Abstract
Receptor activity-modifying protein-2 (RAMP2) is a single-pass transmembrane protein that can regulate the trafficking, ligand binding, and signaling of several G protein-coupled receptors (GPCR). The most well-characterized role of RAMP2 is in the regulation of adrenomedullin (AM) binding to calcitonin receptor-like receptor (CLR), and our previous studies using knockout mouse models support this canonical signaling paradigm. For example, Ramp2−/− mice die at midgestation with a precise phenocopy of the AM−/− and Calcrl−/− mice. In contrast, Ramp2+/− mice are viable and exhibit an expanded variety of phenotypes that are distinct from those of Calcrl+/− mice. Using Ramp2+/− female mice, we demonstrate that a modest decrease in Ramp2 expression causes severe reproductive defects characterized by fetal growth restriction, fetal demise, and postnatal lethality that is independent of the genotype and gender of the offspring. Ramp2+/− female mice also exhibit hyperprolactinemia during pregnancy and in basal conditions. Consistent with hyperprolactinemia, Ramp2+/− female mice have enlarged pituitary glands, accelerated mammary gland development, and skeletal abnormalities including delayed bone development and decreased bone mineral density. Because RAMP2 has been shown to associate with numerous GPCR, it is likely that signaling of one or more of these GPCR is compromised in Ramp2+/− mice, yet the precise identification of these receptors remains to be elucidated. Taken together, this work reveals an essential role for RAMP2 in endocrine physiology and provides the first in vivo evidence for a physiological role of RAMP2 beyond that of AM/CLR signaling.
Receptor activity modifying proteins (RAMP) are single-pass transmembrane proteins that can influence G protein-coupled receptor (GPCR) pharmacology by either altering the cell-surface trafficking of receptors, dictating ligand-binding specificity, regulating receptor desensitization, or modulating second messenger signaling (1). There are three mammalian RAMPs (RAMP1, -2, and -3), each encoded by a separate gene. The RAMPs were originally identified and characterized because they could potentiate the translocation of the calcitonin receptor-like receptor (CLR) from the endoplasmic reticulum to the plasma membrane (2). In addition, each RAMP was shown to associate with CLR and change its ligand-binding affinity for different peptide ligands (2). For example, if CLR associates with RAMP1, then a functional receptor for calcitonin gene-related peptide is formed, but if either RAMP2 or RAMP3 associates with CLR, then a functional receptor for adrenomedullin (AM) is made. Therefore, the spatial and temporal expression of RAMPs determines the cell surface expression and receptor pharmacology of RAMP-interacting GPCR.
Importantly, this added level of receptor complexity offers unique opportunities for the design of small-molecule compounds and drugs that are specifically targeted to the RAMP-receptor interface. For example, BIBN4096BS (olcegepant), MK-0974 (telcagepant), and MK-3207 are nonpeptide, small-molecule antagonists that have clinical efficacy in targeting the CLR-RAMP1 interface to inhibit the functions of calcitonin gene-related peptide in migraine pain (3–7). Whether the RAMP-receptor paradigm can be exploited for pharmacological drug targeting of other GPCRs remains to be determined but is currently hindered by our lack of knowledge regarding other physiologically relevant GPCRs that functionally associate with RAMPs.
The broader cell and tissue expression pattern of RAMPs compared with that of CLR and its ligands suggests that RAMPs are likely to interact with other GPCR and modulate their properties. Indeed, numerous studies have demonstrated that the calcitonin receptor (CTR) preferentially binds amylin (AMY), rather than calcitonin, when the receptor is associated with any of the three RAMPs. Moreover, in vitro biochemical studies in which the subcellular trafficking of overexpressed, fluorescently labeled RAMP is changed by receptor overexpression suggest that many other GPCRs functionally interact with RAMPs. These include several members of the class II GPCRs: the PTH receptors (PTHR) 1 and 2, the vasointestinal peptide/pituitary adenylate cyclase-activating peptide 1 (VIP/VPAC1) receptor, the glucagon receptor (GCGR) (8), and more recently the secretin receptor (9). Bouschet and colleagues also showed that a class III GPCR, the calcium-sensing receptor (CaSR), requires RAMP for efficient cell surface expression in transfected clonal cells (10). However, it remains largely unclear whether these in vitro interactions have any physiological relevance either in normal or pathological conditions.
To address this gap in our knowledge, we have undertaken a phenotype-driven approach to identify the physiologically relevant functions of RAMP, and ultimately their GPCR partners, by generating and characterizing gene knockout mice for each of the three mammalian Ramp genes. The overt phenotypes of various mouse models of RAMP, including those generated by others, has recently been reviewed (11). Here, we describe a constellation of endocrine-related phenotypes that are present in Ramp2+/− mice but are notably absent in mice heterozygous for the gene encoding CLR. Therefore, these data provide in vivo evidence for an important role of RAMP2 in endocrine physiology and extend the physiologically relevant functions of RAMP2 beyond the canonical CLR paradigm.
Results
Genetic reduction of maternal Ramp2 causes fetal loss throughout pregnancy
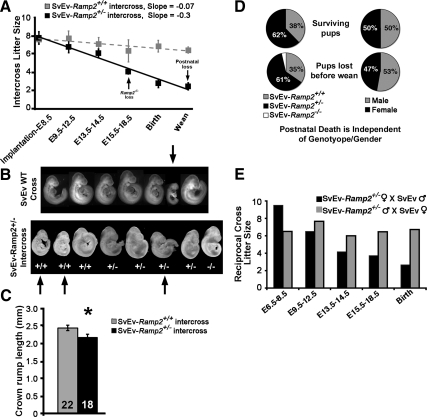
We have previously shown that Ramp2-null embryos exhibit interstitial edema due to defective lymphangiogenesis and die at midgestation [embryonic d 14.5 (E14.5)] on either a 129SvEV/S6 or an F2-129SvEv/S6-C57BL6 genetic background (12). We have also noticed that haploinsufficient Ramp2+/− mice on a 129SvEv/S6 background survive to adulthood but have extremely small litter sizes, worse than what would be predicted from the Mendelian loss of Ramp2−/− embryos (13). To determine the gestational age of fetal loss, we established natural timed matings between SvEv-Ramp2+/− parents and scored the following: 1) number of viable embryos at various gestational ages beyond implantation, 2) number of live pups at birth, and 3) number of live pups at weaning. These data were then compared with data obtained from crosses between wild-type SvEv parents. As shown in the gray dashed line of Fig. 1A, wild-type SvEv intercrosses maintained within our animal colony resulted in average litter sizes that are consistent with our previously published observations and those reported for the 129SvEv/S6-Taconic line (14) (www.taconic.com/wmspage.cfm?parm1=426), with no appreciable decline in litter size throughout gestation, at birth, or at weaning. In contrast, SvEv-Ramp2+/− intercrosses (black solid line, Fig. 1A) showed a gradual and continuous decrease in litter size from implantation to weaning.
Fig. 1.
Reduced fertility in SvEv-Ramp2+/− females and high incidence of postnatal lethality SvEv-Ramp2+/− litters. A, Average number of pups per litter after implantation to weaning (SvEv-Ramp2+/+ intercrosses R2 value = 0.74; SvEv-Ramp2+/− intercrosses R2 value = 0.97). A total of 140 litters were scored for SvEv-Ramp2+/− intercrosses at birth and weaning, and 55 litters were scored for SvEv-Ramp2+/+ intercrosses. Unless otherwise mentioned, three to 17 litters per gestational time point were scored. The black arrows indicate the gestational age at which Ramp2−/− embryos die from lymphatic vascular defects and at which postnatal pup loss is observed in SvEv-Ramp2+/− intercrosses. B, Representative litters from wild-type SvEv and SvEv-Ramp2+/− intercrosses at E9.5 of pregnancy, showing a high incidence of fetal growth restriction that is independent of fetuses' genotype in SvEv-Ramp2+/− intercrosses. Arrows point to severely growth-restricted embryos. Embryos were imaged at the same magnification and later grouped digitally based on their genotype. C, Crown to rump length (millimeters) of E9.5 embryos from SvEv-Ramp2+/+ intercrosses and SvEv-Ramp2+/− intercrosses; *, P < 0.05. Number in the column represents number of embryos measured. D, Pie charts demonstrating that postnatal death observed from 140 SvEv-Ramp2+/− intercross litters was independent of pup genotype (left) and pup gender (right). Note that the white slice of the lower left pie chart represents a single Ramp2−/− mouse that we discovered alive after birth. Consistent with the knockout phenotype, this animal was severely hydropic. E, Reciprocal crosses of SvEv-Ramp2+/− mice. An average of two to three litters were scored for each time point. Loss of pups throughout pregnancy was observed only when the female was SvEv-Ramp2+/−.
Morphological evaluation of embryos from SvEv-Ramp2+/− intercrosses revealed a high incidence of fetal growth restriction compared with embryos from wild-type SvEv crosses. Figure 1B shows representative images of litters from E9.5 wild-type SvEV and SvEv-Ramp2+/− intercrosses. Although we noticed occasional fetal growth restriction in wild-type SvEV crosses, SvEv-Ramp2+/− intercrosses exhibited a high proportion of severe fetal growth restriction. In the representative example shown, three of eight embryos are severely growth restricted (marked by arrows). Moreover, the fetal growth restriction was independent of the genotype of the embryo, because two of the three growth-restricted embryos were wild type. Consistent with these morphological observations, crown to rump length of E9.5 embryos from SvEv-Ramp2+/− intercrosses was significantly reduced compared with that of embryos from wild-type SvEv intercrosses (Fig. 1C). Therefore, the dramatic and progressive decline in litter size in SvEv-Ramp2+/− intercrosses is not solely due to the embryonic lethality of Ramp2−/− embryos but is also caused by a high incidence of fetal growth restriction that affects both wild-type and Ramp2+/− embryos.
Genetic reduction of maternal Ramp2 causes postnatal pup and litter lethality
In addition to fetal loss during gestation, a large percentage of individual pups, as well as entire litters, born to SvEv-Ramp2+/− females died within 1–2 d after birth. Within the 140 born litters we characterized from SvEv-Ramp2+/− intercrosses, we observed a 12% postnatal lethality of individual pups, double that of the 6% postnatal lethality rate observed for SvEV wild-type crosses (Fig. 1A). Most striking was our observation that nearly one quarter, 24%, of litters born to Ramp2+/− females lost all of the pups (12 complete litters died among the 50 litters examined). This unusually high rate of postnatal litter loss was not observed in wild-type SvEv mice maintained in our colony (one litter died among 30 litters examined, 3.3%) or in heterozygote intercrosses of Calcrl+/− or Adm+/− mice that are maintained on an identical isogenic 129SvEv/S6 background in our colony. Moreover, our observations of average litter size at birth, at weaning, and percentage of entire litter loss from wild-type SvEv intercrosses are similar to the breeding characteristics established by Taconic Farms for the 129S6/SvEvTac strain (www.taconic.com/wmspage.cfm?parm1=426). Therefore, compared with control wild-type SvEV intercrosses, these data reveal an unusually high incidence of postnatal lethality for individual pups, as well as entire litters, born to SvEv-Ramp2+/− intercrosses.
To determine whether this postnatal lethality was associated with the genotype or gender of the offspring, we tabulated the genotype and sex of both the surviving and dead pups from the 140 litters generated from SvEv-Ramp2+/− intercrosses. As shown in Fig. 1D (left pie charts), the genotypes of pups, both surviving and dead, fit the expected Mendelian ratio of 1:2 (or 33:66%) for SvEv-Ramp2+/+ and SvEv-Ramp2+/−, respectively, and do not show any association with lethality. Dead pups in which the quality of genomic DNA was not suitable for genotyping by PCR were not included in our analysis. Furthermore, we found no correlation between the sex of the pups and their lethality (Fig. 1D, right pie charts). These data reveal an unusually high incidence of postnatal pup lethality in SvEv-Ramp2+/− intercrosses that is independent of the genotype or gender of the offspring, suggesting that female Ramp2+/− mice have either abnormal rearing behavior and/or problems with lactation. Consistent with this hypothesis, we noticed that most of the postnatal dead pups had no milk in their stomachs.
To eliminate the confounding effects of Ramp2−/− embryos generated from heterozygote intercrosses and to determine whether the pup loss was dependent on the gender of either parent, we established and analyzed genetic reciprocal crosses in which only one parent was heterozygote for the Ramp2 gene. As shown in the black bars of Fig. 1E, fetal loss throughout pregnancy and small litter sizes at birth were evident only when the dam was heterozygote for Ramp2. In contrast, litter sizes throughout pregnancy and at birth were not significantly affected when the male parent was heterozygote for Ramp2 (gray bars, Fig. 1E). Thus, genetic reduction of maternal Ramp2, but not of paternal Ramp2, causes fetal loss throughout pregnancy. Taken together, these data demonstrate that a modest genetic reduction in the maternal Ramp2 gene causes remarkable fetal growth restriction and fetal loss throughout pregnancy that is independent of fetal genotype.
Ramp2+/− female mice exhibit hyperprolactinemia and anterior pituitary gland hyperplasia
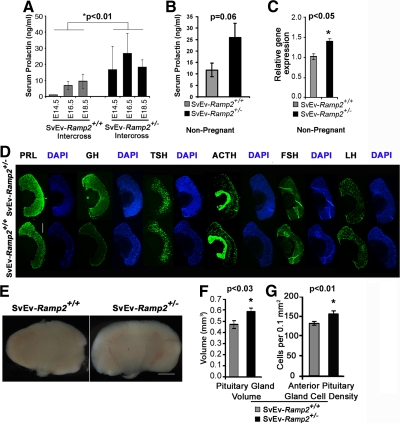
The absence of milk in the stomachs of postnatal dead pups born to SvEv-Ramp2+/− female mice prompted us to measure serum prolactin in female mice during late gestation when mammary glands prepare for lactation (15). As expected, serum prolactin gradually increased with advancing gestation (from E14.5 to E18.5) in SvEv-Ramp2+/+ females bred to SvEv-Ramp2+/+ males (Fig. 2A), with an average level of 3.8 ng/ml. However, SvEv-Ramp2+/− females bred with SvEv-Ramp2+/− males had a significantly more robust increase in serum prolactin, averaging 20.5 ng/ml from E14.5 to E18.5, compared with controls (P < 0.01, Fig. 2A). Moreover, Fig. 2B shows that basal levels of serum prolactin were 2-fold higher in nonpregnant SvEv-Ramp2+/− females compared with nonpregnant wild-type females, although this trend failed to reach statistical significance (11.7 ± 3.0 ng/ml SvEv-Ramp2+/+ vs. 25.8 ± 6.3 ng/ml SvEv-Ramp2+/−; P = 0.06). The expression of prolactin mRNA was also significantly elevated 1.4-fold in the pituitary glands of 6-month old, virgin SvEv-Ramp2+/− female mice compared with SvEv-Ramp2+/+ female mice (Fig. 2C).
Fig. 2.
SvEv-Ramp2+/− females exhibit hyperprolactinemia and hyperplastic pituitary glands. A, Maternal serum prolactin levels in wild-type SvEv-Ramp2+/+ and SvEv-Ramp2+/− intercrosses on E14.5, E16.5, and E18.5 of gestation. *, P < 0.01, by one-way ANOVA; n ≥ 5 mice per group and time point. B, Basal serum prolactin levels in nonpregnant mice; n = 25 for wild-type SvEv-Ramp2+/+ females, and n = 14 for SvEv-Ramp2+/− females. P = 0.06. C, Quantitative RT-PCR of prolactin gene expression, normalized to GAPDH expression, in the anterior pituitary glands of 6-month-old, virgin SvEv-Ramp2+/− female mice (black bars) compared with the wild-type SvEv-Ramp2+/+ controls (gray bars); n ≥ 8 mice per genotype. *, P < 0.05. D, Immunohistochemistry on cryosections of pituitary glands from virgin wild-type and SvEv-Ramp2+/− female mice using antibodies against six anterior pituitary hormones: ACTH, prolactin (PRL), GH, TSH, FSH, and LH. Images are representative of n = 8 pituitaries analyzed for each genotype. E, Images of dissected pituitary glands from wild-type and SvEv-Ramp2+/− females, showing enlarged pituitary glands in SvEv-Ramp2+/− mice. F, Volume of the pituitary gland is significantly larger in SvEv-Ramp2+/− female mice (black bars) compared with the controls (gray bars). *, P < 0.03. Scale bar, 500 μm. G, Cell density of anterior pituitary gland is significantly increased in SvEv-Ramp2+/− female mice (black bars) compared with the controls (gray bars). *, P < 0.01. Mice were 18-wk-old virgin females at time of euthanasia; n ≥ 5 mice per genotype.
Immunohistochemistry on pituitary gland sections of 18-wk-old, virgin SvEv-Ramp2+/+ and SvEv-Ramp2+/− female mice confirmed the basal hyperprolactinemia phenotype. As shown in Fig. 2D, the lactotropes of the anterior pituitary gland of SvEv-Ramp2+/− female mice showed intense staining with an anti-prolactin antibody compared with moderate staining intensity in wild-type control pituitaries. GH expression appeared somewhat elevated in the SvEv-Ramp2+/− mice compared with controls, a finding that may not be unexpected because lactotropes and somatotropes share a common lineage precursor during embryogenesis. Therefore, we also evaluated relative levels of pituitary GH by quantitative real time PCR (SvEv-Ramp2+/+ = 1.04 ± 0.14, n = 9, vs. SvEv-Ramp2+/− = 0.76 ± 0.07, n =8) and serum levels of GH by RIA (SvEv-Ramp2+/+ = 3.49 ng/ml ± 0.69, n =10, vs. SvEv-Ramp2+/− = 3.98 ng/ml ± 0.33, n =8) and found no significant differences between genotypes. In addition, Fig. 2D shows that there were no remarkable differences in staining intensity for any of the other hormone-producing cell types of the anterior pituitary (anti-TSH, ACTH, LH, and FSH).
Because increased prolactin gene expression, synthesis, and secretion are often associated with lactotrope hyperplasia, we evaluated the size of the pituitary glands from 18-wk-old, virgin SvEv-Ramp2+/+ and SvEv-Ramp2+/− female mice. As shown in Fig. 2E, the pituitary gland of SvEv-Ramp2+/− mice appeared larger and was appreciably more dense and firm upon dissection compared with pituitaries from SvEv-Ramp2+/+ mice. Consistent with these morphological observations, SvEv-Ramp2+/− female mice had significant increases in pituitary gland volume and cell density compared with the pituitaries of wild-type females (Fig. 2, F and G, respectively). The increase in pituitary cell density in SvEv-Ramp2+/− mice was also readily appreciated in 4′,6-diamidino-2-phenylindole-stained sections of pituitaries, shown in Fig. 2D. Taken together, these results demonstrate that genetic reduction of Ramp2 in female mice leads to mild hyperprolactinemia, both basally and during pregnancy, which is associated with enlarged and hyperplastic pituitary glands producing increased levels of prolactin. In this regard, when we used the Fisher method (16) to test for the combined significance of these related but independent variables, we found a highly significant (P < 0.0001) overall difference in prolactin measures between SvEv-Ramp2+/− mice and controls.
Accelerated mammary gland development in Ramp2+/− mice
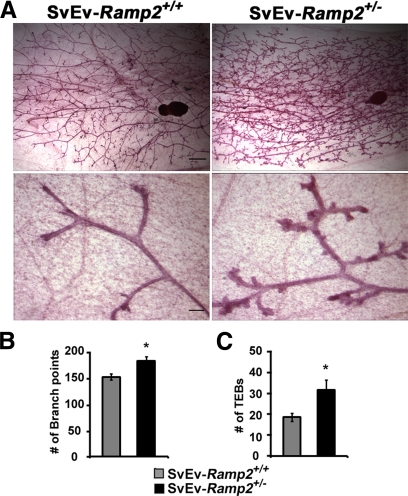
To determine whether the mild hyperprolactinemia of SvEv-Ramp2+/− female mice might contribute to abnormalities in mammary gland development and ultimately lactation defects, we performed whole-mount analyses of mammary glands from 18-wk-old, virgin SvEv-Ramp2+/− and SvEv-Ramp2+/+ female mice. We chose to evaluate mammary gland development of virgin mice rather than pregnant or lactating mice because variable litter sizes and suckling stimulus are known to influence mammary gland development and would confound the results for SvEv-Ramp2+/− mice. As shown in Fig. 3A, SvEv-Ramp2+/− mice had accelerated branching of secondary and tertiary ducts (low magnification) as well as remarkably increased number of terminal end buds (high magnification) compared with SvEv-Ramp2+/+ female mice. Quantitation of mammary gland ductal branch points as well as terminal end buds showed a marked and significant increase in SvEv-Ramp2+/− mice compared with control animals (Fig. 3, B and C, respectively). This precocious mammary gland development in SvEv-Ramp2+/− females was further supported by an increase in prolactin gene expression in the mammary glands (data not shown).
Fig. 3.
Accelerated mammary gland development in SvEv-Ramp2+/− female mice. A, Fourth inguinal mammary glands of 18-wk-old SvEv-Ramp2+/− and wild-type virgin female mice were dissected, fixed in Carnoy's fixative, and stained with Carmine dye. Lymph nodes were used as a reference point; n = 15–20 mice per genotype. Scale bars, 1 mm (low magnification) and 100 μm (high magnification). B, Quantitation of ductal branch points within two independent 30-mm2 areas per sample/animal. *, P < 0.01; n = 9 for SvEv-Ramp2+/+, and n = 6 for SvEv-Ramp2+/−. C, Quantitation of terminal end buds (TEBs) along a 5-mm peripheral duct. *, P < 0.05; n = 9 for SvEv-Ramp2+/+, and n = 6 for SvEv-Ramp2+/−.
Ramp2+/− mice have delayed mineralization during development and decreased bone mineral content and density
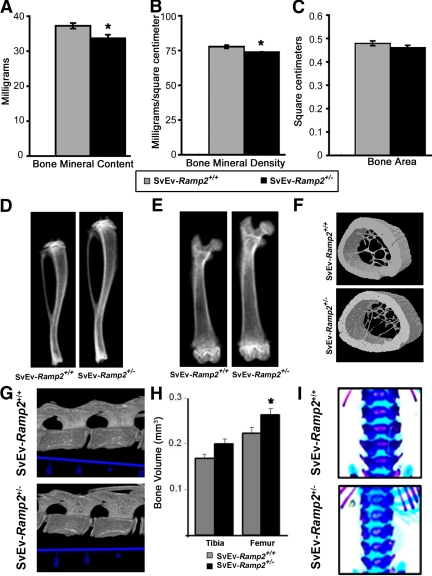
To determine whether the mild hyperprolactinemia of SvEv-Ramp2+/− mice contributed to other endocrine phenotypes, we evaluated the bones of SvEv-Ramp2+/− mice. Dual-energy x-ray absorptiometry (DEXA) analysis of excised femurs of 18-wk-old, virgin female mice revealed significantly reduced bone mineral content and bone mineral density in SvEv-Ramp2+/− mice compared with wild-type controls (Fig. 4, A and B). Total bone area did not change significantly between the two groups of mice (Fig. 4C). Radiographic images of tibiae and femurs of SvEv-Ramp2+/− mice showed significantly longer bones compared with their wild-type controls (Fig. 4, D and E). The tibiae of Ramp2+/− mice were significantly longer than wild-type controls (14.7 ± 0.3 mm for wild type and 15.89 ± 0.24 mm for SvEv-Ramp2+/−, P < 0.04), and the femurs generally appeared longer, although the difference did not reach statistical significance (11.9 ± 0.3 mm for wild type and 12.5 ± 0.06 mm for SvEv-Ramp2+/−). Consistent with the DEXA analysis, microcomputed tomography (micro-CT) analysis of femurs from 18-wk-old virgin mice showed that the reduced bone volume and density of SvEv-Ramp2+/− mice were associated with reduced cortical thickness and fewer, thinner trabecular structures compared with SvEv-Ramp2+/+ controls (Fig. 4F). The lumbar vertebrae of SvEv-Ramp2+/− mice were similar in length to SvEv-Ramp2+/+ mice but appeared underdeveloped with wider growth plates, wider intervertebral discs, and reduced mineralization (Fig. 4G).
Fig. 4.
Ramp2+/− mice have skeletal abnormalities. A–C, DEXA analyses of femurs showing decreased bone mineral content in milligrams (A), decreased bone mineral density in milligrams per square centimeters (B) (*, P < 0.01) and C) unchanged bone area in square centimeters (C); D, radiographs of tibiae; E, radiographs of femurs; F, micro-CT of midregion of femurs; G, 3D model of lumbar vertebrae; H, tibial and femoral bone volume (*, P < 0.05); I, skeletal staining by Alcian blue; Alizarin red (unmineralized bone and cartilage in blue, mineralized bone in purple). Animals used for the experiments in A–G are 18-wk-old virgin female SvEv-Ramp2+/+ and SvEv-Ramp2+/− mice; n = 8–10 per genotype for DEXA, and n =3 for micro-CT analysis and radiographs. Animals used in H and I are 2-d-old pups of SvEv-Ramp2+/+ (n = 5) and SvEv-Ramp2+/− genotypes (n = 3).
To distinguish whether these skeletal abnormalities were caused by the modest hyperprolactinemia or whether they arose independently of prolactin dysregulation, we evaluated the hind limb bones and lumbar spines of SvEv-Ramp2+/− pups 2 d postnatally. The femurs of the hind legs of SvEv-Ramp2+/− pups exhibited modest, yet statistically significant increases in bone volume compared with the wild-type pups, whereas the tibia volumes also trended to be greater in SvEv-Ramp2+/− pups (Fig. 4H). In the spine, there was also delayed development of the lumbar vertebrae as indicated by reduced mineralization of the epiphyseal plates of the vertebrae and delayed development of lumbar transverse processes (Fig. 4I).
Despite these skeletal changes, SvEv-Ramp2+/− mice had no significant differences in circulating levels of calcium when compared with SvEv-Ramp2+/+ controls (SvEv-Ramp2+/+ = 11.88 ± 0.96 mg/dl, and SvEv-Ramp2+/− = 13.55 ± 0.11 mg/dl). Nevertheless, these results clearly demonstrate that haploinsufficiency for Ramp2 results in significant defects in bone homeostasis and development.
Endocrine phenotypes of Ramp2+/− mice are strain dependent
We have previously shown that the reduced fertility phenotype of Ramp2+/− mice was not present in Ramp2+/− intercrosses on an F2 129SvEv/S6-C57BL6 genetic background (12). Consistent with this observation, we also found no significant increase in serum prolactin levels in nonpregnant F2-Ramp2+/− mice (data not shown). We also evaluated mammary gland development in F2-Ramp2+/− females and found no significant differences compared with strain-matched controls (data not shown). Therefore, the endocrine-related phenotypes of reduced fertility, postnatal pup loss, hyperprolactinemia, and accelerated mammary gland development in SvEv-Ramp2+/− mice can be rescued by crossing one generation onto a C57BL6 genetic background. These findings imply that the Ramp2 gene must have potent genetic modifiers that vary between common laboratory mouse strains.
Discussion
We have previously shown that global knockout mice for either Ramp2, Calcrl (gene name for CLR), and Adm (gene name for AM peptide) share a strikingly similar phenotype of embryonic lethality associated with impaired lymphatic vascular development and interstitial edema (12, 17, 18). This striking similarity in phenotypes allowed us to conclude that the CLR/RAMP2/AM signaling complex is an essential mediator of lymphatic vascular development during embryogenesis. However, because the global knockouts die at midgestation, we have been limited in our ability to extend our phenotypic comparison of these mice to other phenotypes, particularly those that might be present in Ramp2−/− mice but not in Calcrl−/− or Adm−/− mice.
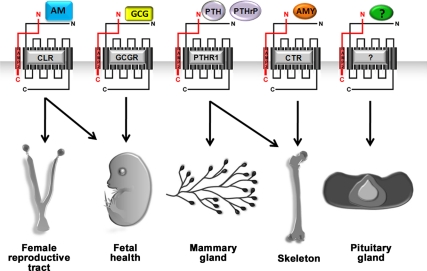
In this study, we have addressed this caveat by taking advantage of the viability of Ramp2+/− mice and characterized a constellation of endocrine-related phenotypes that include fetal loss throughout gestation, postnatal lethality of offspring, maternal hyperprolactinemia, accelerated mammary gland development, and altered skeletal properties and development. If these phenotypes were due solely to a reduction in signaling of the canonical CLR, then the logical expectation would be that female SvEv-Calcrl+/− mice should exhibit the same, or possibly exacerbated, phenotypes. However, SvEv-Calcrl+/− mice (which we generated and maintained on the identical genetic background as SvEv-Ramp2+/− mice) do not exhibit postnatal pup lethality, do not have elevated serum prolactin, do not have hyperplastic pituitaries, and do not have accelerated mammary gland development (data not shown). Therefore, this divergence in phenotypes allows us to conclude that in adults, RAMP2 must have physiologically relevant functions that extend beyond its well-established role as a modulator of CLR function. This conclusion is entirely consistent with in vitro biochemical and pharmacological studies that show associations between RAMP2 and several other GPCRs involved in endocrine physiology, like PTHR1, CTR, and GCGR (Fig. 5).
Fig. 5.
Proposed model for phenotypic outcomes in Ramp2+/− mice. The constellation of phenotypes observed in SvEv-Ramp2+/− mice is likely due to a combined effect of reduced signaling for many GPCR and ligands and perhaps others that have yet to be identified.
Determining exactly which receptors, or more likely which combination of receptors, ultimately underlies the endocrine phenotypes of SvEv-Ramp2+/− mice remains a challenging task. Once again, a comparison of phenotypes from genetic mouse models of RAMP2-interacting GPCRs and their ligands may provide the best clues. For example, PTHrP, via the receptor PTHR1, plays essential roles in fetal and placental development (19, 20) and mammary gland development (21, 22) and in the endochondral ossification process of bone development (23–27). Glucagon signaling through GCGR also plays a critical role in fertility and fetal development because Gcgr−/− female mice exhibit reduced fertility with embryonic death and perinatal lethality (28). Mice deficient for AMY, a peptide ligand for CTR, display bone phenotypes similar to Ramp2+/− mice (29). Last but not least, AM, the ligand for the canonical CLR-RAMP2 complex, can induce osteoblast proliferation in vitro and increase bone formation in vivo (30) and is fundamentally required for normal female reproduction. And so, considered all together, it is most likely that the endocrine phenotypes of Ramp2-heterozygote mice are the combined result of reduced signaling for a cohort of previously identified, and yet to be identified, RAMP2-interacting GPCRs (Fig. 5).
Additional genetic lines in which the compound haploinsufficient loss of RAMP2 with either CLR or other RAMP2-associating GPCR could potentially shed light on which endocrine organ system is predominantly affected by a 50% reduction in RAMP2. For example, a compound RAMP2/CLR heterozygote mouse may show predominant phenotypes in the female reproductive system, whereas a compound RAMP2/PTHR1 heterozygote mouse may exhibit predominant phenotypes in the skeletal system.
Another important aspect of these findings, which further implies the involvement of multiple RAMP2-interacting GPCR, is that the SvEv-Ramp2+/− phenotype is manifested on a haploinsufficient background. Although robust endocrine phenotypes are revealed in global knockouts and transgenic overexpression mouse models for many individual RAMP2-interacting GPCR and their ligands, it is remarkable that a modest 50% reduction in RAMP2 can result in similarly robust phenotypes. This constellation of phenotypes is also likely to be influenced by the very broad spatiotemporal expression pattern of RAMP2 compared with individual GPCR. Therefore, these results demonstrate that the appropriate genetic dosage of Ramp2 is required for normal female reproduction and endocrine homeostasis and that this requirement is likely to involve numerous GPCRs.
In summary, we have demonstrated that a modest genetic reduction in the GPCR accessory protein Ramp2 causes severe female subfertility, mild hyperprolactinemia, and endocrine-related defects in mammary gland and bone target organs. Although we cannot solely attribute these phenotypes to hyperprolactinemia, the presence of bone phenotypes as early as postnatal d 2 argues for a direct role of RAMP2 in bone development that is independent of hyperprolactinemia. In a broader context, the constellation of endocrine phenotypes demonstrates that RAMP2 has physiological functions that extend well beyond its interaction with the canonical CLR. The elucidation of the specific receptors (or combination of receptors) that underlie the Ramp2+/− phenotype in different tissues will ultimately require more sophisticated mouse modeling approaches. However, these phenotypic data provide a valuable research resource from which to begin exploring novel pharmacological and biochemical GPCR-RAMP2 interactions. Given the pharmacological tractability of GPCR-RAMP2 interfaces, these and future studies have the potential to identify receptor targets for the therapeutic treatment of numerous endocrine-related conditions such as infertility, osteoporosis, and hyperprolactinemia.
Materials and Methods
Animals
Mice with a targeted deletion of Ramp2 were generated and characterized as described previously (13). Briefly, chimeric mice with Ramp2 gene targeted were generated using targeting vectors and embryonic stem cells of the SvEV129/6-TC1 background. Isogenic colonies were established by breeding chimeric male mice with SvEV129/6-TC1 female mice to generate SvEv-Ramp2+/− mice. We previously published that subfertility seen in SvEv-Ramp2+/− mice is completely rescued by backcrossing to the C57BL6 background (12). To obtain F2-Ramp2+/− mice, we bred SvEv-Ramp2+/− mice to C57BL6 wild-type mice to generate F1-hybrid offspring. F1 siblings heterozygous for Ramp2+/− were intercrossed to yield F2-Ramp2+/−. Genotyping was performed by PCR using a three-primer-based approach: primer 1, 5′-TCTGTCTGGATGCTGCCTTGC-3′; primer 2, 5′-GAAGTCAGGCAGTCAGGGTTG-3′; and primer 3, 5′-GACGAGTTCTTCTGAGGGGA-3′. Primers 1 and 2 amplified a 900-bp wild-type fragment, whereas primers 1 and 3 amplified a 650-bp targeted fragment. For timed matings, crosses were established, and the morning of vaginal plug detection was considered as E0.5. Control animals were wild type with similar age, gender, and genetic background. All experiments performed on animals were approved by the Institutional Animal Care and Use Committee of The University of North Carolina at Chapel Hill.
Serum hormone measurements
Serum was collected by mandibular vein bleed at 0600 h where the light cycle was from 0700–1900 h. Prolactin and GH were assayed from serum by RIA by A. F. Parlow at the National Hormone and Peptide Program (Torrance, CA). Serum for calcium measurement was collected at 1000 h and was processed at the Animal Clinical Chemistry and Gene Expression Laboratories core facility at the University of North Carolina at Chapel Hill.
Antibodies
The antibodies used in this study were obtained from National Institute of Diabetes and Digestive and Kidney Diseases and are the following: mouse prolactin (AFP-131078), mouse GH (AFP5672099), rat β-TSH (AFP-1274789), rat ACTH (AFP-156102789), rat β-LH (AFP-C697071P), and rat β-FSH (AFP7798-1289). Corresponding fluorescent secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). Nuclei were stained with Hoechst 33258 dye (Sigma-Aldrich, St. Louis, MO).
Histology and immunofluorescence microscopy
Tissues were fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose, embedded in Tissue-Tek Optimal Cutting Temperature Compound (Sakura Finetek, Torrance, CA) and cryosectioned at 8–10 μm. For immunostaining, sections were rehydrated in PBS, permeabilized in 0.2% Triton X-100, blocked in 4% BSA, and incubated in primary antibody overnight. After washing, sections were incubated in secondary antibody for 1–2 h, washed, and mounted for imaging. Images were acquired on a Nikon E800 microscope with a Hamamatsu ORCA-ER charge-coupled device camera with Metamorph software (Molecular Devices Corp., Sunnyvale, CA) and processed with Photoshop.
Pituitary gland volume and density measurements
Pituitary volume was calculated from two-dimensional images by making the assumption that the parameter of depth is proportional to the parameter of height for an ellipsoid shape. For measurement of pituitary cell density, cells were manually counted in 4′,6-diamidino-2-phenylindole-stained sections and represented as number of cells per square millimeter.
RNA extraction, cDNA synthesis, and quantitative RT-PCR analyses
RNA was extracted using the Trizol method following the manufacturer's protocol (Ambion, Austin, TX). Isolated RNA was deoxyribonuclease treated and reverse transcribed using the Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA), and gene expression was measured by MX3000 Q-PCR machine from Stratagene (Santa Clara, CA). All the primer-probe sets used in this study were TaqMan probes purchased from Applied Biosystems (Carlsbad, CA). mGAPDH was used as a normalizing gene, and the comparative Ct method (ΔΔCt) method was used to analyze the relative levels of gene expression (31).
Mammary gland whole mounts
Mouse mammary glands were collected for whole-mount analysis. Fourth inguinal glands were carefully dissected, placed on a glass slide, air dried for 5 min, and fixed overnight in Carnoy's solution (three parts absolute ethanol and one part glacial acetic acid) and stained in Carmine alum (0.2% carmine dye and 0.5% aluminum potassium sulfate) overnight. Slides were destained in 70% ethanol containing 2% HCl until the background was minimal and then dehydrated in increasing concentrations of ethanol. Toluene was used to defat, and the slides were mounted with Permount. Mammary gland branch points were manually scored in two images per animal along three major ducts that surrounded the lymph node within a 30-mm2 area. Terminal end buds located along a 5-mm length of a major peripheral duct were counted.
DEXA densitometry
DEXA (Lunar PIXImus densitometer, Madison WI) was used to measure bone mineral density and content of right femurs. In vivo DEXA scans were performed by anesthetizing animals with tribromoethanol and laying the animal flat on the DEXA machine.
Micro-CT
Micro-CT was performed to analyze femurs and vertebrae as described previously (32). Briefly, femurs were fixed, dehydrated, and scanned using a high-resolution x-ray source to generate three-dimensional (3D) reconstructions. Virtual transverse sections at the mid-diaphysis of the femur 3D reconstructions were made that revealed the cortical and trabecular bone architecture.
Statistical analyses
All quantitative analyses were performed using unpaired, two-tailed Student's t test, unless otherwise specified. Data are represented as sem unless indicated otherwise. Differences were considered significant when the P value was <0.05.
Acknowledgments
We thank Kunjie Hua and the University of North Carolina Clinical Nutrition Research Unit (National Institutes of Health Grant DK056350) for assistance with DEXA measurements and Xiu Xu, Helen Willcockson, and Kirk McNaughton for technical assistance.
This work was supported by the Burroughs Wellcome Fund Career Awards in the Biomedical Sciences; NIH HD46970, HL091973, and HD060860 grants to K.M.C.; and an American Heart Association Predoctoral Fellowship to M.K.
Disclosure Summary: T.S. and G.R. are shareholders in Medella Therapeutics and have patents field on agents modulating RAMP activity.
Footnotes
- AM
- Adrenomedullin
- AMY
- amylin
- CLR
- calcitonin receptor-like receptor
- CTR
- calcitonin receptor
- 3D
- three-dimensional
- DEXA
- dual-energy x-ray absorptiometry
- E14.5
- embryonic d 14.5
- GCGR
- glucagon receptor
- GPCR
- G protein-coupled receptor
- micro-CT
- microcomputed tomography
- PTHR
- PTH receptor
- RAMP
- receptor activity modifying protein.
References
- 1. Sexton PM, Morfis M, Tilakaratne N, Hay DL, Udawela M, Christopoulos G, Christopoulos A. 2006. Complexing receptor pharmacology: modulation of family B G protein-coupled receptor function by RAMPs. Ann NY Acad Sci 1070:90–104 [DOI] [PubMed] [Google Scholar]
- 2. McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. 1998. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339 [DOI] [PubMed] [Google Scholar]
- 3. Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. 2004. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350:1104–1110 [DOI] [PubMed] [Google Scholar]
- 4. Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. 2008. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 372:2115–2123 [DOI] [PubMed] [Google Scholar]
- 5. Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM. 2008. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 70:1304–1312 [DOI] [PubMed] [Google Scholar]
- 6. Paone DV, Shaw AW, Nguyen DN, Burgey CS, Deng JZ, Kane SA, Koblan KS, Salvatore CA, Mosser SD, Johnston VK, Wong BK, Miller-Stein CM, Hershey JC, Graham SL, Vacca JP, Williams TM. 2007. Potent, orally bioavailable calcitonin gene-related peptide receptor antagonists for the treatment of migraine: discovery of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4- (2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin- 1-yl)piperidine-1-carboxamide (MK-0974). J Med Chem 50:5564–5567 [DOI] [PubMed] [Google Scholar]
- 7. Salvatore CA, Moore EL, Calamari A, Cook JJ, Michener MS, O'Malley S, Miller PJ, Sur C, Williams DL, Jr, Zeng Z, Danziger A, Lynch JJ, Regan CP, Fay JF, Tang YS, Li CC, Pudvah NT, White RB, Bell IM, Gallicchio SN, Graham SL, Selnick HG, Vacca JP, Kane SA. 2010. Pharmacological properties of MK-3207, a potent and orally active calcitonin gene-related peptide receptor antagonist. J Pharmacol Exp Ther 333:152–160 [DOI] [PubMed] [Google Scholar]
- 8. Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM. 2003. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem 278:3293–3297 [DOI] [PubMed] [Google Scholar]
- 9. Harikumar KG, Simms J, Christopoulos G, Sexton PM, Miller LJ. 2009. Molecular basis of association of receptor activity-modifying protein 3 with the family B G protein-coupled secretin receptor. Biochemistry 48:11773–11785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouschet T, Martin S, Henley JM. 2005. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci 118:4709–4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kadmiel M, Fritz–Six K, Caron K. 2010. Understanding RAMPs through genetically engineered mouse models. Austin, TX: Landes Bioscience; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fritz-Six KL, Dunworth WP, Li M, Caron KM. 2008. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest 118:40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dackor R, Fritz-Six K, Smithies O, Caron K. 2007. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem 282:18094–18099 [DOI] [PubMed] [Google Scholar]
- 14. Li M, Yee D, Magnuson TR, Smithies O, Caron KM. 2006. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest 116:2653–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grattan DR, Steyn FJ, Kokay IC, Anderson GM, Bunn SJ. 2008. Pregnancy-induced adaptation in the neuroendocrine control of prolactin secretion. J Neuroendocrinol 20:497–507 [DOI] [PubMed] [Google Scholar]
- 16. Fisher RA. 1972. Statistical methods, experimental design, and scientific inference. 14th ed. New York: Hafner Publishing [Google Scholar]
- 17. Caron KM, Smithies O. 2001. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci USA 98:615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dackor RT, Fritz-Six K, Dunworth WP, Gibbons CL, Smithies O, Caron KM. 2006. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol Cell Biol 26:2511–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El-Hashash AH, Esbrit P, Kimber SJ. 2005. PTHrP promotes murine secondary trophoblast giant cell differentiation through induction of endocycle, upregulation of giant-cell-promoting transcription factors and suppression of other trophoblast cell types. Differentiation 73:154–174 [DOI] [PubMed] [Google Scholar]
- 20. El-Hashash AH, Kimber SJ. 2006. PTHrP induces changes in cell cytoskeleton and E-cadherin and regulates Eph/Ephrin kinases and RhoGTPases in murine secondary trophoblast cells. Dev Biol 290:13–31 [DOI] [PubMed] [Google Scholar]
- 21. Wysolmerski JJ, McCaughern-Carucci JF, Daifotis AG, Broadus AE, Philbrick WM. 1995. Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development 121:3539–3547 [DOI] [PubMed] [Google Scholar]
- 22. Wysolmerski JJ, Philbrick WM, Dunbar ME, Lanske B, Kronenberg H, Broadus AE. 1998. Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development 125:1285–1294 [DOI] [PubMed] [Google Scholar]
- 23. Amizuka N, Warshawsky H, Henderson JE, Goltzman D, Karaplis AC. 1994. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J Cell Biol 126:1611–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amizuka N, Karaplis AC, Henderson JE, Warshawsky H, Lipman ML, Matsuki Y, Ejiri S, Tanaka M, Izumi N, Ozawa H, Goltzman D. 1996. Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Dev Biol 175:166–176 [DOI] [PubMed] [Google Scholar]
- 25. Karaplis AC. 2001. PTHrP: novel roles in skeletal biology. Curr Pharm Des 7:655–670 [DOI] [PubMed] [Google Scholar]
- 26. Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. 1999. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest 104:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo J, Chung UI, Yang D, Karsenty G, Bringhurst FR, Kronenberg HM. 2006. PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev Biol 292:116–128 [DOI] [PubMed] [Google Scholar]
- 28. Vuguin PM, Kedees MH, Cui L, Guz Y, Gelling RW, Nejathaim M, Charron MJ, Teitelman G. 2006. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology 147:3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dacquin R, Davey RA, Laplace C, Levasseur R, Morris HA, Goldring SR, Gebre-Medhin S, Galson DL, Zajac JD, Karsenty G. 2004. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol 164:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cornish J, Callon KE, Coy DH, Jiang NY, Xiao L, Cooper GJ, Reid IR. 1997. Adrenomedullin is a potent stimulator of osteoblastic activity in vitro and in vivo. Am J Physiol 273:E1113–E1120 [DOI] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 32. Gomez C, David V, Peet NM, Vico L, Chenu C, Malaval L, Skerry TM. 2007. Absence of mechanical loading in utero influences bone mass and architecture but not innervation in Myod-Myf5-deficient mice. J Anat 210:259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]