Abstract
Prolactin (PRL) regulates cytoskeletal rearrangement and cell motility. PRL-activated Janus tyrosine kinase 2 (JAK2) phosphorylates the p21-activated serine-threonine kinase (PAK)1 and the Src homology 2 (SH2) domain-containing adapter protein SH2B1β. SH2B1β is an actin-binding protein that cross-links actin filaments, whereas PAK1 regulates the actin cytoskeleton by different mechanisms, including direct phosphorylation of the actin-binding protein filamin A (FLNa). Here, we have used a FLNa-deficient human melanoma cell line (M2) and its derivative line (A7) that stably expresses FLNa to demonstrate that SH2B1β and FLNa are required for maximal PRL-dependent cell ruffling. We have found that in addition to two actin-binding domains, SH2B1β has a FLNa-binding domain (amino acids 200–260) that binds directly to repeats 17–23 of FLNa. The SH2B1β-FLNa interaction participates in PRL-dependent actin rearrangement. We also show that phosphorylation of the three tyrosines of PAK1 by JAK2, as well as the presence of FLNa, play a role in PRL-dependent cell ruffling. Finally, we show that the actin- and FLNa-binding-deficient mutant of SH2B1β (SH2B1β 3Δ) abolished PRL-dependent ruffling and PRL-dependent cell migration when expressed along with PAK1 Y3F (JAK2 tyrosyl-phosphorylation-deficient mutant). Together, these data provide insight into a novel mechanism of PRL-stimulated regulation of the actin cytoskeleton and cell motility via JAK2 signaling through FLNa, PAK1, and SH2B1β. We propose a model for PRL-dependent regulation of the actin cytoskeleton that integrates our findings with previous studies.
Prolactin (PRL) is a pituitary-secreted polypeptide hormone that was named for its stimulatory action on lactation. To date, more than 300 separate biological activities have been attributed to PRL (for review, please see Refs. 1–4). Recent evidence suggests that PRL is locally produced by several target organs, including the mammary gland, prostate, skin, brain, some immune cells, adipocytes, and others. PRL may act as an autocrine/paracrine factor within mammary tissue, and a number of animal and in vitro studies have suggested that PRL may be involved in mammary tumorigenesis by promoting cell proliferation, survival, and development of metastasis (5; for review, please see Refs. 6–13). PRL was shown to act as a chemoattractant for human breast carcinoma (14), and the serine-threonine kinase NIMA-related kinase 3 was implicated in PRL-mediated breast cancer motility through a mechanism involving the Rho guanine nucleotide exchange factor Vav2, Ras-related C3 botulinum toxin substrate 1 (Rac1) activation, and paxillin phosphorylation (15, 16). These data, combined with animal studies reporting increased metastases with PRL administration (17), suggest that PRL is involved in the development of metastasis and tumor progression.
We have recently shown that the Src homology 2 (SH2) domain-containing adapter protein SH2B1β enhances tyrosyl phosphorylation of Janus tyrosine kinase 2 (JAK2) in response to PRL (18). The widely expressed SH2B1β was originally identified as a JAK2-binding protein in a yeast two-hybrid screen (19). In cultured cells, SH2B1β not only is tyrosyl phosphorylated by JAK2 but also potentiates JAK2 activation, in response to GH (19–21) and leptin (22). SH2-B is a member of the SH2B family [SH2-B, adapter protein containing a PH and SH2 domain, and lymphocyte-specific adapter protein (Lnk)], SH2-B, adapter protein containing a PH and SH2 domain and Lnk were renamed recently by HUGO Gene Nomenclature Committee as SH2B1, SH2B2, and SH2B3, respectively. The SH2B1 (SH2-B) gene encodes four isoforms (α, β, γ, and δ) by alternative mRNA splicing (20, 23). Deletion of the SH2B1 gene results in severe obesity and both leptin and insulin resistance, as well as infertility (24–26). Thus, knockout mice support a role for SH2B1 as a positive regulator of JAK2 signaling pathways initiated by leptin, as well as of pathways initiated by insulin and IGF-I. SH2B1β is involved in signaling to the actin cytoskeleton. First, SH2B1β increases membrane ruffling and pinocytosis induced by GH and platelet-derived growth factor (PDGF) (27). Second, SH2B1β is required for optimum actin-based cell motility and binds Rac (28). Third, SH2B1β is also required for maximal actin-based motility of Listeria monocytogenes. SH2B1β increases the rate of bacteria propulsion in infected cells and in cell extracts in a vasodilator-stimulated phosphoprotein (VASP)-dependent fashion (29). Both SH2B2 and SH2B3 have also been implicated as regulators of actin; thus, SH2B3 (Lnk) was pulled down in a yeast two-hybrid screen as a partner for Filamin A (FLNa) (30). We have recently shown that SH2B1β directly binds to and cross-links actin filaments and that this activity is required for PRL-dependent cell ruffling (18).
Another substrate of JAK2 in PRL-dependent signaling is the p21-activated serine-threonine kinase (PAK)1 (31). PAK1, a downstream effector for the small GTPases cell division control protein 42 homolog and Rac1, regulates actin and microtubule cytoskeletons and is implicated in breast cancer (for review, please see Ref. 32). Heregulin-activated PAK1 increased invasiveness of breast cancer cells (33), whereas expression of a kinase-dead PAK1 mutant in highly invasive breast cancer cells led to stabilization of stress fibers, enhanced cell spreading, and reduction in invasiveness (34). Conversely, hyperactivation of the PAK1 pathway in the noninvasive breast cancer MCF7 cells promotes cell migration and anchorage-independent growth (35). Additionally, the constitutively active PAK in breast cancer cells is the result of mislocalization of PAK to focal adhesions (36). PAK1 stimulates the activity of LIM motif-containing protein kinase 1, which in turn increases the phosphorylation and inactivation of cofilin, leading to a reduction in the depolymerization of actin filaments (37). PAK1 also directly phosphorylates other cytoskeletal proteins, including myosin light chain kinase (38), paxillin (39), FLNa, merlin, and p41-Arc (40–42). We have recently shown that PAK1 is a novel substrate of JAK2 tyrosine kinase and that PRL-activated JAK2 phosphorylates PAK1 in vivo. PAK1 tyrosines 153, 201, and 285 were identified as sites of JAK2 tyrosyl phosphorylation by mass spectrometry and two-dimensional peptide mapping. Our findings indicated that JAK2 phosphorylates PAK1 at these specific tyrosines and that this phosphorylation plays an important role in cell survival and cell motility (31).
PAK1 has been shown to directly phosphorylate FLNa at serine 2152, and FLNa, in turn, stimulates both the autophosphorylation and the kinase activity of PAK1 (40). FLNa is an actin-binding protein that binds actin filaments to the plasma membrane (43). FLNa plays important parts in cross-linking cortical actin filaments into a dynamic three-dimensional structure. The N-terminal region of filamin contains an actin-binding domain, followed by a rod-like domain consisting of 24 tandem repeats (44). Some of these repeats (repeats 9–15) contain another actin binding domain (45). Dimerization of FLNa through the last C-terminal repeat allows the formation of a V-shaped flexible structure that is essential for function (44, 46). Given that SH2B3 (Lnk) was pulled down in the yeast two-hybrid screen as a partner for FLNa (30), and PAK1 phosphorylates FLNa (11), we hypothesized that FLNa may associate with the adapter protein SH2B1β and JAK2 tyrosyl-phosphorylated PAK1, and thereby contribute to the PRL-dependent motility of breast cancer cells.
In the present study, we use a FLNa-deficient human melanoma cell line (M2) and its derivative line (A7), which stably expresses FLNa (47), and demonstrate that in addition to two actin-binding domains, SH2B1β has a FLNa-binding domain. [For wild-type (WT) and mutant forms of rat SH2B1β used in the study, see Fig. 1]. We demonstrate that amino acids 200–260 of SH2B1β directly interact with repeats 17–23 (amino acids 1863–2522) of FLNa. The SH2B1β-FLNa interaction participates in PRL-dependent actin rearrangement. We also show that phosphorylation of the three tyrosines of PAK1 by JAK2, as well as the presence of FLNa, plays a role in PRL-dependent cell ruffling. Finally, we show that the actin- and FLNa-binding-deficient (SH2B1β 3Δ) mutant of SH2B1β abolished PRL-dependent ruffling and PRL-dependent cell migration when expressed along with PAK1 Y3F (JAK2 tyrosyl-phosphorylation deficient mutant). We confirm the role of SH2B1β, PAK1, and FLNa in PRL-dependent migration of T47D breast cancer cells.
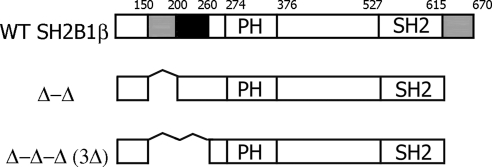
Fig. 1.
Schematic representation of WT and mutant forms of rat SH2B1β used in the study. Actin-binding domains (amino acids 150-200 and 615-670) are shown in gray. Filamin-binding domain (amino acids 200-260) is shown in black. PH is the plekstrin homology domain (amino acids 274-376), and SH2 is the SH2 domain (amino acids 527-620). Proline-rich regions (amino acids 13–24, 89-103, and 469-496) and dimerization domain (amino acids 24-85) are not shown.
Results
FLNa, PAK1, and SH2B1β are required for maximal PRL-dependent ruffling
Generation of membrane ruffles rich in polymerized actin is one of the earliest detectable events when cells are stimulated with growth factors or hormones. Membrane ruffles are found on the leading edge of motile cells and are thought to be required for cell motility (48). Both FLNa and SH2B1β participate in actin-dependent cell ruffling. FLNa plays a critical role in heregulin-induced ruffling (40), whereas SH2B1β regulates GH-, PDGF-, and PRL-dependent ruffling, and the two actin-binding domains of SH2B1β are required for maximal ruffling (18, 27). To assess the role of both FLNa and SH2B1β in PRL-dependent cell ruffling, we used the FLNa-deficient human melanoma cell line (M2) and its derivative line (A7), which stably expresses FLNa (47). Because these cell lines have detectable levels of JAK2 and SH2B1β but not PRL receptor (PRLR), we overexpresseed green fluorescent protein (GFP)-containing human long PRLR in A7 and M2 cells and showed that serum deprivation abolishes tyrosyl phosphorylation of endogenous JAK2 in both cell lines, whereas PRL activates JAK2 (Fig. 2).
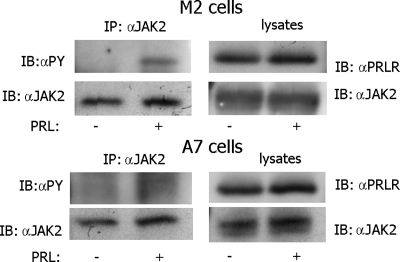
Fig. 2.
JAK2 is tyrosyl phosphorylated in M2 and A7 cells overexpressing PRLR in response to PRL. Human PRLR was overexpressed in M2 and A7 cells. The cells were deprived of serum and treated with or without 400 ng/ml PRL for 15 min. JAK2 was immunoprecipitated with αJAK2. The immunoprecipitates and whole-cell lysates were immunoblotted with indicated antibodies. Each experiment was performed at least three times with similar results. IP, Immunoprecipitation.
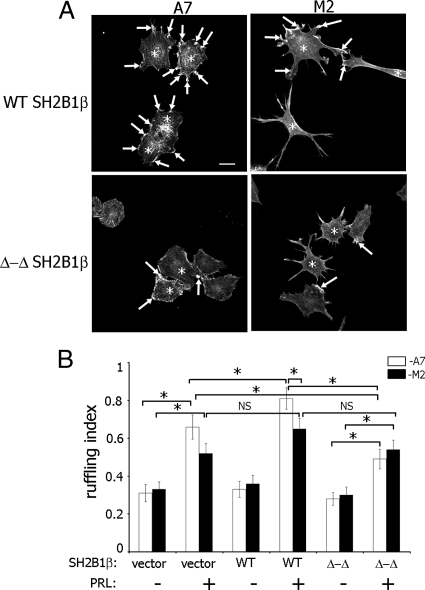
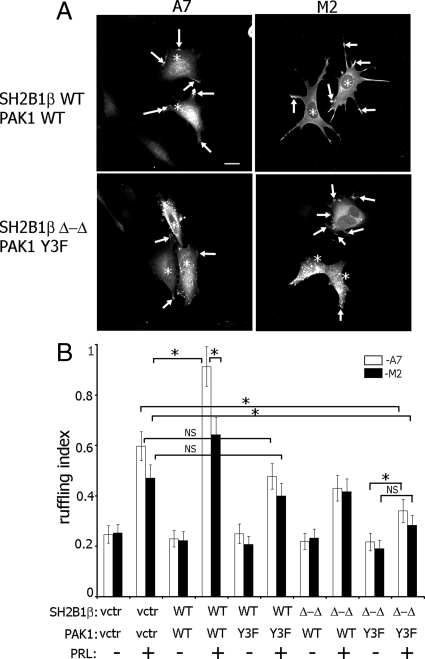
To determine whether FLNa and SH2B1β work together in PRL-dependent actin reorganization, PRL-dependent ruffling was assessed in M2 and A7 cells overexpressing GFP-PRLR. Myc-tagged SH2B1β wild type (WT) or SH2B1β Δ-Δ mutant, which has deletion of two actin-binding sites, were expressed in M2 and A7 cells, and actin ruffling was assessed (number of phalloidin-positive ruffles per cell) after treatment with PRL. PRL induces the formation of cell ruffles in both cell lines (Fig. 3A; compare the first and the second pair of bars in Fig. 3B), although the ruffling index in A7 cells was higher than in M2 cells (Fig. 3B, second pair of bars), suggesting that FLNa participates in PRL-induced ruffling. As expected, A7 cells overexpressing WT SH2B1β exhibited more pronounced ruffling in response to PRL compared with the cells expressing vector alone, whereas WT SH2B1β failed to enhance ruffling in M2 cells (Fig. 3B, black bars for vector and WT SH2B1β-expressing cells in the presence of PRL). The ruffling index for A7 cells overexpressing WT SH2B1β was significantly higher than for M2 cells overexpressing WT SH2B1β, suggesting that the presence of both FLNa and SH2B1β are required for maximal PRL-dependent cell ruffling. Expression of the SH2B1β Δ-Δ mutant inhibited cell ruffling in A7 but not in M2 cells, suggesting that the actin-binding sites of SH2B1β play a role in actin regulation only in the presence of FLNa.
Fig. 3.
Actin-binding domains of SH2B1β and FLNa are required for maximal PRL-induced membrane ruffling. A, The FLNa-deficient human melanoma M2 cell line and its derivative cell line (A7), which stably expresses FLNa, were cotransfected with GFP-PRLR and myc-tagged WT SH2B1β or SH2B1β Δ-Δ mutant. Cells were serum deprived and treated with 400 ng/ml PRL for 15 min. Filamentous actin was visualized by staining with Alexa Fluor 350 phalloidin, and the transfected cells were visualized by both GFP fluorescence for PRLR and staining with αmyc for SH2B1β. Arrows indicate ruffles, and asterisks denote transfected cells. Scale bar, 20 μm. B, Ruffling index as a number of ruffles per cell was counted. White bars represent A7 cells, and black bars represent M2 cells. Bars represent mean ± se. *, P < 0.05. Each experiment was repeated three times. n = 300 for each experimental condition. NS, Not significant.
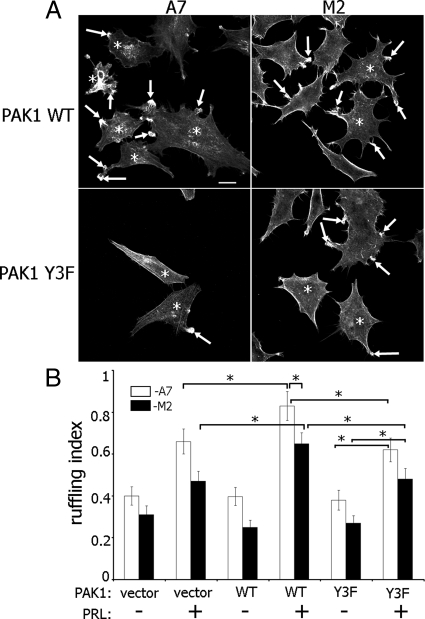
The serine-threonine kinase PAK1 phosphorylates FLNa, and this phosphorylation is required for PAK1-mediated cell ruffling (40). We have previously shown that PRL-activated JAK2 tyrosine kinase phosphorylates PAK1. Tyrosines 153, 201, and 285 of PAK1 are the sites of JAK2 phosphorylation, and mutation of these three tyrosines causes a decrease in cell motility (31). Here, we sought to determine whether PRL-induced cell ruffling is regulated by FLNa and tyrosyl phosphorylation of PAK1. PAK1 WT-expressing A7 cells demonstrated the greatest level of PRL-stimulated ruffling as compared with A7 cells expressing PAK1 Y3F (JAK2 tyrosyl-phosphorylation-deficient mutant) and M2 cells expressing PAK1 WT, whereas expression of PAK1 Y3F in M2 cells resulted in a decrease in membrane ruffling (Fig. 4, A and B). These data suggest that both pTyr-PAK1 and FLNa participate in PRL-dependent cell ruffling.
Fig. 4.
Tyrosyl-phosphorylated PAK1 and FLNa are required for maximal PRL-induced membrane ruffling. A, M2 and A7 cell lines were cotransfected with GFP-PRLR and myc-tagged WT PAK1 or Y3F PAK1 mutant. Cells were treated as in Fig. 3. Filamentous actin was visualized by staining with Alexa Fluor 350 phalloidin, and the transfected cells were visualized by both GFP fluorescence for PRLR and staining with αmyc for PAK1. Arrows indicate ruffles, and asterisks denote transfected cells. Scale bar, 20 μm. B, Ruffling index as a number of ruffles per cell was counted. White bars represent A7 cells, and black bars represent M2 cells. Bars represent mean ± se. *, P < 0.05. Each experiment was repeated three times. n = 300 for each experimental condition.
To examine whether SH2B1β works together with pTyr-PAK1 and FLNa to mediate PRL-stimulated actin rearrangement, we coexpressed GFP-containing PRLR, myc-tagged WT PAK1 or PAK1 Y3F, and monomeric red fluorescent protein (mRFP)-tagged WT SH2B1β or SH2B1β Δ-Δ in M2 and A7 cells, treated with PRL and assessed the ruffling index (Fig. 5, A and B). We demonstrated that A7 cells expressing WT PAK1 and WT SH2B1β showed the greatest amount of PRL-induced membrane ruffles, whereas the absence of either tyrosyl phosphorylation in PAK1 Y3F, actin-binding activity in SH2B1β Δ-Δ, or FLNa in M2 cells caused a decrease in PRL-dependent membrane ruffling. Most importantly, A7 cells overexpressing PAK1 Y3F and SH2B1β Δ-Δ were still able to respond to PRL through cell ruffling, whereas M2 cells failed to do so (Fig. 5B, two last pairs of bars), demonstrating that all three proteins pTyr-PAK1, SH2B1β, and FLNa are required for the PRL-induced membrane ruffling.
Fig. 5.
The actin-binding domains of SH2B1β, pTyr-PAK1, and FLNa are required for maximal PRL-induced membrane ruffling. A, A7 and M2 cell lines were cotransfected with GFP-PRLR, mRFP-tagged WT SH2B1β or SH2B1β Δ-Δ mutant, and myc-tagged WT PAK1 or PAK1 Y3F mutant. Cells were treated as in Fig. 3. Myc-PAK1 was visualized by αmyc, whereas PRLR was visualized by GFP fluorescence, and mRFP fluorescence for SH2B1β. PAK1 WT and/or SH2B1β WT or Δ-Δ localization in ruffles was used to count total number of ruffles per transfected cell. Arrows indicate ruffles, and asterisks denote transfected cells. Scale bar, 20 μm. B, Ruffling index as a number of ruffles per cells was counted. White bars represent A7 cells, and black bars represent M2 cells. Bars represent mean ± se. *, P < 0.05. Each experiment was repeated three times. n = 300 for each experimental condition. NS, Not significant; vctr, vector.
SH2B1β is a FLNa-binding protein
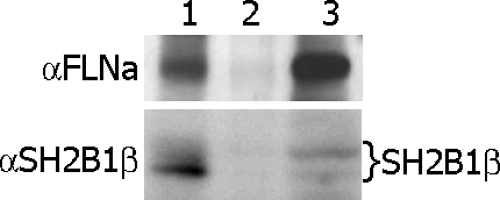
To assess the mechanism by which SH2B1β may regulate the actin cytoskeleton in a FLNa-dependent manner, we sought to provide direct evidence for an association between FLNa and SH2B1β. We immunoprecipitated endogenous SH2B1β and immunoblotted with αFLNa antibody. The same blot was reprobed with αSH2B1β, which demonstrated that endogenous SH2B1β coimmunoprecipitates with endogenous FLNa (Fig. 6).
Fig. 6.
Endogenous SH2B1β associates with endogenous FLNa. Whole-cell lysates of 293T cells (lane 3) were immunoprecipitated with αSH2B1β antibody (lane 1) or with control IgG (lane 2) and immunoblotted with αFLNa antibody (upper). Endogenous FLNa was detected in the whole-cell lysate (lane 3) and in the αSH2B1β immunoprecipitate (lane 1) but not the control lane (lane 2). The same blot was reprobed with αSH2B1β (bottom). Several bands representing different levels of SH2B1β phosphorylation (indicated on the right) were detected in the cell lysate (lane 3) and αSH2B1β immunoprecipitate (lane 1) but not in the control lane (lane 2). Each experiment was performed at least three times with similar results.
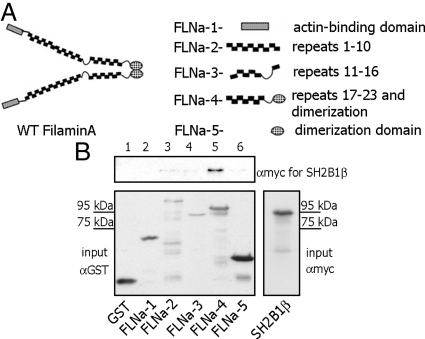
To further examine the SH2B1β-FLNa interaction, we used glutathione S-transferase (GST)-tagged truncation mutants of FLNa: FLNa-1 (FLNa actin-binding domain; amino acids 1-278), FLNa-2 (FLNa repeats 1-10; amino acids 279-1252), FLNa-3 (FLNa repeats 11-16; amino acids 1253-1862), FLNa-4 (repeats 17-23 and dimerization domain; amino acids 1863-2647), and FLNa-5 (dimerization domain; amino acids 2523-2647). These proteins are schematically depicted in Fig. 7A (modified from Ref. 49). Myc-tagged WT SH2B1β was in vitro translated and incubated with GST or various GST-tagged truncation mutants of FLNa. We were able to identify repeats 17-23 of FLNa as a SH2B1β-binding domain, because SH2B1β bound directly to FLNa-4 (repeats 17-23 and dimerization domain) but not FLNa-5 (dimerization domain) only (Fig. 7B).
Fig. 7.
FLNa repeats 17–23 is the site for SH2B1β interaction. A, Schematic depiction of FLNa truncations [modified from Cukier et al. (49)]. B, Different GST-tagged truncated FLNa mutants were purified from bacterial lysates and immobilized on glutathione-agarose beads. Myc-tagged SH2B1β was translated in vitro using TNT Coupled Reticulocyte Lysate System. The mixture of GST-tagged FLNa mutants and in vitro translated SH2B1β was rotated for 1 h. The glutathione-agarose beads were washed, GST-FLNa constructs-bound myc-SH2B1β was detected by IB with αmyc (upper panel). SH2B1β was detected only in lane 5, indicating that only FLNa-4 construct (repeats 17–23 and dimerization domain) directly binds to SH2B1β. Amount of GST or GST-FLNa mutants input was detected by IB with αGST (left bottom panel). Migration of myc- SH2B1β alone is shown on the bottom right panel. Each experiment was performed at least three times with similar results. Lane 1, GST; lane 2, FLNa-1 (actin-binding domain of FLNa); lane 3, FLNa-2 (repeats 1–10); lane 4, FLNa-3 (repeats 11–16); lane 5, FLNa-4 (repeats 17–23 and dimerization domain); and lane 6, FLNa-5 (dimerization domain).
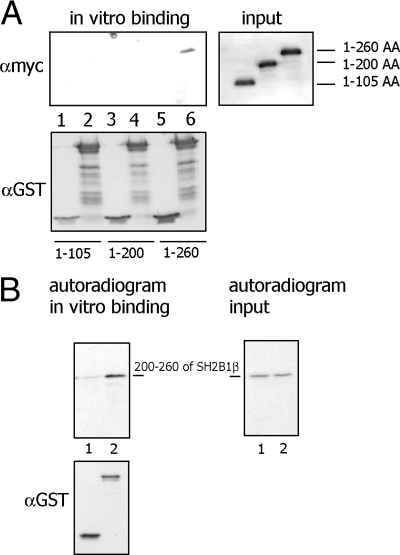
To determine which site of SH2B1β is responsible for binding FLNa, we performed GST-pull-down experiments using GST-tagged FLNa-4 as bait and different in vitro translated myc-tagged truncated SH2B1β mutants. We found that only the SH2B1β (1-260 amino acids) mutant was pulled down by GST-FLNa-4, whereas SH2B1β (1-105 amino acids) and SH2B1β (1-200 amino acids) mutants were undetectable in the pellets (Fig. 8A). To confirm that amino acids 200-260 of SH2B1β are a site of interaction with FLNa, we created mRFP-tagged SH2B1β (200-260 amino acids) mutant, translated it in vitro, and incubated with affinity purified GST or GST-FLNa-4. The data in Fig. 8B demonstrate that SH2B1β (200-260) was pulled down with GST-FLNa-4 but not with GST. Overall, these observations demonstrate that SH2B1β is a novel FLNa-binding protein and that amino acids 200-260 of SH2B1β interact directly with repeats 17-23 (amino acids 1863-2522) of FLNa.
Fig. 8.
Amino acids 200–260 of SH2B1β is the site for FLNa interaction. A, Different in vitro translated myc-tagged truncated SH2B1β mutants were incubated with either GST (lanes 1, 3, and 5) or GST-tagged FLNa-4 (lanes 2, 4, and 6). Bound myc-SH2Bβ mutant was detected by IB with αmyc. Lanes 1 and 2, Amino acids 1-105 of SH2B1β; lanes 3 and 4, amino acids 1-200 of SH2B1β; and lanes 5 and 6, amino acids 1-260 of SH2B1β. B, mRFP-tagged (200–260) amino acids mutant of SH2B1β was translated in vitro in the presence of 35S methionine and incubated with either GST (lane 1) or GST-tagged FLNa-4 (lane 2). Bound mRFP-SH2Bβ mutant was detected by autoradiogram (48 h of film exposure). Equal amounts of 35S methionine-labeled SH2B1β input is shown on the right (24 h of film exposure). Amount of GST or GST-FLNa-4 was detected by IB with αGST. Each experiment was performed at least three times with similar results.
Interaction between SH2B1β and FLNa is required for PRL-dependent ruffling
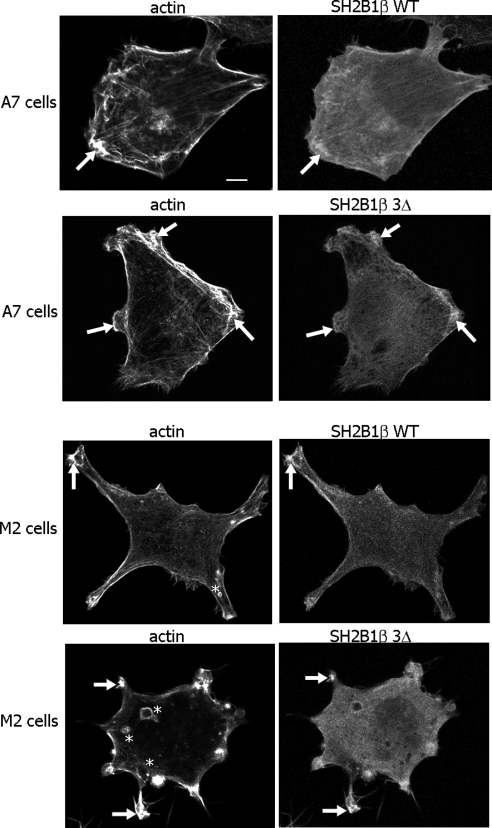
Our previous experiments in 3T3-F442A cells showed that both WT SH2B1β and Δ-Δ mutant localize to membrane ruffles (18). To examine the role of FLNa-binding site of SH2B1β in this localization, we overexpressed mRFP-tagged WT or 3Δ mutant (SH2B1β mutant deficient in both actin-binding domains and FLNa-binding domain) together with GFP-PRLR in M2 or A7 cells. Both WT SH2B1β and 3Δ mutant colocalize with actin in the membrane ruffles in A7 and M2 cells (Fig. 9, arrows). Interestingly, in addition to ruffles, M2 cells often demonstrated bleb formation as described previously (50).
Fig. 9.
SH2B1β WT and SH2B1β 3Δ colocalize with actin in the ruffles of A7 and M2 cells. M2 and A7 cells overexpressing mRFP-tagged SH2B1β WT or 3Δ mutant were stained with Alexa Fluor 647 phalloidin for actin visualization. Arrows indicate ruffles, and asterisks denote blebs. Each experiment was performed at least three times with similar results. Scale bar, 20 μm.
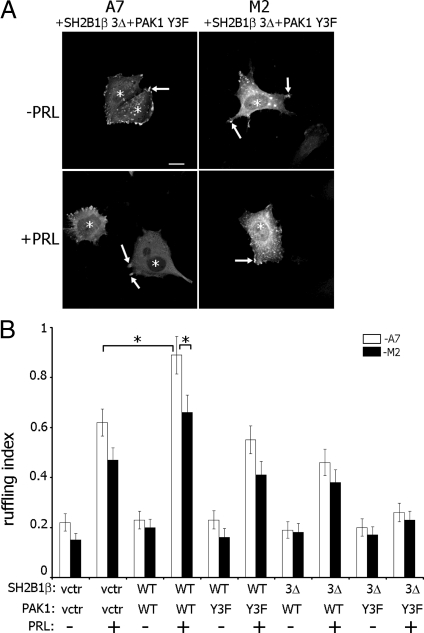
To test the role of the FLNa-binding activity of SH2B1β in PRL-dependent actin rearrangement, we overexpressed GFP-PRLR, myc-tagged PAK1 WT or PAK1 Y3F, and mRFP-tagged SH2B1β WT or SH2B1β 3Δ in M2 and A7 cells (Fig. 10A). M2 and A7 cells transfected with only GFP-PRLR were stained with Texas Red phalloidin. M2 and A7 cells expressing GFP-PRLR, myc-tagged PAK WT or Y3F, and mRFP-tagged SH2B1β WT or 3Δ mutant were visualized by fluorescence microscopy, and the total number of ruffles for 100 cells for each transfection condition was counted using mRFP-SH2B1β and/or myc-PAK1 as a marker for ruffles, because both proteins localize in the ruffles. As expected, A7 cells expressing WT PAK1 and WT SH2B1β exhibited maximal PRL-stimulated membrane ruffling, and both cell lines expressing PAK1 Y3F and SH2B1β 3Δ failed to ruffle in response to PRL, suggesting that pTyr-PAK1 and SH2B1β work in complex with FLNa downstream of JAK2 activation, to stimulate PRL-dependent actin rearrangement (Fig. 10B).
Fig. 10.
The FLNa-binding domain of SH2B1β, pTyr-PAK1 and FLNa are required for PRL-induced membrane ruffling. A, A7 and M2 cell lines were cotransfected with GFP-PRLR, mRFP-tagged WT SH2B1β (data not shown) or SH2B1β 3Δ mutant (shown), and myc-tagged WT PAK1 (data not shown) or PAK1 Y3F mutant (shown). Cells were treated as in Fig. 3. Myc-PAK1 was visualized by αmyc, whereas PRLR was visualized by GFP fluorescence and mRFP fluorescence for SH2B1β. PAK1 and SH2B1β localization in ruffles was used to count total number of ruffles per transfected cell. Arrows indicate ruffles, and asterisks denote transfected cells. Scale bar, 2 0 μm. B, Ruffling index as a number of ruffles per cell was counted. White bars represent A7 cells, and black bars represent M2 cells. Bars represent mean ± se. *, P < 0.05. Each experiment was repeated three times. n = 300 for each experimental condition. vctr, Vector.
Interaction between SH2B1β and FLNa is required for PRL-dependent cell motility
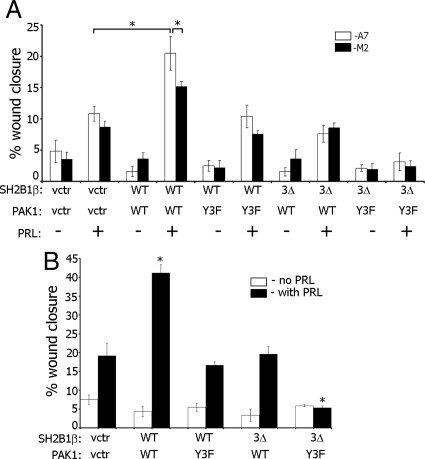
Migration of M2 and A7 cells was previously assessed either in a Transwell migration assay or in a wounding assay (51–53). To provide further evidence that tyrosyl-phosphorylated PAK1 and the FLNa-binding activity of SH2B1β are required for PRL-dependent cell motility, we assessed cell movement with a wounding assay. Migration of M2 and A7 cells expressing GFP-PRLR, myc-tagged PAK1 WT or PAK1 Y3F, and mRFP-tagged SH2B1βWT or SH2B1β 3Δ into the wound was assessed after 24 h of cell deprivation, followed by wounding in the presence or absence of PRL. Percentage of wound closure in 8 h was calculated and plotted for each condition (Fig. 11A). A7 cells expressing WT PAK1 and WT SH2B1β exhibited maximal PRL-stimulated movement cells into the wound, whereas both cell lines expressing PAK1 Y3F and SH2B1β 3Δ failed to move in response to PRL, suggesting that PRL-dependent cell migration is regulated by pTyr-PAK1 and SH2B1β in a complex with FLNa downstream of JAK2 activation.
Fig. 11.
The FLNa-binding domains of SH2B1β, pTyr-PAK1, and FLNa are required for PRL-induced cell migration. A, A7 and M2 cell lines were cotransfected with GFP-PRLR, mRFP-tagged WT SH2B1β or SH2B1β 3Δ mutant, and myc-tagged WT PAK1 or PAK1 Y3F mutant. After deprivation, monolayers of cells were scarified and incubated in deprivation media with or without 400 ng/ml PRL. The percentage wound closure in 8 h was calculated for each condition. Bars represent mean ± se. *, P < 0.05. B, Clones of T47D cells stably expressing GFP, GFP-containing PAK1 WT, or PAK1 Y3F were transiently transfected with vector, WT SH2B1β, or SH2B1β 3Δ mutant. Monolayers of cells were wounded in the presence or absence of 200 ng/ml PRL and assessed after 18 h. Bars represent mean ± se. *, P < 0.05 compared with cells expressing vectors and treated with PRL. Each experiment was repeated three times. n = 30 for each experimental condition. vctr, Vector.
To gain insight into the role of the SH2B1β-PAK1-FLNa complex in motility of breast cancer cells, we used T47D cell clones stably expressing GFP, GFP-containing PAK1 WT, or PAK1 Y3F. We transiently overexpressed mRFP, mRFP-tagged SH2B1β WT, or SH2B1β 3Δ in these clones and assessed cell migration into the wound as described above (Fig. 11B). The T47D PAK1 WT clone expressing SH2B1β WT exhibited stronger migration into the wound in response to PRL than the T47D GFP clone expressing mRFP (vector). In contrast, the T47D PAK1 Y3F clone expressing SH2B1β 3Δ failed to move in response to PRL, suggesting that PAK1, SH2B1β, and FLNa play an important role in PRL-dependent migration of breast cancer cells.
Discussion
PRL has dual functions, working both as a circulating hormone and as a cytokine. The effects of PRL are mediated by its cell surface receptor, the PRLR, which is a member of the cytokine receptor superfamily (54). Several PRLR forms exist, including the long (85–90 kDa), intermediate (65 kDa), and short (42 kDa) PRLR. PRL binding to its receptor activates JAK2, PRLR phosphorylation, and phosphorylation of signal transducer and activator of transcription (Stat)5, Stat3, and Stat1 (55–62). PRL activates other pathways in mammary epithelial cells, including the Ras-MAPK (61, 63, 64), protein kinase C (65, 66), Src family kinases, and phosphatidylinositol 3-kinases (67). PRL also stimulates cell motility and thereby contributes to metastasis (for review, please see Ref. 9).
Recent data have implicated a significant role for PRL in the pathogenesis of human breast cancer. PRL increases cell motility and/or invasiveness of breast cancer cells (14, 68). These data, combined with animal studies reporting increased metastases with PRL administration (17), suggest that PRL is involved in the development of metastasis and tumor progression. However, the precise molecular mechanism by which PRL induces actin cytoskeletal rearrangements remains poorly understood. One mechanism involves the serine-threonine kinase NIMA-related kinase 3, Rac1 activation, and paxillin phosphorylation (15, 16). However, a process as complicated as cell migration may be mediated by distinct signaling cascades. The data presented here provide insight into the novel mechanism of PRL-stimulated regulation of the actin cytoskeleton and cell motility via JAK2 signaling through FLNa, PAK1, and SH2B1β.
The widely expressed SH2 domain-containing protein SH2B1β was initially identified as a binding partner and substrate of JAK2 (19; for review, please see Ref. 22). In cultured cells, SH2B1β is tyrosyl phopshorylated by JAK2 on tyrosines 439 and 494 (69) and potentiates JAK2 activation in response to PRL (18), GH (19, 21, 70), and leptin (71). The role of SH2B1β in actin regulation is well established (27–29, 69), and our laboratory has recently shown that SH2B1β directly binds to and cross-links actin filaments through its two actin-binding domains, and these domains are required for maximal PRL-dependent ruffling (18). Membrane ruffling has been observed in many cell types in response to various extracellular factors and on motile cells, where they are believed to be required for directed cell migration. Thus, the formation of membrane ruffles may be a sign of increased response to external stimuli and of elevated cell migration (for review, please see Refs. 48, 72).
Because another member of the SH2-B family of proteins, SH2B3 (Lnk), was pulled down in the yeast two-hybrid screen as a partner for FLNa (30), we hypothesized that PRL-activated JAK2 and SH2B1β could regulate the actin-cytoskeleton through FLNa. To test this hypothesis, we overexpressed PRLR in the FLNa-deficient melanoma cell line M2 and in A7 cells, which are M2 cells stably reexpressing FLNa. PRL induced membrane ruffling in both cell lines, whereas WT SH2B1β enhanced this effect only in A7 cells. The amplifying effect of SH2B1β on cell ruffling, which our current data suggest is FLNa dependent, has been previously described for GH, PDGF (27), and PRL (18).
We demonstrated that overexpression of SH2B1β WT enhanced membrane ruffling in A7 but not in M2 cells, implying a role for FLNa in SH2B1β-dependent regulation of actin cytoskeleton. Our current findings that deletion of the actin-binding sites in SH2B1β conferred dominant-negative activity upon SH2B1β in A7 cells (Fig. 3B, last white bar) support our previous data obtained with PRL-treated T47D cells (18) and suggest a role for the WT protein in PRL-dependent cell ruffling. In contrast, the SH2B1β Δ-Δ mutant does not block the ability of PRL to induce ruffling in FLNa-deficient M2 cells (Fig. 3B, last black bar), suggesting that a FLNa-independent pathway is still functioning but also that FLNa plays a role in SH2B1β-dependent regulation of the actin cytoskeleton. Based upon our findings, we propose that upon ligation of PRLR, SH2B1β translocates to activated membrane receptor-JAK2 complexes, where it cross-links actin filaments and facilitates cytokine-dependent cell ruffling in a FLNa-dependent manner. Thus, the dominant negative Δ-Δ mutant might still be able to bind and therefore sequester JAK2 and FLNa from binding to endogenous SH2B1β but is unable to bind and cross-link actin filaments for cell ruffling.
Here, we have confirmed that the actin-binding sites of SH2B1β participate in PRL-induced ruffling. However, both cell lines expressing SH2B1β Δ-Δ were still able to respond to PRL, although with impaired ruffling (compare two last pairs of bars in Fig. 3). It suggests that another mechanism might be involved in the regulation of the actin cytoskeleton. We decided to test the role of the serine-threonine kinase PAK1 in this process for two reasons. First, we have recently shown that PAK1 is a substrate for JAK2, which directly phosphorylates PAK1 on tyrosines 153, 201, and 285 (31). Second, PAK1 can directly phosphorylate FLNa at serine 2152, and FLNa, in turn, stimulates both the autophosphorylation and the kinase activity of PAK1 (40). In Fig. 4, we have shown that WT PAK1 indeed stimulates PRL-induced ruffling, and the FLNa does not participate in this process, because the ruffling index in both cell lines was significantly higher than in vector-transfected cells. Similar observations have been published previously, for example, when serum-deprived M2 cells were treated with serum, the cells produced ruffles around the entire cell periphery within 10 min; however, within hours, ruffles revert to blebbing (50). Epidermal growth factor-induced membrane ruffles were visible in M2 cells in 5 min after EGF treatment (73). Cell ruffling was also evident in M2 cells stimulated or not stimulated with different ligands (74–82). In contrast, M2 cells treated with heregulin (which binds to epidermal growth factor receptor) and sphingosine (which activates PAK1) failed to induce ruffle formation (40). It is possible that heregulin, sphingosine, and PRL engage different downstream pathways to regulate the actin-cytoskeleton. The JAK2-dependent phosphorylation of PAK1 was clearly important for formation of PRL-dependent ruffles, because the mutant of PAK1 that does not have tyrosines 153, 201, and 285 (PAK1 Y3F) failed to amplify the ruffling in both cell lines (Fig. 4, last two bars). These data suggest that PAK1 can regulate actin rearrangement not only by phosphorylation of downstream targets but also by other mechanism(s), for example, by regulating protein-protein interaction(s) via phosphorylated tyrosines 153, 201, and 285. We are currently investigating which actin-regulating proteins associate with tyrosyl-phosphorylated PAK1. PAK1 Y3F failed to amplify ruffling like PAK1 WT (Fig. 4). However, PAK1 Y3F acted as a dominant negative inhibitor when coexpressed with actin-binding-deficient SH2B1β Δ-Δ (Fig. 5, last two bars). Because SH2B1β Δ-Δ can still bind to and sequester possible PAK1 Y3F target(s), it may be that these downstream targets are more dramatically affected when both PAK1 and SH2B1β are mutated. One obvious candidate is Rac1. SH2B1β coimmunoprecipitated with Rac1, and amino acids 85–106 of SH2B1β (which are different from the actin-binding sites) are required for this binding (28). Rac1 is a well-known activator of the kinase activity of PAK1. Another possible protein is growth factor receptor-bound protein 2 (Grb2), because it binds to both SH2B1β (38) and PAK1 (83). Thus, SH2B1β Δ-Δ may bind and sequester Rac1, Grb2, and/or other effector molecules away from PAK1 Y3F and from endogenous PAK1, thereby preventing the assembly of active signaling complexes at sites of actin rearrangement near the plasma membrane.
Finally, we have shown that SH2B1β can directly bind to FLNa, mapped the binding sites on both molecules, and created a mutant of SH2B1β that is both actin- and FLNa-binding deficient (SH2B1β 3Δ). This mutant abolished PRL-dependent ruffling when expressed in M2 and A7 cells along with PAK1 Y3F (Fig. 10, last two pairs of bars). SH2B1β Δ-Δ, coexpressed with PAK1 Y3F, is still able to produce PRL-dependent ruffling in A7 cells (Fig. 5, last two white bars), whereas SH2B1β 3Δ, coexpressed with PAK1 Y3F, failed to do it (Fig. 10, last two white bars), perhaps because the SH2B1β Δ-Δ mutant can still bind to FLNa and help maintain a functional JAK2-SH2B1β Δ-Δ -FLNa-PAK1 Y3F-actin complex. Once the FLNa-binding site is deleted from SH2B1β, SH2B1β 3Δ can bind neither actin nor FLNa, presumably leading to the disruption of this complex and inhibition of PRL signal to the actin cytoskeleton. Importantly, disruption of the JAK2-SH2B1β-PAK1-FLNa complex abolished not only cell ruffling but also PRL-induced migration of the cells into the wound (Fig. 11).
It is interesting to note that studies with M2 and A7 cells indicated that FLNa is critical not only for actin-dependent function but also for the intracellular localization, intracellular sorting and recycling, and/or signaling of various receptors, including integrins, insulin receptor, overexpressed receptors to calcitonin, dopamine, opioid, and class I IgG (FcγRI) (78, 84–89). We cannot exclude the possibility that some of these FLNa-dependent functions can influence the PRL-dependent response in A7 and M2 cells. Furthermore, using an overexpression system has some limitations, and further experiments with endogenous proteins are needed.
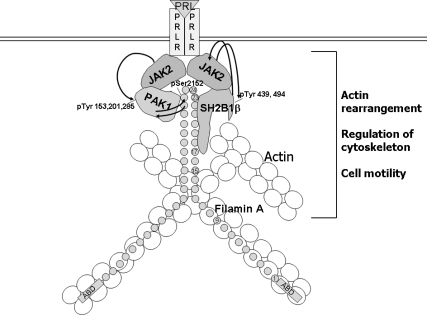
We propose a model for PRL-dependent regulation of the actin cytoskeleton that integrates our findings with previous studies (Fig. 12). Upon ligation of PRLR and activation of JAK2, SH2B1β translocates to activated PRLR-JAK2 complexes, where it cross-links actin filaments and binds to FLNa. PRL-activation of JAK2 also leads to tyrosyl phosphorylation of PAK1, thereby increasing PAK1's activity and stimulating phosphorylation of FLNa. FLNa, in turn, activates PAK1, binds to SH2B1β and relocates more SH2B1β to the JAK2-PAK1-FLNa complex. Because SH2B1β enhances the tyrosine kinase activity of JAK2 (19), the formation of this multiprotein complex results in enhancement of JAK2 activation and further activation of the JAK2-PAK1-FLNa-actin complex, leading to actin cytoskeleton reorganization. Upon tyrosyl phosphorylation by JAK2, SH2B1β and PAK1 may also create additional docking sites to recruit actin-regulating SH2-domain-containing proteins to the plasma membrane to facilitate membrane ruffling and actin rearrangement. Indeed, JAK2-phosphorylated tyrosines 439 and 494 of SH2B1β participate in GH-dependent ruffling (69). Additionally, Grb2 and/or other SH3 domain-containing proteins (such actin-binding proteins as cortactin, spectrin, and myosin I) may bind to a proline-rich domain of SH2B1β and also facilitate PRL-dependent membrane ruffling. Because PRL acts as a chemoattractant for human breast carcinoma (14), our current data may provide insight into one mechanism of PRL-stimulated metastatic distribution of breast cancer.
Fig. 12.
PRL-activated JAK2 promotes formation of multiprotein complex. Schematic representation of the proposed working model. PRL-activation of JAK2 leads to tyrosyl phosphorylation of PAK1 on tyrosines 153, 201, and 285, thereby increasing PAK1 activities (both the serine/threonine kinase activity and ability to create potential protein-protein interactions) and stimulating phosphorylation of FLNa. Phosphorylated FLNa stimulates the kinase activity of PAK1 and has increased actin-regulating activity. FLNa, which directly binds to SH2B1β, relocates SH2B1β to the JAK2-PAK1-FLNa complex. Because SH2B1β is the enhancer of the kinase activity of JAK2, the formation of the complex results in enhancement of JAK2 activation and further activation of the JAK2-PAK1-FLNa complex that leads to actin cytoskeleton reorganization via actin-regulating proteins PAK1, FLNa, and SH2B1β. Binding of FLNa to the plasma membrane is not shown.
Materials and Methods
Plasmids
cDNA encoding myc-tagged WT SH2B1β, SH2B1β (1-105), or SH2B1β Δ-Δ mutant were described previously (18, 28, 70). To generate myc-tagged SH2B1β (1-200) and (1-260) mutants, restriction sites were inserted at appropriate positions in myc-tagged WT SH2B1β by using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). mRFP-tagged SH2B1β WT and SH2B1β Δ-Δ mutant were generated using the mRFP plasmid as a gift of Pugacheva (West Virginia University, Morgantown, WV). To generate mRFP-tagged SH2B1β 3Δ, restriction sites were inserted at appropriate positions in the mRFP-tagged SH2B1β Δ-Δ mutant by using a QuikChange site-directed mutagenesis kit (Stratagene). Mutations were confirmed by sequencing at the University of Michigan DNA Sequencing Core (Ann Arbor, MI). Schematic representation of WT and mutant forms of rat SH2B1β used in the study is shown in Fig. 1. cDNA encoding myc-tagged PAK1 WT and PAK1 Y3F mutant in which tyrosines 153, 201, and 285 in PAK1 WT were mutated to phenylalanines were described previously (31). cDNA encoding the GFP-containing long form of PRLR was described previously (90, 91). cDNA encoding GST-tagged truncated forms of FLNa were also described previously (49).
Cell cultures
FLNa-deficient human melanoma cell line (M2) and its derivative line (A7), which stably expresses FLNa, were described previously (47). M2 cells were maintained in MEM (Mediatech, Inc., Manassas, VA) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 1% nonessential amino acids (HyClone, Logan, UT), 50 U/ml penicillin (Mediatech, Inc.), 50 μg/ml streptomycin (Mediatech, Inc.), and supplemented with 4 mm l-glutamine (Mediatech, Inc.). A7 cells were maintained in the same medium as M2 cells but additionally supplemented with 500 μg/ml G418 (InvivoGen, San Diego, CA). HEK 293T cells were maintained in DMEM (Mediatech, Inc.) containing10% calf serum, 50 U/ml penicillin, 50 μg/ml streptomycin, and supplemented with 4 mm l-glutamine. T47D cells were maintained in RPMI (Mediatech, Inc.) supplemented with 10% fetal bovine serum, bovine insulin (0.2 units/ml; Sigma-Aldrich), 50 U/ml penicillin, 50 μg/ml streptomycin, and supplemented with 4 mm l-glutamine. To generate stable T47D cell clones overexpressing PAK1 WT and PAK1 Y3F mutant, the T47D cells were transfected with a construction of PAK1 WT in pLNCX2 retroviral vector containing internal ribosome entry site 2-enhanced GFP element (92). To make PAK1 Y3F construct, tyrosines 153, 201, and 285 in PAK1 WT were mutated to phenylalanines using the QuikChange site-directed mutagenesis kit (Stratagene) (31).
Immunocytochemistry
For localization studies, M2 and A7 cells were transfected with cDNA encoding GFP-PRLR, and mRFP-tagged either SH2B1β WT or 3Δ using Nucleofector kit V (Lonza, Basel, Switzerland) according to the manufacturer's instruction. The cells were replated on coverslips and processed for immunocytochemistry (28). The coverslips were fixed and incubated with Alexa Fluor 647 phalloidin for actin visualization. Confocal imaging was performed with an Olympus 1X70 laser scanning confocal microscope (Olympus, Tokyo, Japan).
Assessment of membrane ruffling
Transient transfections were performed using Nucleofector kit V (Lonza) according to the manufacturer's instruction. The cells were replated on coverslips and processed for immunocytochemistry (28). To measure the effect of SH2B1β and PAK1 mutants on membrane ruffling, M2 and A7 cells expressing the indicated proteins were deprived of serum [deprivation media is MEM supplemented with 1% BSA (Millipore, Bedford, MA), 50 U/ml penicillin, 50 μg/ml streptomycin, and 4 mm l-glutamine] and treated as indicated in the figure legends. The coverslips were incubated with αmyc (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) followed by goat αmouse-Alexa Fluor 594 (Invitrogen, Carlsbad, CA) in the case of myc-PAK1 only transfection. F-actin was stained with Alexa Fluor 350 phalloidin (Invitrogen). When both myc-PAK1 and mRFP-SH2B1β were transfected, myc-PAK1 was stained with αmyc, followed by rabbit-αmouse and goat αrabbit-Alexa Fluor 350 (Invitrogen). The number of ruffles per transfected cell was determined using a Zeiss Axiovert 200 microscope (Zeiss, Oberkochen, Germany). Ruffling index was calculated as the number of total ruffles, counted for 100 transfected cells, divided by total number of assessed cells (100 cells for each experimental condition). Confocal imaging was performed with an Olympus 1X70 laser scanning confocal microscope. Each transfection was repeated at least three times with similar results.
Wounding assay
M2 and A7 cells were transfected with cDNA encoding the indicated proteins using the Nucleofector kit V (Lonza) according to the manufacturer's instruction. After 24 h, cells were replated at high density (190,000 M2 cells/well and 95,000 A7 cells/well) onto 24-well plates. After 1 d, cells were deprived of serum for 24 h. After deprivation, monolayers of cells were scarified using a plastic pipette tip, washed extensively, and incubated in deprivation media with or without 400 ng/ml human PRL [National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases (Parlow)]. Ten measurements along each wound were taken using a phase-contrast microscope with a calibrated eyepiece at the initial time of wounding and 8 h later, to calculate the percentage wound closure in 8 h for each condition. For some experiments, T47D cells stably expressing either GFP or GFP-containing PAK1 or PAK1 Y3F were transiently transfected with cDNA encoding indicated proteins using the Nucleofector kit V (Lonza) and processed as above.
In vitro binding assay
GST or GST-tagged FLNa truncated mutants were purified using a glutathione-agarose affinity column (Sigma-Aldrich). The purity of the proteins was monitored by SDS-PAGE. Myc- or mRFP-tagged WT or truncated forms of SH2B1β were translated in vitro using TNT Coupled Reticulocyte Lysate System T7 or SP6 (Promega, Madison, WI) with or without 35S-methionine according to the manufacturer's instruction. The mixture of GST-tagged FLNa mutants and in vitro translated SH2B1β mutants was rotated for 1 h or overnight at 4 C. The glutathione-agarose beads were washed, bound myc-SH2Bβ was detected by immunoblotting (IB) with αmyc, and bound mRFP-SH2Bβ mutant was detected by autoradiogram. Amount of GST or GST-FLNa mutants was detected by IB with αGST.
Coimmunoprecipitation and IB
SH2B1β were immunoprecipitated from 293T cell lysates using αSH2B1β (19) and protein A-agarose. Proteins were resolved by SDS-PAGE followed by IB with αFLNa (Cell Signaling, Inc., Beverly, MA). The same blot was reprobed with αSH2B1β. M2 and A7 cells were transiently transfected with cDNA encoding indicated proteins using the Nucleofector kit V (Lonza) and processed as described in the figure legends.
Statistical analysis
Data from at least three separate transfections per each condition were pooled and analyzed using an ANOVA test. For each experimental condition, 100 cells were counted in the ruffling assay, and 10 independent measurements were performed in the wounding assay. When individual experiments were analyzed, the results were indistinguishable from those obtained from the pooled data. Differences were considered to be statistically significant at P < 0.05. Results are expressed as the mean ± se. For wounding experiments, measurements were taken before determining the identity of the cells on the plate.
Acknowledgments
We thank Dr. Matter (University of Hawaii at Manoa, Honolulu, HI) for providing M2 cells and to Dr. Stossel (Harvard Medical School, Boston, MA) for sending A7 cells. We thank Drs. Clevenger (Northwestern University, Chicago, IL), Lee (University of Ottawa, Ottawa, ON, Canada), Chernoff (Fox Chase Cancer Institute, Philadelphia, PA), Pugacheva (West Virginia University, Morgantown, WV) for cDNA encoding PRLR, GST-tagged forms of FLNa, myc-PAK1 WT, and mRFP, respectively. We thank Dr. Mattingly (Wayne State University, Detroit, MI) for construction of PAK1 WT in the pLNCX2 retroviral vector containing the internal ribosome entry site 2-enhanced GFP element. We are grateful to Dr. Carter-Su (University of Michigan, Ann Arbor, MI) for providing the cDNA encoding WT and (1-105) mutant of SH2B1β and αSH2B1β and αJAK2 serums. We are grateful to Dr. Leaman (University of Toledo, Toledo, OH) for very helpful discussion.
This work was supported by National Institutes of Health Grants R01 DK88127 and R15 CA135378 (to M.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FLNa
- Filamin A
- GFP
- green fluorescent protein
- Grb2
- growth factor receptor-bound protein 2
- GST
- glutathione S-transferase
- IB
- immunoblotting
- JAK2
- Janus tyrosine kinase 2
- Lnk
- lymphocyte-specific adapter protein
- mRFP
- monomeric red fluorescent protein
- PAK
- p21-activated serine-threonine kinase
- PAK1 Y3F
- JAK2 tyrosyl-phosphorylation deficient mutant of PAK1
- PDGF
- platelet-derived growth factor
- PRL
- prolactin
- PRLR
- PRL receptor
- Rac1
- Ras-related C3 botulinum toxin substrate 1
- SH2
- Src homology 2
- Stat
- signal transducer and activator of transcription
- WT
- wild type.
References
- 1. Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. 1998. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- 2. Horseman ND. 1999. Prolactin and mammary gland development. J Mammary Gland Biol Neoplasia 4:79–88 [DOI] [PubMed] [Google Scholar]
- 3. Goffin V, Bernichtein S, Touraine P, Kelly PA. 2005. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev 26:400–422 [DOI] [PubMed] [Google Scholar]
- 4. Bernichtein S, Touraine P, Goffin V. 2010. New concepts in prolactin biology. J Endocrinol 206:1–11 [DOI] [PubMed] [Google Scholar]
- 5. Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE. 1995. Expression of prolactin and prolactin receptor in human breast carcinoma. Evidence for an autocrine/paracrine loop. Am J Pathol 146:695–705 [PMC free article] [PubMed] [Google Scholar]
- 6. Vonderhaar BK. 1998. Prolactin: the forgotten hormone of human breast cancer. Pharmacol Ther 79:169–178 [DOI] [PubMed] [Google Scholar]
- 7. Vonderhaar BK. 1999. Prolactin involvement in breast cancer. Endocr Relat Cancer 6:389–404 [DOI] [PubMed] [Google Scholar]
- 8. Welch DR, Steeg PS, Rinker-Schaeffer CW. 2000. Molecular biology of breast cancer metastasis. Genetic regulation of human breast carcinoma metastasis. Breast Cancer Res 2:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clevenger CV, Furth PA, Hankinson SE, Schuler LA. 2003. The role of prolactin in mammary carcinoma. Endocr Rev 24:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clevenger CV. 2003. Role of prolactin/prolactin receptor signaling in human breast cancer. Breast Dis 18:75–86 [DOI] [PubMed] [Google Scholar]
- 11. Harvey PW, Everett DJ, Springall CJ. 2008. Adverse effects of prolactin in rodents and humans: breast and prostate cancer. J Psychopharmacol 22:20–27 [DOI] [PubMed] [Google Scholar]
- 12. Clevenger CV, Gadd SL, Zheng J. 2009. New mechanisms for PRLr action in breast cancer. Trends Endocrinol Metab 20:223–229 [DOI] [PubMed] [Google Scholar]
- 13. Wagner KU, Rui H. 2008. Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia 13:93–103 [DOI] [PubMed] [Google Scholar]
- 14. Maus MV, Reilly SC, Clevenger CV. 1999. Prolactin as a chemoattractant for human breast carcinoma. Endocrinology 140:5447–5450 [DOI] [PubMed] [Google Scholar]
- 15. Miller SL, DeMaria JE, Freier DO, Riegel AM, Clevenger CV. 2005. Novel association of Vav2 and Nek3 modulates signaling through the human prolactin receptor. Mol Endocrinol 19:939–949 [DOI] [PubMed] [Google Scholar]
- 16. Miller SL, Antico G, Raghunath PN, Tomaszewski JE, Clevenger CV. 2007. Nek3 kinase regulates prolactin-mediated cytoskeletal reorganization and motility of breast cancer cells. Oncogene 26:4668–4678 [DOI] [PubMed] [Google Scholar]
- 17. Liby K, Neltner B, Mohamet L, Menchen L, Ben-Jonathan N. 2003. Prolactin overexpression by MDA-MB-435 human breast cancer cells accelerates tumor growth. Breast Cancer Res Treat 79:241–252 [DOI] [PubMed] [Google Scholar]
- 18. Rider L, Tao J, Snyder S, Brinley B, Lu J, Diakonova M. 2009. Adapter protein SH2B1β cross-links actin filaments and regulates actin cytoskeleton. Mol Endocrinol 23:1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C. 1997. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol 17:6633–6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelms K, O'Neill TJ, Li S, Hubbard SR, Gustafson TA, Paul WE. 1999. Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm Genome 10:1160–1167 [DOI] [PubMed] [Google Scholar]
- 21. Nishi M, Werner ED, Oh BC, Frantz JD, Dhe-Paganon S, Hansen L, Lee J, Shoelson SE. 2005. Kinase activation through dimerization by human SH2-B. Mol Cell Biol 25:2607–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maures TJ, Kurzer JH, Carter-Su C. 2007. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab 18:38–45 [DOI] [PubMed] [Google Scholar]
- 23. Yousaf N, Deng Y, Kang Y, Riedel H. 2001. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J Biol Chem 276:40940–40948 [DOI] [PubMed] [Google Scholar]
- 24. Ohtsuka S, Takaki S, Iseki M, Miyoshi K, Nakagata N, Kataoka Y, Yoshida N, Takatsu K, Yoshimura A. 2002. SH2-B is required for both male and female reproduction. Mol Cell Biol 22:3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duan C, Yang H, White MF, Rui L. 2004. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol 24:7435–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ren D, Li M, Duan C, Rui L. 2005. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab 2:95–104 [DOI] [PubMed] [Google Scholar]
- 27. Herrington J, Diakonova M, Rui L, Gunter DR, Carter-Su C. 2000. SH2-B is required for growth hormone-induced actin reorganization. J Biol Chem 275:13126–13133 [DOI] [PubMed] [Google Scholar]
- 28. Diakonova M, Gunter DR, Herrington J, Carter-Su C. 2002. SH2-Bβ is a Rac-binding protein that regulates cell motility. J Biol Chem 277:10669–10677 [DOI] [PubMed] [Google Scholar]
- 29. Diakonova M, Helfer E, Seveau S, Swanson JA, Kocks C, Rui L, Carlier MF, Carter-Su C. 2007. Adapter protein SH2-Bβ stimulates actin-based motility of Listeria monocytogenes in a vasodilator-stimulated phosphoprotein (VASP)-dependent fashion. Infect Immun 75:3581–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He X, Li Y, Schembri-King J, Jakes S, Hayashi J. 2000. Identification of actin binding protein, ABP-280, as a binding partner of human Lnk adaptor protein. Mol Immunol 37:603–612 [DOI] [PubMed] [Google Scholar]
- 31. Rider L, Shatrova A, Feener EP, Webb L, Diakonova M. 2007. JAK2 tyrosine kinase phosphorylates PAK1 and regulates PAK1 activity and functions. J Biol Chem 282:30985–30996 [DOI] [PubMed] [Google Scholar]
- 32. Vadlamudi RK, Kumar R. 2003. P21-activated kinases in human cancer. Cancer Metastasis Rev 22:385–393 [DOI] [PubMed] [Google Scholar]
- 33. Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. 1998. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem 273:28238–28246 [DOI] [PubMed] [Google Scholar]
- 34. Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. 2000. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J Biol Chem 275:12041–12050 [DOI] [PubMed] [Google Scholar]
- 35. Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. 2000. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem 275:36238–36244 [DOI] [PubMed] [Google Scholar]
- 36. Stofega MR, Sanders LC, Gardiner EM, Bokoch GM. 2004. Constitutive p21-activated kinase (PAK) activation in breast cancer cells as a result of mislocalization of PAK to focal adhesions. Mol Biol Cell 15:2965–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards DC, Sanders LC, Bokoch GM, Gill GN. 1999. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1:253–259 [DOI] [PubMed] [Google Scholar]
- 38. Qian X, Riccio A, Zhang Y, Ginty DD. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21:1017–1029 [DOI] [PubMed] [Google Scholar]
- 39. Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. 1999. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J Cell Biol 145:851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. 2002. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol 4:681–690 [DOI] [PubMed] [Google Scholar]
- 41. Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. 2004. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep 5:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xiao GH, Beeser A, Chernoff J, Testa JR. 2002. p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem 277:883–886 [DOI] [PubMed] [Google Scholar]
- 43. Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. 2001. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 2:138–145 [DOI] [PubMed] [Google Scholar]
- 44. Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, Hartwig JH. 1990. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol 111:1089–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakamura F, Osborn TM, Hartemink CA, Hartwig JH, Stossel TP. 2007. Structural basis of filamin A functions. J Cell Biol 179:1011–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weihing RR. 1988. Actin-binding and dimerization domains of HeLa cell filamin. Biochemistry 27:1865–1869 [DOI] [PubMed] [Google Scholar]
- 47. Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, Stossel TP. 1992. Actin-binding protein requirement for cortical stability and efficient locomotion. Science 255:325–327 [DOI] [PubMed] [Google Scholar]
- 48. Ridley AJ. 1994. Membrane ruffling and signal transduction. Bioessays 16:321–327 [DOI] [PubMed] [Google Scholar]
- 49. Cukier IH, Li Y, Lee JM. 2007. Cyclin B1/Cdk1 binds and phosphorylates filamin A and regulates its ability to cross-link actin. FEBS Lett 581:1661–1672 [DOI] [PubMed] [Google Scholar]
- 50. Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP. 2001. Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol 155:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klaile E, Müller MM, Kannicht C, Singer BB, Lucka L. 2005. CEACAM1 functionally interacts with filamin A and exerts a dual role in the regulation of cell migration. J Cell Sci 118:5513–5524 [DOI] [PubMed] [Google Scholar]
- 52. Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y. 2006. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol 175:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. 2008. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem 283:27973–27981 [DOI] [PubMed] [Google Scholar]
- 54. Bazan JF. 1990. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA 87:6934–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Campbell GS, Argetsinger LS, Ihle JN, Kelly PA, Rillema JA, Carter-Su C. 1994. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci USA 91:5232–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lebrun JJ, Ali S, Sofer L, Ullrich A, Kelly PA. 1994. Prolactin-induced proliferation of Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J Biol Chem 269:14021–14026 [PubMed] [Google Scholar]
- 57. Rui H, Lebrun JJ, Kirken RA, Kelly PA, Farrar WL. 1994. JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology 135:1299–1306 [DOI] [PubMed] [Google Scholar]
- 58. Goffin V, Struman I, Mainfroid V, Kinet S, Martial JA. 1994. Evidence for a second receptor binding site on human prolactin. J Biol Chem 269:32598–32606 [PubMed] [Google Scholar]
- 59. Gouilleux F, Wakao H, Mundt M, Groner B. 1994. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J 13:4361–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. DaSilva L, Rui H, Erwin RA, Howard OM, Kirken RA, Malabarba MG, Hackett RH, Larner AC, Farrar WL. 1996. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol Cell Endocrinol 117:131–140 [DOI] [PubMed] [Google Scholar]
- 61. Schaber JD, Fang H, Xu J, Grimley PM, Rui H. 1998. Prolactin activates Stat1 but does not antagonize Stat1 activation and growth inhibition by type I interferons in human breast cancer cells. Cancer Res 58:1914–1919 [PubMed] [Google Scholar]
- 62. Yu-Lee L, Luo G, Moutoussamy S, Finidori J. 1998. Prolactin and growth hormone signal transduction in lymphohaemopoietic cells. Cell Mol Life Sci 54:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Das R, Vonderhaar BK. 1996. Involvement of SHC, GRB2, SOS and RAS in prolactin signal transduction in mammary epithelial cells. Oncogene 13:1139–1145 [PubMed] [Google Scholar]
- 64. Das R, Vonderhaar BK. 1996. Activation of raf-1, MEK, and MAP kinase in prolactin responsive mammary cells. Breast Cancer Res Treat 40:141–149 [DOI] [PubMed] [Google Scholar]
- 65. Waters SB, Rillema JA. 1989. Role of protein kinase C in the prolactin-induced responses in mouse mammary gland explants. Mol Cell Endocrinol 63:159–166 [DOI] [PubMed] [Google Scholar]
- 66. Banerjee R, Vonderhaar BK. 1992. Prolactin-induced protein kinase C activity in a mouse mammary cell line (NOG-8). Mol Cell Endocrinol 90:61–67 [DOI] [PubMed] [Google Scholar]
- 67. Rane SG, Reddy EP. 2000. Janus kinases: components of multiple signaling pathways. Oncogene 19:5662–5679 [DOI] [PubMed] [Google Scholar]
- 68. Gutzman JH, Rugowski DE, Nikolai SE, Schuler LA. 2007. Stat5 activation inhibits prolactin-induced AP-1 activity: distinct prolactin-initiated signals in tumorigenesis dependent on cell context. Oncogene 26:6341–6348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Brien KB, Argetsinger LS, Diakonova M, Carter-Su C. 2003. YXXL motifs in SH2-Bβ are phosphorylated by JAK2, JAK1, and platelet-derived growth factor receptor and are required for membrane ruffling. J Biol Chem 278:11970–11978 [DOI] [PubMed] [Google Scholar]
- 70. Rui L, Carter-Su C. 1999. Identification of SH2-bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA 96:7172–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li Z, Zhou Y, Carter-Su C, Myers MG, Jr, Rui L. 2007. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol 21:2270–2281 [DOI] [PubMed] [Google Scholar]
- 72. Borm B, Requardt RP, Herzog V, Kirfel G. 2005. Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp Cell Res 302:83–95 [DOI] [PubMed] [Google Scholar]
- 73. Fiori JL, Zhu TN, O'Connell MP, Hoek KS, Indig FE, Frank BP, Morris C, Kole S, Hasskamp J, Elias G, Weeraratna AT, Bernier M. 2009. Filamin A modulates kinase activation and intracellular trafficking of epidermal growth factor receptors in human melanoma cells. Endocrinology 150:2551–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li C, Yu S, Nakamura F, Pentikäinen OT, Singh N, Yin S, Xin W, Sy MS. 2010. Pro-prion binds filamin A, facilitating its interaction with integrin β1, and contributes to melanomagenesis. J Biol Chem 285:30328–30339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. 2009. Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell 20:4531–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. 2009. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J 96:5095–5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kasza KE, Nakamura F, Hu S, Kollmannsberger P, Bonakdar N, Fabry B, Stossel TP, Wang N, Weitz DA. 2009. Filamin A is essential for active cell stiffening but not passive stiffening under external force. Biophys J 96:4326–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Beekman JM, van der Poel CE, van der Linden JA, van den Berg DL, van den Berghe PV, van de Winkel JG, Leusen JH. 2008. Filamin A stabilizes FcγRI surface expression and prevents its lysosomal routing. J Immunol 180:3938–3945 [DOI] [PubMed] [Google Scholar]
- 79. Kim EY, Ridgway LD, Dryer SE. 2007. Interactions with filamin A stimulate surface expression of large-conductance Ca2+-activated K+ channels in the absence of direct actin binding. Mol Pharmacol 72:622–630 [DOI] [PubMed] [Google Scholar]
- 80. Park JH, Kim HJ, Choy HE, Kim K. 2005. Presence of presenilin 1/2 affects the invasion and replication of Salmonella typhimurium. Biochem Biophys Res Commun 332:1081–1085 [DOI] [PubMed] [Google Scholar]
- 81. Meng X, Yuan Y, Maestas A, Shen Z. 2004. Recovery from DNA damage-induced G2 arrest requires actin-binding protein filamin-A/actin-binding protein 280. J Biol Chem 279:6098–6105 [DOI] [PubMed] [Google Scholar]
- 82. Lin R, Canfield V, Levenson R. 2002. Dominant negative mutants of filamin A block cell surface expression of the D2 dopamine receptor. Pharmacology 66:173–181 [DOI] [PubMed] [Google Scholar]
- 83. Puto LA, Pestonjamasp K, King CC, Bokoch GM. 2003. p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J Biol Chem 278:9388–9393 [DOI] [PubMed] [Google Scholar]
- 84. Meyer SC, Sanan DA, Fox JE. 1998. Role of actin-binding protein in insertion of adhesion receptors into the membrane. J Biol Chem 273:3013–3020 [DOI] [PubMed] [Google Scholar]
- 85. Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, Campbell ID, Ylänne J, Calderwood DA. 2006. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell 21:337–347 [DOI] [PubMed] [Google Scholar]
- 86. He HJ, Kole S, Kwon YK, Crow MT, Bernier M. 2003. Interaction of filamin A with the insulin receptor alters insulin-dependent activation of the mitogen-activated protein kinase pathway. J Biol Chem 278:27096–27104 [DOI] [PubMed] [Google Scholar]
- 87. Seck T, Baron R, Horne WC. 2003. Binding of filamin to the C-terminal tail of the calcitonin receptor controls recycling. J Biol Chem 278:10408–10416 [DOI] [PubMed] [Google Scholar]
- 88. Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. 2001. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci USA 98:5258–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Onoprishvili I, Andria ML, Kramer HK, Ancevska-Taneva N, Hiller JM, Simon EJ. 2003. Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol Pharmacol 64:1092–1100 [DOI] [PubMed] [Google Scholar]
- 90. Kline JB, Roehrs H, Clevenger CV. 1999. Functional characterization of the intermediate isoform of the human prolactin receptor. J Biol Chem 274:35461–35468 [DOI] [PubMed] [Google Scholar]
- 91. Zheng J, Koblinski JE, Dutson LV, Feeney YB, Clevenger CV. 2008. Prolyl isomerase cyclophilin A regulation of Janus-activated kinase 2 and the progression of human breast cancer. Cancer Res 68:7769–7778 [DOI] [PubMed] [Google Scholar]
- 92. Li Q, Mullins SR, Sloane BF, Mattingly RR. 2008. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia 10:314–329 [DOI] [PMC free article] [PubMed] [Google Scholar]