] | ||
| MV-EGFR, MV-CD38 | 4 (EGFR, CD38) | [21] | |||
| MV-αFR | 4 (αFR) | [89] | |||
| Newcastle disease virus PV701 | 2 (IFN defects) | [90] | |||
| Piconarviridae | +ssRNA | Non-enveloped | Poliovirus PV1(RIPO) | 2 (HRV2 IRES) | [91] |
| 7.2–8.4 | Icosahedral | Echovirus EV1 | 4 (α2β1) | [92] | |
| 28–30 | Coxsackie CVA21 | 4 (ICAM-1, DAF) | [93] | ||
| Seneca Valley virus | 4 (unknown receptor) | c | |||
| Reoviridae | dsRNA | Non-enveloped | Reovirus (Reolysin®) | 2,4 (Ras, PKR, protease cleavage) | [94,95] |
| 22–27 | Icosahedral | ||||
| 60–80 | |||||
| Retroviridae | +ssRNA | Enveloped | MLV (ACE-CD) | Dividing cells | [96] |
| 8 | Icosahedral | MLV (ACE-Pb) | 1 (probasin) | [97] | |
| 80–100 | MLV (AZE-ZZ-GFP) | 4 (HER2) | [98] | ||
| Rhabdoviridae | −ssRNA | Enveloped | VSV-MΔ51 | 2 (matrix mutant) | [14] |
| 11–12 | Helical | VSV-IFN-β | 2 (IFN defects) | [99] | |
| 180 × 75 | |||||
| Togaviridae | +ssRNA | Enveloped | Sindbis virus | 4 (laminin receptor) | [100] |
| 12 | 60–70 |
Abbreviations: DAF, decay accelerating factor; EGFR, epidermal growth factor receptor; FR, folate receptor; HRV2 IRES, human rhinovirus type 2; hTERT, human telomerase promoter; ICAM, intracellular cell adhesion molecule-1; ICP, infected cell protein; NS1, nonstructural 1; PSA, prostate-specific antigen; SP1,2, viral serine protease inhibitors; TK, thymidine kinase.
Key: 1, transcriptional targeting; 2, translational targeting; 3, pro-apoptotic targeting; 4, transductional targeting.
Presentation at the 4th International Conference on Oncolytic Viruses and Cancer Therapeutics, Arizona.
Box 1. Virus replication.
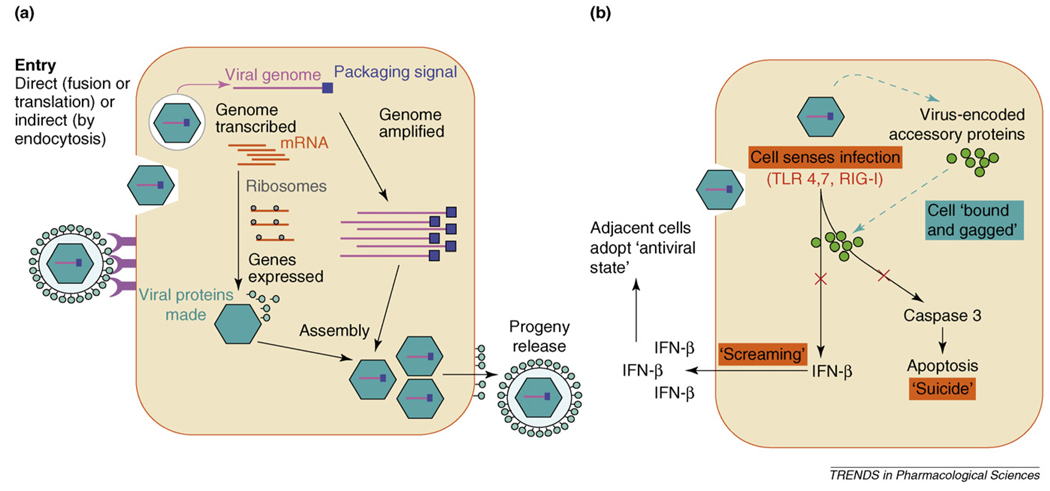
Attachment is the first step in the virus life cycle (Figure I). Attachment proteins on the surface of the virion interact with specific receptors on the surface of the target cell. Attachment provides the trigger for entry, wherein the viral genome is delivered into the cytoplasm of the target cell. Entry occurs by membrane fusion for enveloped viruses and by endosomal disruption or particle translocation across the target cell membrane for non-enveloped viruses. Once inside the infected cell, viral genomes are transported to specific nuclear or cytoplasmic destinations where they can be expressed and replicated. The viral genome is then copied and amplified, the viral genes are expressed and the structural proteins are assembled to form new virus particles, which interact with the packaging signal sequences in the progeny viral genomes to form fully infectious, nucleic-acid-carrying progeny virus particles that are released from the cell. Viral genes encoding nonstructural and structural proteins are typically expressed sequentially. The nonstructural proteins, which are expressed earliest, have several functions. They drive the amplification of the viral genome, induce the expression of the structural (late) proteins and maintain the viability of the target cell long enough so that it can make viable progeny viruses.
The first response of a cell, upon sensing that it has been infected by a virus, is to ’scream’ by releasing IFN-β. Surrounding uninfected cells respond to the interferon signal by activating their viral defense mechanisms and suppressing their translational machinery so that they become poor substrates to support virus propagation. The second response of the infected cell is to activate its apoptotic program so that it will die before it releases progeny viruses. Unsurprisingly, viruses have evolved to suppress both apoptosis and the interferon response using many different mechanisms. Infected cells are, thereby, ‘bound’, ‘gagged’ and exploited as virus-producing factories.
Figure 1.
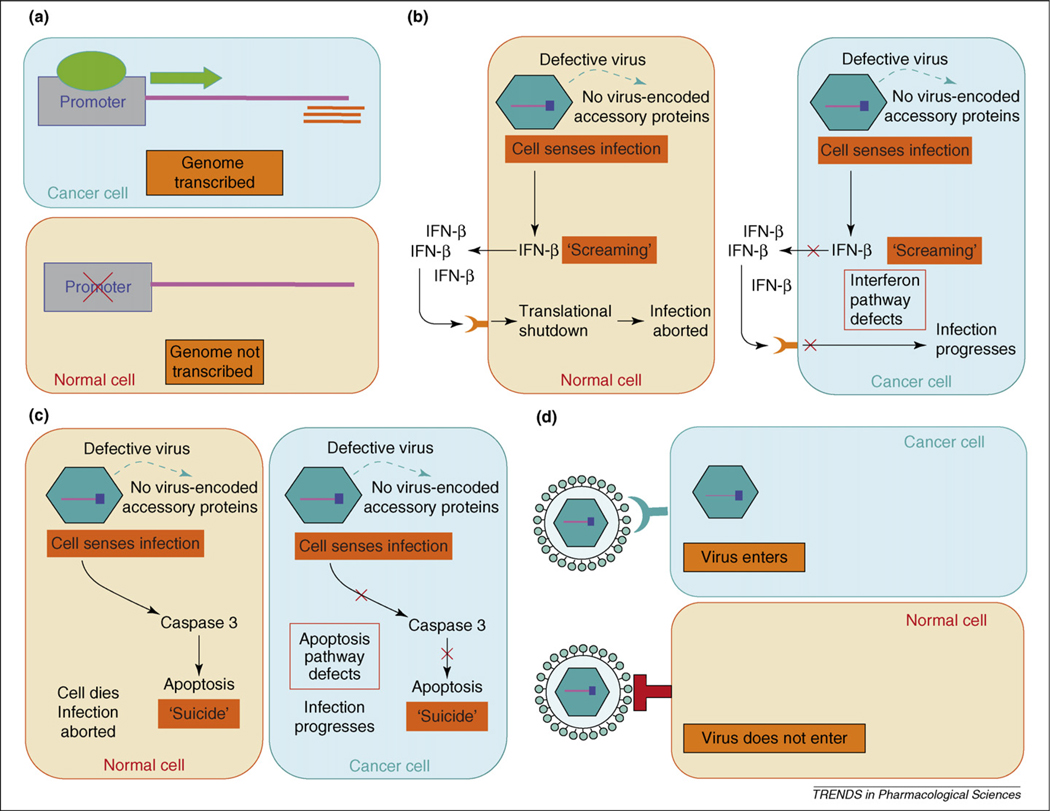
Mechanisms of tumor targeting by oncolytic viruses. (a) Transcriptional targeting. An essential viral gene is placed under the control of a tumor-specific promoter (some virus promoters are naturally tumor specific). Typically, the selected gene encodes an early viral protein that is essential for successful completion of the virus life cycle. This is applicable only to DNA viruses (excluding poxviruses) and retroviruses. (b) Translational targeting. The virus is engineered (or adapted) to disable viral proteins that antagonize the cellular interferon (IFN) response. Normal cells then release interferon upon infection, causing neighboring cells to shut-off translation. Infected cancer cells are impaired in their ability to release or respond normally to interferon. (c) Pro-apoptotic targeting. The virus is engineered (or adapted) to disable viral proteins that prevent apoptosis. Normal cells then die quickly upon infection before progeny viruses can be produced. Infected cancer cells are impaired in their ability to undergo apoptosis. Hence, the virus can generate progeny and spread only in the cancer cells. (d) Transductional targeting. The virus gains entry to its target cells through a receptor expressed more abundantly on tumor cells than on normal cells. The natural receptors for several viruses fall into this category. Alternatively, the attachment specificity of the virus can be reprogrammed towards tumor antigens by the display of single-chain antibodies or other polypeptide-binding ligands on the viral surface.
Pro-apoptotic targeting
Adenoviruses can be rendered tumor selective by mutating or deleting their E1 genes, whose products are essential for virus propagation in normal cells but not in tumor cells [10,11]. The E1a and E1b gene products prevent apoptosis and cell-cycle arrest in the infected cells by inhibiting p53 and Rb, respectively. Normal cells undergo rapid Rb-induced cell-cycle arrest and p53-induced apoptosis when infected by an oncolytic adenovirus lacking E1 functions, and the propagation of the virus is, thereby, aborted. However, in tumor cells in which the p53 and Rb proteins are frequently nonfunctional, the E1-deleted adenovirus can still propagate efficiently without fear of triggering premature apoptosis [12,13].
Translational targeting
This targeting strategy provides a way to exploit defects in interferon signaling pathways in tumor cells. Virus infections tend to provoke the induction of type I interferons but successful viruses suppress this response (Box 1). When viruses are mutated to destroy their interferon suppression mechanisms, the release of interferon from the infected cell signals to neighboring cells to shut down protein synthesis, leading to the establishment of an antiviral state. In contrast to normal cells, tumor cells have defective interferon signaling pathways and, therefore, remain susceptible to the virus, even after exposure to interferon. Vesicular stomatitis virus (VSV) infection provokes transcription of interferon genes but the virally encoded matrix (M) protein, by inhibiting mRNA transport, blocks the synthesis and release of active interferons. VSVs can, therefore, be rendered nonpathogenic by mutating the M protein so that it no longer inhibits interferon release [14] or by inserting an interferon gene into the VSV genome [15]. These nonpathogenic VSVs are still oncolytic because tumor cells are defective in their ability to produce and respond to interferon and, therefore, efficiently support the propagation of the defective viruses [16]. A second translational targeting approach, employed recently and with good effect to develop an oncolytic poliovirus, is to restrict the expression of viral genes by placing them under the control of a tissue-specific internal ribosome entry site [17].
Transcriptional targeting
Adenoviruses, herpes viruses and retroviruses can be rendered tumor selective by inserting tissue-specific or tumor-specific promoters in their genomes to regulate the expression of viral genes, whose products are essential for virus propagation. For adenoviruses, this has been achieved by placing the E1 gene under the control of a foreign promoter [10,11]. For oncolytic herpes viruses, transcriptional targeting efforts have focused on γ34.1, one of the key early genes [18]. For retroviruses, the U3 promoter–enhancer region in the long terminal repeat has been replaced [19].
Transductional targeting
Virus attachment proteins can be adapted to use receptors that are expressed preferentially or exclusively on tumor cell surfaces or to mediate virus entry only when exposed to proteases that are abundant in the tumor microenvironment [20]. Both of these approaches have been extensively validated using recombinant measles viruses [21,22]. The measles virus has two envelope glycoproteins: the hemagglutinin (H) attachment protein and the fusion (F) protein. To redirect virion binding to tumor-associated receptors, ligands such as growth factors and single-chain antibodies are inserted as C-terminal extensions of the H protein, mutated to ablate its interactions with measles receptors, CD46 and SLAM (signaling lymphocyte activation molecule). These fully re-targeted viruses can infect and kill only those cells expressing the cognate receptor for the displayed ligand. In a second transductional targeting approach, protease-sensitive linkers are inserted into the F protein (between F1 and F2) so that proteolytic maturation of the F protein occurs exclusively at sites at which the protease is abundantly expressed. Measles viruses that can be selectively activated by tumor-associated matrix metalloproteinases have recently been described [22].
Evolving viruses
Viruses evolve by mutation and selection [23]. Viral mutation rates are generally relatively high (higher for RNA viruses than for DNA viruses) such that many, if not most, viruses in a ‘pure’ virus preparation carry a point mutation somewhere in their genome. A virotherapy product cannot, therefore, be so precisely defined as a small molecule because it is actually a swarm of quasispecies that are closely related, but not identical, to a consensus product [24]. Under selective pressure in the treated cancer patient, these viruses or their in vivo progeny might acquire the ability to infect non-target tissues, to evade the host immune response or to spread from the patient to their carers. These risks are likely to vary greatly depending on the biology and prevalence of the oncolytic virus in question. The risk of oncolytic virus transmission from a treated patient to their carer is expected to be higher when the virus is derived from an animal pathogen than when it is derived from a human vaccine. Oncolytic measles viruses are considered extremely safe in this regard. The measles virus was originally isolated in 1954 from the throat swab of a measles-infected patient (David Edmonston) and was attenuated by extensive tissue culture passage [25]. Most of the live attenuated measles vaccines in current use were derived from this Edmonston lineage, as were the oncolytic strains of measles virus that are currently under investigation. Because of the successful global deployment of the measles vaccine, most humans are immune to measles [26]. Hence, even if an oncolytic measles virus were to evolve new pathogenic potential, transmission from patient to (measles-immune) carer would be highly unlikely. The scenario of an oncolytic measles virus evolving to become a pathogen is highly unlikely because reversion to pathogenicity of the closely related Edmonston-derived measles vaccine has not been documented in more than a billion vaccinees, the vaccine strain is not shed in saliva or urine, and person-to-person vaccine transmission has not been convincingly documented [27]. Finally, in contrast to other viruses such as influenza, measles is monotypic and has an extremely slow rate of evolution. This explains how anti-measles immunity acquired by vaccination with the Edmonston vaccine strain, originally isolated in 1954 [25], is still strongly protective against all currently circulating measles strains, more than 50 years later.
Efficacy
For efficacy, oncolytic viruses must be capable of penetrating host defenses to access growing tumors, whether primary or metastatic. They must also be capable of propagating sufficiently at the target site to destroy infected tumors before the infection is controlled and eliminated by the immune system.
Virus delivery via the bloodstream
Cancer patients have many antiviral defense mechanisms that all need to be penetrated or overcome for the oncolytic virus to be effective. For example, viruses in the bloodstream can be neutralized by antibodies and complement, bound by receptor-positive non-target cells or cleared by phagocytes in the liver and the spleen [3]. Intravenously administered viruses are, therefore, eliminated rapidly from the circulation, and this process becomes faster after each subsequent exposure because of increasing antiviral immunity. So formidable are the barriers to efficient and accurate vascular delivery of viruses that skepticism has been expressed as to whether these agents will ever be exploitable as systemic therapies. However, against this uninformed negativity, the virotherapy research community is developing a broad range of viable solutions to the intravascular (intravenous or intra-arterial) delivery problem.
Serotype switching
One approach is to administer a different viral serotype at each successive treatment cycle. For many viruses (e.g. adenovirus and VSV), there are several naturally occurring serotypes that, by definition, are resistant to neutralization by antisera against the other serotypes [28,29]. For certain viruses, it is also possible to modify the viruses by engineering or evolving them so that they are no longer neutralized by antibodies raised against the original virus [30]. The serotype-switching approach is not applicable to monotypic viruses such as measles.
Polymer coating
Polymer coating of viruses is another way to block antibody recognition [31]. 90% of primary amino groups on the surface of adenovirus particles became modified by the polymer when adenoviruses were mixed with poly [N-(2-hydroxypropyl)methacrylamide] (pHPMA) bearing reactive 4-nitrophenyl (ONp) esters on pendent diglycyl side-chains. The polymer-coated viruses have extended circulation times in vivo but this prevents the virus from binding to its cellular receptors. Infectivity can be restored, at least partially, by incorporating cell-targeting ligands into the polymer coating [32].
Antibody depletion
An alternative to virus modification is to reduce the concentration of circulating antiviral antibodies in the patient before infusing the virus [33]. This might be achieved by infusion of soluble viral antigens, passing blood through antigen-loaded columns or immunizing with antiviral antibodies to provoke an anti-idiotypic antibody response that sequesters antiviral antibodies and prevents them from interacting with infused viral particles Another approach to reducing the concentration of antiviral antibodies is through the destruction of virus-specific B lymphocytes or antibody-producing plasma cells with steroids, cyclophosphamide or anti-B-cell antibodies [34]. However, reductions in the concentration of neutralizing antibodies after terminating their production are very slow because the serum half-life of antiviral IgG is longer than 20 days [35,36]. Plasmapheresis is routinely used in the clinic for depletion of IgM antibodies or immune complexes but the approach, although straightforward, is relatively ineffective for depleting circulating IgG.
Antibody evasion
As an alternative to changing the virus coat or to suppressing antibody responses, recent data indicate that it could be possible to ‘hide’ a virus so that it cannot be seen or bound by antiviral antibodies as it transits the blood-stream to gain access to the tumor cell population [37]. This can be achieved either by using virus-infected cells as carriers to transport the virus to its target cell population [33,38–40] or by delivering the virus genome to target cells as non-immunogenic infectious nucleic acid [41]. Both of these approaches have the potential to circumvent phagocytic clearance mechanisms that sequester viruses in the liver and the spleen, but they must be used in conjunction with effective targeting strategies to minimize the transduction of non-target tissues.
Virus extravasation
The extravasation of intravenously administered viruses into the parenchyma of a tumor is another important variable, which is influenced by both the size of the viral particles (smaller nanoparticles extravasate better) [42] and the permeability of the tumor blood vessels. In mouse xenograft models, and perhaps in some primary and metastatic human cancers, the permeability of blood vessels is greater at the tumor periphery, and oncolytic viruses extravasate more efficiently at that location [43–45]. Vascular permeability can be increased by local expression of vascular permeability factors such as vascular endothelial growth factor or by local inflammation secondary to treatment with external beam radiation or chemotherapy [46]. An alternative strategy for enhancing viral extravasation from tumor blood vessels is to engineer their attachment proteins to bind to receptors expressed on tumor blood vessel endothelium [47,48]. Viruses with dual specificity for neovessel endothelium and antigens expressed on tumor cells are required to ensure that intratumoral propagation can proceed after the virus has been transported across the endothelial lining of the tumor blood vessels.
Intratumoral spread of the virus
Depending on the virus, the time from infection to target-cell death can range from a few hours to a few days, and the number of progeny released from a single infected cell (burst size) can range from1 to 100 000. Thus, in the absence of an immune system, oncolytic viruses spread through tumors at widely differing speeds [49]. In the presence of an immune system, antiviral immunity is the major host factor that serves to modulate the speed of intratumoral virus propagation [50].Most important in this regard is the cellular arm of the immune system, which controls the spread of infection by destroying infected cells before they have a chance to release their viral progeny.
Immunosuppression
Antiviral cytotoxic T lymphocyte responses can be suppressed by immunosuppressive drugs, thereby promoting the intratumoral spread of an oncolytic virus. Cyclophosphamide is an attractive immunosuppressive drug to use in combination with oncolytic virotherapy because rapidly dividing lymphocytes are exquisitely sensitive to its cytotoxic actions and it has proven activity as an effective anti-neoplastic agent used extensively in the treatment of human malignancy [51]. Cyclophosphamide is well tolerated, available at low cost and strongly inhibits antiviral immune responses, whether primary or anamnestic, B cell or T cell, when administered at the appropriate time and dose following virus exposure [52–55]. Thus, cyclophosphamide can greatly increase the efficiency of virus spread from infected tumor cells to adjacent uninfected cells [56] and can help to limit increases in the antiviral antibody titer between successive viral doses.
Pharmacological monitoring
Pharmacokinetics describes the fate of a drug in the body, including its absorption, distribution, biotransformation and excretion, and has not been adequately addressed in previous human virotherapy studies. Key pharmacokinetic questions, usually left unanswered in Phase I virotherapy trials, are: (i) where do the virus particles go and how many reach the target site? (ii) How many target cells get infected, where are they located and what is their identity? (iii) Is the viral genome expressed in the infected cells and, if so, at what level and for how long? (iv) How many progeny viruses are released by infected cells, when are they released and where do they go? (v) How fast and how far does the virus spread and when is it eliminated from the body? Answers to these important questions are routinely obtained in preclinical studies by direct analysis of tissue samples harvested from multiple sites at multiple time-points but such analyses are not feasible in human trials. To study the pharmacokinetics of oncolytic viruses in a clinical setting, we need convenient, noninvasive monitoring strategies that can be used to quantify the expression level of virus-encoded proteins and to map the distribution of virus-infected cells. Repeat testing should be undertaken in each treated patient to track the spread of the virus and to determine the time course of viral gene expression. Marker genes can be incorporated into the viral genome to facilitate this type of clinical monitoring.
Oncolytic measles viruses coding for the soluble extracellular domain of human carcinoembryonic antigen (MV-CEA) were recently generated to facilitate the noninvasive monitoring of virus propagation in human subjects through serial measurement of serum carcinoembryonic antigen (CEA) concentrations [57,58]. A second recombinant oncolytic measles virus (MV-NIS) – coding for the human thyroidal sodium iodide symporter (NIS), a membrane ion channel responsible for transporting iodine into thyroid follicular cells – was then generated [59]. MV-NIS-infected cells concentrate radioactive iodine from the bloodstream, enabling the status of an infection to be monitored by serial noninvasive single-photon-emission computed tomography (SPECT) or positron-emission tomography (PET) imaging using 123I or 124I as tracers. These two recombinant measles viruses are currently being tested in Phase I dose-escalation clinical trials in patients with ovarian cancer (MV-CEA), glioma (MV-CEA) or multiple myeloma (MV-NIS) [59–62].
Transgenes encoding certain intracellular enzymes or non-signaling cell-surface receptors can also facilitate imaging. Thus, the herpes simplex virus thymidine kinase enzyme can phosphorylate and trap a radioactive 2′-fluoro-2′-deoxy-1-β-d-β-arabinofuranosyl-5-iodouracil (FIAU) tracer in virus-infected cells, so viruses expressing this transgene can be imaged by PET or SPECT [63–65]. Also, a mutated dopamine D2 receptor through which dopamine is unable to signal can trap a PET-labeled ligand tracer at sites of virus propagation [65,66]. These new monitoring strategies are expected to enhance the quality of the pharmacological information gained from early-stage clinical virotherapy trials and this will provide a rational basis for intelligent protocol modifications that will hasten the inevitability of clinical success.
Concluding remarks
After a long incubation period, oncolytic viruses are finally emerging as potentially useful anticancer drugs. Data currently emerging from ongoing Phase I and Phase II clinical trials are extremely encouraging, showing that tumor regressions can occur even after systemic virus delivery [67]. However, significant challenges remain. In particular, the clinical potency of oncolytic viruses must be increased if they are to become a truly effective cancer therapy, and it seems likely that their clinical potential will be fully realized only when they can be safely administered to patients receiving concurrent immunosuppressive therapy. To ensure their safe deployment in the setting of antibody depletion and transient immunosuppression, it is anticipated that future oncolytic viruses will have to be not only more potent but also more tumor specific than those currently tested, and amenable to noninvasive pharmacological monitoring.
Figure I.
Virus replication. (a) Viral invasion of the cell. (b) The cellular response to viral infection and the suppression of this response by viral accessory proteins. Abbreviations: RIG, retinoic-acid-inducible protein-1; TLR, Toll-like receptor.
Acknowledgements
We are supported by NIH grants (CA100634 and HL66958 to S.J.R., and CA118488 to K-W.P.).
Footnotes
Disclosure statement
S.J.R. and K-W.P. are named inventors on patents owned by the Mayo Clinic regarding oncolytic measles that have been licensed to a biotechnology company (Houston Pharma).
References
- 1.Condit RC. Principles of virology. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams and Wilkins; 2001. pp. 19–51. [Google Scholar]
- 2.Harrison SC. Principles of virus structure. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams and Wilkins; 2001. pp. 53–85. [Google Scholar]
- 3.Parato KA, et al. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 4.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 5.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 6.Russell SJ. Replicating vectors for cancer therapy: a question of strategy. Semin. Cancer Biol. 1994;5:437–443. [PubMed] [Google Scholar]
- 7.Russell SJ. Replicating vectors for gene therapy of cancer: risks, limitations and prospects. Eur. J. Cancer. 1994;30A:1165–1171. doi: 10.1016/0959-8049(94)90477-4. [DOI] [PubMed] [Google Scholar]
- 8.Geletneky K, et al. Oncolytic potential of rodent parvoviruses for cancer therapy in humans: a brief review. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2005;52:327–330. doi: 10.1111/j.1439-0450.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- 9.Roberts MS, et al. Naturally oncolytic viruses. Curr. Opin. Mol. Ther. 2006;8:314–321. [PubMed] [Google Scholar]
- 10.Dobbelstein M. Replicating adenoviruses in cancer therapy. Curr. Top. Microbiol. Immunol. 2004;273:291–334. doi: 10.1007/978-3-662-05599-1_9. [DOI] [PubMed] [Google Scholar]
- 11.Ko D, et al. Development of transcriptionally regulated oncolytic adenoviruses. Oncogene. 2005;24:7763–7774. doi: 10.1038/sj.onc.1209048. [DOI] [PubMed] [Google Scholar]
- 12.Genovese C, et al. Cell cycle control and beyond: emerging roles for the retinoblastoma gene family. Oncogene. 2006;25:5201–5209. doi: 10.1038/sj.onc.1209652. [DOI] [PubMed] [Google Scholar]
- 13.Bouchet BP, et al. p53 as a target for anti-cancer drug development. Crit. Rev. Oncol. Hematol. 2006;58:190–207. doi: 10.1016/j.critrevonc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Stojdl DF, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 15.Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 16.Stojdl DF, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 17.Gromeier M, et al. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung RY, et al. B-myb promoter retargeting of herpes simplex virus γ34.5 gene-mediated virulence toward tumor and cycling cells. J. Virol. 1999;73:7556–7564. doi: 10.1128/jvi.73.9.7556-7564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalba C, et al. Beyond oncolytic virotherapy: replication-competent retrovirus vectors for selective and stable transduction of tumors. Curr. Gene Ther. 2005;5:655–667. doi: 10.2174/156652305774964659. [DOI] [PubMed] [Google Scholar]
- 20.Peng KW, Russell SJ. Viral vector targeting. Curr. Opin. Biotechnol. 1999;10:454–457. doi: 10.1016/s0958-1669(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 22.Springfeld C, et al. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006;66:7694–7700. doi: 10.1158/0008-5472.CAN-06-0538. [DOI] [PubMed] [Google Scholar]
- 23.DeFilippis VR, Villarreal LP. Virus evolution. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams and Wilkins; 2001. pp. 353–370. [Google Scholar]
- 24.Domingo E, et al. Viruses as quasispecies: biological implications. Curr. Top. Microbiol. Immunol. 2006;299:51–82. doi: 10.1007/3-540-26397-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enders JF, Peebles TC. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 1954;86:277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- 26.de Quadros CA. Is global measles eradication feasible? Curr. Top. Microbiol. Immunol. 2006;304:153–163. doi: 10.1007/3-540-36583-4_9. [DOI] [PubMed] [Google Scholar]
- 27.Hilleman MR. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine. 2001;20:651–665. doi: 10.1016/s0264-410x(01)00384-x. [DOI] [PubMed] [Google Scholar]
- 28.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr. Gene Ther. 2006;6:215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez I, et al. Recombinant vesicular stomatitis (Indiana) virus expressing New Jersey and Indiana glycoproteins induces neutralizing antibodies to each serotype in swine, a natural host. Vaccine. 2004;22:4035–4043. doi: 10.1016/j.vaccine.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 30.Novella IS, et al. Adaptability costs in immune escape variants of vesicular stomatitis virus. Virus Res. 2005;107:27–34. doi: 10.1016/j.virusres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Green NK, et al. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson M, et al. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via α6-integrins. Cancer Gene. Ther. 2007;14:335–345. doi: 10.1038/sj.cgt.7701022. [DOI] [PubMed] [Google Scholar]
- 33.Ong HT, et al. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 34.Sperr WR, et al. Rituximab for the treatment of acquired antibodies to factor VIII. Haematologica. 2007;92:66–71. doi: 10.3324/haematol.10553. [DOI] [PubMed] [Google Scholar]
- 35.Thurmann P, Harder S. Criteria for the appropriate drug utilisation of immunoglobulin. Pharmacoeconomics. 1996;9:417–429. doi: 10.2165/00019053-199609050-00005. [DOI] [PubMed] [Google Scholar]
- 36.Thurmann PA, et al. Pharmacokinetics of viral antibodies after administration of intravenous immunoglobulin in patients with chronic lymphocytic leukaemia or multiple myeloma. Eur. J. Clin. Pharmacol. 2001;57:235–241. doi: 10.1007/s002280100305. [DOI] [PubMed] [Google Scholar]
- 37.Power AT, Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol. Ther. 2007;15:660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- 38.Power AT, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 39.Iankov ID, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol. Ther. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 40.Komarova S, et al. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 41.Carlisle RC, et al. Use of synthetic vectors for neutralising antibody resistant delivery of replicating adenovirus DNA. Gene Ther. 2006;13:1579–1586. doi: 10.1038/sj.gt.3302814. [DOI] [PubMed] [Google Scholar]
- 42.Yuan F, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 43.Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9:253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- 44.Liu P, et al. Study of non-uniform nanoparticle liposome extravasation in tumour. Int. J. Hyperthermia. 2005;21:259–270. doi: 10.1080/02656730500068643. [DOI] [PubMed] [Google Scholar]
- 45.Demers GW, et al. Pharmacologic indicators of antitumor efficacy for oncolytic virotherapy. Cancer Res. 2003;63:4003–4008. [PubMed] [Google Scholar]
- 46.Monsky WL, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–4135. [PubMed] [Google Scholar]
- 47.Oh P, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 48.Hallak LK, et al. Targeted measles virus vector displaying echistatin infects endothelial cells via αvβ3 and leads to tumor regression. Cancer Res. 2005;65:5292–5300. doi: 10.1158/0008-5472.CAN-04-2879. [DOI] [PubMed] [Google Scholar]
- 49.Novozhilov AS, et al. Mathematical modeling of tumor therapy with oncolytic viruses: regimes with complete tumor elimination within the framework of deterministic models. Biol. Direct. 2006;1:6. doi: 10.1186/1745-6150-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wein LM, et al. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res. 2003;63:1317–1324. [PubMed] [Google Scholar]
- 51.Hill DA. A Review of Cyclophosphamide. Springerfield; 1975. [Google Scholar]
- 52.Camenga DL, et al. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J. Infect. Dis. 1974;130:634–641. doi: 10.1093/infdis/130.6.634. [DOI] [PubMed] [Google Scholar]
- 53.Worthington M, et al. Mechanism of recovery from systemic vaccinia virus infection. I. The effects of cyclophosphamide. J. Exp. Med. 1972;136:277–290. doi: 10.1084/jem.136.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurd J, Heath RB. Effect of cyclophosphamide on infections in mice caused by virulent and avirulent strains of influenza virus. Infect. Immun. 1975;11:886–889. doi: 10.1128/iai.11.5.886-889.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rager-Zisman B, Allison AC. Effects of immunosuppression on coxsackie B-3 virus infection in mice, and passive protection by circulating antibody. J. Gen. Virol. 1973;19:339–351. doi: 10.1099/0022-1317-19-3-339. [DOI] [PubMed] [Google Scholar]
- 56.Fulci G, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng KW, et al. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 58.Peng KW, et al. Pharmacokinetics of oncolytic measles virotherapy: eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer Gene Ther. 2006;13:732–738. doi: 10.1038/sj.cgt.7700948. [DOI] [PubMed] [Google Scholar]
- 59.Dingli D, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 60.Peng KW, et al. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 61.Phuong LK, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 62.Peng KW, et al. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- 63.Dempsey MF, et al. Assessment of 123I-FIAU imaging of herpes simplex viral gene expression in the treatment of glioma. Nucl. Med. Commun. 2006;27:611–617. doi: 10.1097/00006231-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Verwijnen SM, et al. Molecular imaging and treatment of malignant gliomas following adenoviral transfer of the herpes simplex virus-thymidine kinase gene and the somatostatin receptor subtype 2 gene. Cancer Biother. Radiopharm. 2004;19:111–120. doi: 10.1089/108497804773391757. [DOI] [PubMed] [Google Scholar]
- 65.Aung W, et al. In vivo PET imaging of inducible D2R reporter transgene expression using [11C]FLB 457 as reporter probe in living rats. Nucl. Med. Commun. 2005;26:259–268. doi: 10.1097/00006231-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Liang Q, et al. Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther. 2001;8:1490–1498. doi: 10.1038/sj.gt.3301542. [DOI] [PubMed] [Google Scholar]
- 67.Liu TC, et al. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 68.O’Shea CC, et al. Heat shock phenocopies E1B-55K late functions and selectively sensitizes refractory tumor cells to ONYX-015 oncolytic viral therapy. Cancer Cell. 2005;8:61–74. doi: 10.1016/j.ccr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 69.Fujiwara T, et al. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Curr. Cancer Drug Targets. 2007;7:191–201. doi: 10.2174/156800907780058835. [DOI] [PubMed] [Google Scholar]
- 70.Small EJ, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol. Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki K, et al. The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin. Cancer Res. 2002;8:3348–3359. [PubMed] [Google Scholar]
- 72.Kemeny N, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum. Gene Ther. 2006;17:1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- 73.Mineta T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 74.Hunter WD, et al. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J. Virol. 1999;73:6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Markert JM, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 76.Liu BL, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 77.Hu JC, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 78.Kanai R, et al. Augmented therapeutic efficacy of an oncolytic herpes simplex virus type 1 mutant expressing ICP34.5 under the transcriptional control of musashi1 promoter in the treatment of malignant glioma. Hum. Gene Ther. 2007;18:63–73. doi: 10.1089/hum.2006.107. [DOI] [PubMed] [Google Scholar]
- 79.Herrero YCM, et al. Parvovirus H-1 infection of human glioma cells leads to complete viral replication and efficient cell killing. Int. J. Cancer. 2004;109:76–84. doi: 10.1002/ijc.11626. [DOI] [PubMed] [Google Scholar]
- 80.Kim JH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 81.Guo ZS, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 2005;65:9991–9998. doi: 10.1158/0008-5472.CAN-05-1630. [DOI] [PubMed] [Google Scholar]
- 82.Wang F, et al. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 2004;5:1266–1274. doi: 10.1038/ni1132. [DOI] [PubMed] [Google Scholar]
- 83.Werden SJ, et al. M-T5, the ankyrin repeat, host range protein of myxoma virus, activates Akt and can be functionally replaced by cellular PIKE-A. J. Virol. 2007;81:2340–2348. doi: 10.1128/JVI.01310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verheije MH, et al. Redirecting coronavirus to a nonnative receptor through a virus-encoded targeting adapter. J. Virol. 2006;80:1250–1260. doi: 10.1128/JVI.80.3.1250-1260.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wurdinger T, et al. Targeting non-human coronaviruses to human cancer cells using a bispecific single-chain antibody. Gene Ther. 2005;12:1394–1404. doi: 10.1038/sj.gt.3302535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bergmann M, et al. A genetically engineered influenza A virus with ras-dependent oncolytic properties. Cancer Res. 2001;61:8188–8193. [PubMed] [Google Scholar]
- 87.Myers R, et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005;12:593–599. doi: 10.1038/sj.cgt.7700823. [DOI] [PubMed] [Google Scholar]
- 88.Asada T. Treatment of human cancer with mumps virus. Cancer. 1974;34:1907–1928. doi: 10.1002/1097-0142(197412)34:6<1907::aid-cncr2820340609>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 89.Hasegawa K, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin. Cancer Res. 2006;12:6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 90.Lorence RM, et al. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr. Cancer Drug Targets. 2007;7:157–167. doi: 10.2174/156800907780058853. [DOI] [PubMed] [Google Scholar]
- 91.Gromeier M, et al. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shafren DR, et al. Oncolysis of human ovarian cancers by echovirus type 1. Int. J. Cancer. 2005;115:320–328. doi: 10.1002/ijc.20866. [DOI] [PubMed] [Google Scholar]
- 93.Au GG, et al. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int. J. Oncol. 2005;26:1471–1476. doi: 10.3892/ijo.26.6.1471. [DOI] [PubMed] [Google Scholar]
- 94.Shmulevitz M, et al. Unshackling the links between reovirus oncolysis, Ras signaling, translational control and cancer. Oncogene. 2005;24:7720–7728. doi: 10.1038/sj.onc.1209041. [DOI] [PubMed] [Google Scholar]
- 95.Alain T, et al. The oncolytic effect in vivo of reovirus on tumour cells that have survived reovirus cell killing in vitro. Br. J. Cancer. 2006;95:1020–1027. doi: 10.1038/sj.bjc.6603363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hiraoka K, et al. Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin. Cancer Res. 2006;12:7108–7116. doi: 10.1158/1078-0432.CCR-06-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Logg CR, et al. Tissue-specific transcriptional targeting of a replication-competent retroviral vector. J. Virol. 2002;76:12783–12791. doi: 10.1128/JVI.76.24.12783-12791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tai CK, et al. Antibody-mediated targeting of replication-competent retroviral vectors. Hum. Gene Ther. 2003;14:789–802. doi: 10.1089/104303403765255174. [DOI] [PubMed] [Google Scholar]
- 99.Obuchi M, et al. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Unno Y, et al. Oncolytic viral therapy for cervical and ovarian cancer cells by Sindbis virus AR339 strain. Clin. Cancer Res. 2005;11:4553–4560. doi: 10.1158/1078-0432.CCR-04-2610. [DOI] [PubMed] [Google Scholar]