SUMMARY
Barrett's esophagus is an intestine-like metaplasia and precursor of esophageal adenocarcinoma. Triggered by gastroesophageal reflux disease, the origin of this metaplasia remains unknown. p63-deficient mice, which lack squamous epithelia, may model acid-reflux damage. We show here that p63 null embryos rapidly develop intestine-like metaplasia with gene expression profiles similar to Barrett's metaplasia. We track its source to a unique embryonic epithelium that is normally undermined and replaced by p63-expressing cells. Significantly, we show that a discrete population of these embryonic cells persists in adult mice and humans at the squamocolumnar junction, the source of Barrett's metaplasia. Upon programmed damage to the squamous epithelium, we show that these embryonic cells migrate towards adjacent, specialized squamous cells in a process that may recapitulate early Barrett's. Our findings suggest that certain precancerous lesions, such as Barrett's, initiate not from genetic alterations but from competitive interactions between cell lineages driven by opportunity.
INTRODUCTION
Esophageal and gastric adenocarcinoma together kill more than a million people each year. Both cancers arise in association with chronic inflammation and are preceded by robust metaplasia with intestinal cell characteristics. Gastric intestinal metaplasia is linked to H. pylori infection, while Barrett's metaplasia of the esophagus can be triggered by gastroesophageal reflux disease (GERD). Although H. pylori suppression therapies have contributed to the recent decline of gastric adenocarcinoma, the incidence of esophageal adenocarcinoma, especially in the West, has increased dramatically in the past several decades (Spechler and Goyal, 1986; Blot et al., 1991; Reid et al., 1991, Raskin et al., 1992; Jankowski et al., 1999; Badreddine and Wang, 2010; Reid et al., 2010). Treatments for late stages of these diseases are challenging and largely palliative, therefore considerable efforts have focused on understanding the earlier, precancerous stages of these diseases as a prerequisite to developing therapeutic approaches. Intestine-like metaplasia is characterized by a columnar epithelium containing prominent goblet cells and cells expressing intestinal markers such as villin and trefoil factors (TFF1–3). Once established, this metaplasia appears to be irreversible without ablative treatments (Naef et al., 1975; Sagar et al., 1995; Barr et al., 1996; Badreddine and Wang, 2010).
Esophageal adenocarcinoma arises from this metaplasia as the result of stereotypic genetic and cytological changes that present as dysplasia, high-grade dysplasia, and finally invasive cancer, all in a process involving clonal evolution (Raskin et al., 1992; Jankowski et al., 1999; Haggitt, 1994; Maley et al., 2006; Leedham et al., 2008). The ontogeny of these metaplasias remains an intriguing mystery with cogent support for hypotheses suggesting transcommitment of resident squamous stem cells, migration of cells from lower gastrointestinal sites, colonization by circulating bone marrow cells, or the reparative emergence of submucosal glands (Souza et al., 2008; Badreddine and Wang, 2010).
p63 is a p53 homolog that is essential for the self-renewal of stem cells of all stratified epithelial tissues, including mammary and prostate glands as well as all squamous epithelia. In p63 null embryos, stratified epithelial tissues are lost or eroded several days after stratification due to the loss of regenerative cell populations (Yang et al., 1998; Yang et al., 1999; Senoo et al., 2007). Indeed the p63 null embryos have been reported to have idiopathic metaplastic changes both the esophagus and proximal stomach, two normally squamous tissues in the mouse (Yang et al., 1999; Daniely et al., 2004). The p63 null mouse therefore offers a unique opportunity to probe the link between tissue damage and metaplasia. This mouse rapidly develops metaplasia with the hallmarks of Barrett's esophagus. We examined the molecular properties and evolution of the metaplasia in the p63 null mouse to understand its origins, and applied this information to an independent mouse model testing the origins of Barrett's metaplasia in adult animals.
RESULTS
Metaplasia in the p63 null mouse
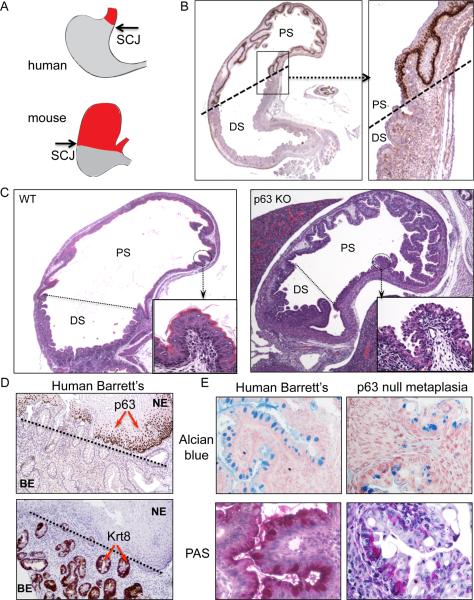
p63 embryos develop to term but show absent or dysmorphic stratified epithelia including epidermis, breast, prostate, and thymus at birth (Yang et al., 1999; Senoo et al., 2007). To determine if similar events occur in the squamous epithelia of the esophagus and proximal stomach of p63 null embryos, we examined these regions by histology. The squamocolumnar junction present at the distal esophagus in humans is shifted posteriorly in mice due to an extension of squamous epithelium to the gastric midline, as marked by the limit of p63 immunohistochemistry (Fig. 1A,B). Although the wild type E18 embryo has a mature squamous epithelium in the proximal stomach, the p63 null embryo shows a remarkably well-developed columnar epithelium at this site (Fig. 1C and Figure S1). Barrett's esophagus in humans also lacks p63 staining unlike adjacent squamous tissues where p63 strongly decorates basal nuclei (Glickman et al., 2001; Fig. 1D, top panel). Antibodies to keratin 8 stain human Barrett's esophagus as well as the metaplasia in the p63 null mouse (Fig. 1D, bottom panel, and Figure S1). Other similarities between human Barrett's esophagus and the metaplasia in the p63 null mouse are the positive staining with Alcian blue and periodic acid-Schiff (PAS), typical histological markers of goblet-like cells (Fig. 1E). We probed the metaplasia and control tissues for known markers (Wang et al., 2009; Botelho et al., 2010) of Barrett's esophagus using antibodies against villin (Vil1) and anterior gradient 2 (Agr2) in addition to keratin 8 (Krt8), all of which showed specific and robust staining of the metaplasia in the p63 null embryos (Figure S1). The histological similarity between the observed metaplasia in p63 null embryos and Barrett's esophagus in humans drove us to investigate these tissues at a molecular level.
Figure 1.
Metaplasia in the proximal stomach of p63 null embryos
A. Schematic representation of the human and murine upper gastrointestinal tract showing the respective positions of the squamous (red) and glandular tissues (gray) and intervening squamocolumnar junction. B. Histological section through the stomach of an E18 wild type mouse stained with anti-p63 antibodies highlighting the p63-positive squamous epithelia of the proximal stomach (PS) and the glandular epithelium of the distal stomach (DS). C. Comparison of histologically-stained sections through stomachs of E18 wild type (left panel) and p63 null (right panel) embryos with high magnification insets showing squamous tissue lining the proximal stomach of the wild type mouse and columnar epithelia in the p63 mutant animals. D. Histological sections through Barrett's esophagus with both squamous islands and glands of intestinal metaplasia stained with antibodies to p63 (top panel) and to the Barrett's marker Krt8 (bottom panel). E. Comparison of Alcian blue and periodic acid-Schiffs staining of human Barrett's esophagus (left panels) and metaplasia in E19 p63 null proximal stomach (right panels). See also Figure S1.
Gene expression profile versus Barrett's
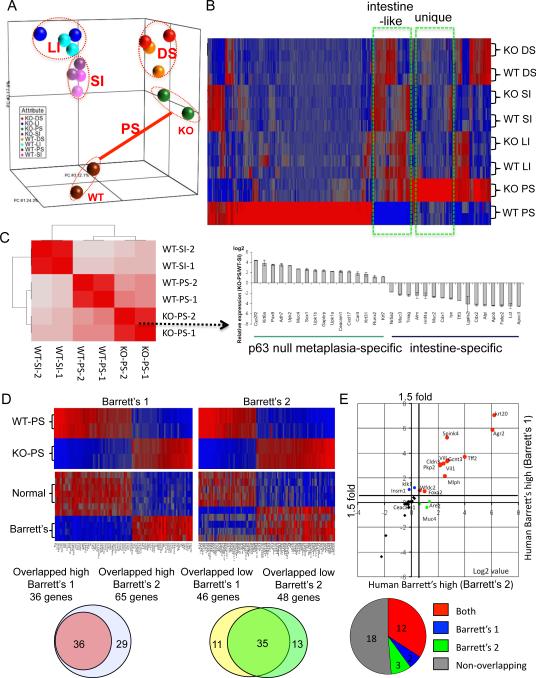
We compared the gene expression profile of the metaplastic epithelia with those of specific regions of the gastrointestinal tract in mutant and wild type animals, comparing RNA from microdissected tissues on expression microarray chips. These data revealed that the wild type and p63 null colon, small intestine, and distal stomach formed concordant pairs of overall gene expression (Fig. 2A). In contrast, comparisons of gene expression between wild type and p63 null proximal stomach revealed stark differences, suggesting that the cells in the observed metaplasia were clearly distinct from those in normal squamous epithelia at this site (Fig. 2A). Moreover, an unsupervised correlation analysis of the whole genome expression profiles of tissues in the p63 null embryos indicated only passing relationships between the metaplastic tissue and the small intestine (Fig. 2B), suggesting that this metaplasia is a unique entity unrelated to other major tissues of the normal mouse gastrointestinal tract. The distinction between the metaplasia and the small intestine is underscored by examining selected genes on both a global level as in the correlation coefficient analysis as well as a selected set of genes expressed in the metaplasia and small intestine (Fig. 2C). Thus although many genes linked to intestinal metaplasia found in Barrett's are present in the p63 null metaplasia, many others, including Caudal-type homeobox 2 (Cdx2), are not. A comparison of Cdx2 expression by microarray intensity across the wild type and p63 null tissues of the gastrointestinal tract revealed Cdx2 expression restricted to the small intestine and colon of these mice (Figure S2). Cdx2 has been strongly implicated in the developmental determination of the intestinal track where it behaves as a haploinsufficient tumor suppressor and key regulator of intestinal differentiation and proliferation (Suh and Traber, 1996; Chawengsaksophak et al., 1997). Significantly, ectopic expression of Cdx2 in parietal cells resulted in an intestinal metaplasia of transgenic mice, suggesting a dominant role for this transcription factor in the transcommitment to intestinal programs (Mutoh et al., 2002). However, a recent analysis of Barrett's esophagus by expression microarray did not reveal enhanced levels of Cdx2 in Barrett's over that of normal esophageal squamous epithelium (Stairs et al., 2008). Cdx2 expression can only be weakly detected in 30–70% of Barrett's tissues, and only becomes strong in cases involving dysplasia (van Baal et al., 2008; Weimann et al., 2010). Moreover, a comparison between human Barrett's esophagus and human small intestine shows a similar divide in gene expression as we found between the p63 null metaplasia and the murine small intestine (Figure S2). Together, these data argue that both Barrett's esophagus and the p63 null metaplasia are different entities from other regions of the gastrointestinal tract.
Figure 2.
Gene expression of metaplasia in p63 null embryos
A. A three-dimensional representation of a Principle Component Analysis of expression microarray data derived from gastrointestinal tract tissues of E18 wild type (WT) and p63 null (KO) embryos. PS, proximal stomach; DS, distal stomach; LI, large intestine; SI, small intestine. B. Heat map of expression microarray data from gastrointestinal tract anchored by a comparison between wild type and p63 null proximal stomach datasets. C. Hierarchical correlation analysis of whole genome data sets from wild type and p63 null tissues. Histogram depicts expression profiles of genes selected to emphasize metaplasia-specific and intestine-specific genes. D. Heat maps of differentially-expressed genes in wild type and p63 null proximal stomach and corresponding expression patterns in two different datasets of normal human esophagus and Barrett's metaplasia (Barrett's #1 from Stairs et al., 2008; Barrett's #2 from Kimchi et al., 2005). Below, Venn diagrams showing the overlap of genes from the two Barrett's dataset that are also high (or low) in the p63 null metaplasia. E. Scatterplot of up-regulated genes in Barrett's esophagus from two different datasets that are also in the top 50 overexpressed genes in metaplasia of the p63 null mouse. Pie chart indicates relative intersection of 35 most overexpressed gene of p63 null metaplasia with the Barrett's datasets. See also Figure S2, Figure S3, Table S1, Table S2, and Table S3.
We next asked how the gene expression differences between wild type and p63 null proximal stomach compared with two available, independent datasets (Stairs et al., 2008; Kimchi et al., 2005) of human Barrett's metaplasia and normal esophagus (Fig. 2D; Table S1). A broad comparison of the mouse data sets with the two human Barrett's datasets showed a strong correlation between the genes differentially expressed in the metaplasia of the p63 null mouse and those differentially expressed in Barrett's esophagus compared with normal human esophagus (Fig. 2D, heatmaps). Interestingly, we found a remarkable similarity in the overlapping genes in the two human datasets (Fig. 2D, Venn diagrams), even thought the Barrett's dataset #1 (Stairs et al., 2008) was from Barrett's biopsies whereas the Barrett's dataset #2 was from metaplasia of patients who had progressed to esophageal adenocarcinoma (Kimchi et al., 2005). Indeed of the top fifty genes overrepresented in the metaplasia of the mouse proximal stomach, approximately 50% of those present on the human arrays were overrepresented in both Barrett's esophagus datasets compared with normal human esophagus (1.5-fold cutoff, p< 0.05; Fig. 2E; Table S2). Within these common genes were many of the markers established for Barrett's esophagus and gastric intestinal metaplasia (Wang et al., 2009; Botelho et al., 2010). In fact, the fold-change in these genes in the p63 null metaplasia (mucin 4 (Muc4, 73×), keratin 20 (Krt20, 61×), trefoil factor 2 (TFF2, 49×), claudin 3 (Cldn3, 46×), Agr2 (120×), and Vil1 (27×); p< 10−5 for all) greatly exceeded those in the Barrett's datasets (Table S2). We used antibodies to Agr2 and Vil1 to validate some of these genes, and both show robust staining of the metaplastic tissue in the p63 null proximal stomach (cf. Figure S1).
To validate some of these markers upregulated in the metaplasia of the p63 null mouse with human Barrett's esophagus, we stained sections of 16 cases of Barrett's esophagus and controls with antibodies to human keratin 7 (Krt7), Vil1, Cldn3, Fc fragment of IgG binding protein (FCGBP), Muc4, and Agr2. All 16 Barrett's esophagus cases were positive for all of these markers, while antibodies to p63 showed staining restricted to squamous islands (Figure S3).
Finally, we compared the gene expression datasets of the murine metaplasia and the human Barrett's esophagus at the level of gene ontology. Of the 63 gene ontology (GO) categories significantly (p<0.01) represented in the metaplasia of the mice and the 42 identified for Barrett's esophagus, 34 or 54% were in common (Figure S3, Table S3). These findings, while broadly based, suggest similar processes are occurring in the cells of the p63 null metaplasia and those of Barrett's esophagus.
Embryonic Origin of Metaplasia in the p63 Null Mouse
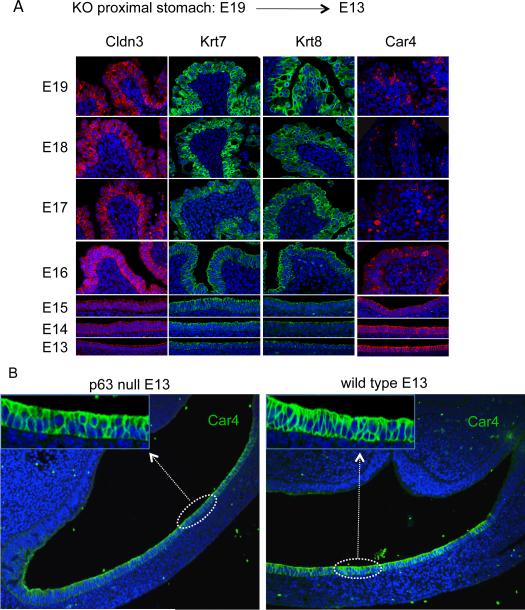
In an effort to clarify the source of the epithelial metaplasia observed in the E18 p63 null mouse, we traced markers common to both Barrett's esophagus and our metaplasia retrospectively through embryonic development. Antibodies to Cldn3, Krt7, Krt8, carbonic anhydrase 4 (Car4), and Muc4 were used to trace the metaplasia from embryonic day 19 to 13 (Fig. 3A, Figure S4). Each of these markers could be traced in the proximal stomach of the E13/E14 p63 null mouse to a monolayer of cells lining the proximal stomach. Curiously, while Cldn3, Krt7, Krt8, and Muc4 signals became weaker as one approached E13, the Car4 antibody signal was more robust and broadly expressed on this monolayer of cells at E13. When we compared the Car4 staining pattern on the p63 null and wild type proximal stomach at E13, both yielded a similarly appearing, Car4-expressing columnar epithelium (Fig. 3B). Thus the Car4-positive, columnar epithelium at E13 must represent a ground state for the metaplasia that emerges later in the proximal stomach of the p63 null mice.
Figure 3.
Retrospective tracing of metaplasia through embryogenesis
A. Fluorescence micrographs of metaplasia in proximal stomach of p63 null embryos from E19 to E13 stained with antibodies to Cldn3, Krt7, Krt8, and Car4 and counterstained with Hoechst dye for DNA (blue). B. Sections through the proximal stomach of E13 p63 null (left) and wild type (right) embryos stained with antibodies to Car4 (green) showing a simple columnar epithelium lining the lumen. Insets at higher magnification. Sections counterstained with Hoechst dye for DNA. See also Figure S4.
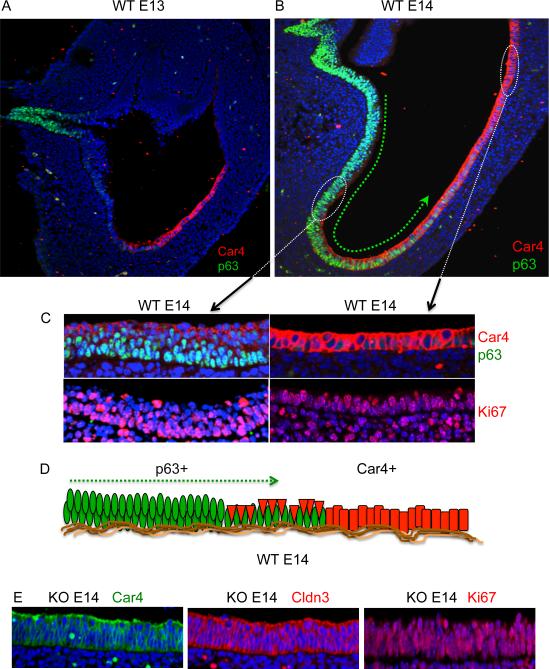
Why do the p63 null embryos go on to develop a Barrett's-like metaplasia while the wild type embryos do not? p63 is a transcription factor required for long-term self-renewal of stem cells of stratified epithelia but not for their commitment to stem cells nor for their differentiation (Yang et al., 1998; Yang et al., 1999; Senoo et al., 2007). In wild type embryos, strong p63 expression is first detected at E13 in a population of cells in the esophagus and this expression is notably weaker in cells that extend distally toward the Car4 cells (Fig. 4A). By E14, the p63-positive cells appear to extend to and actually among and under the Car4/Cldn3 positive cells in an anterior-posterior gradient (Fig. 4B), such that many of the Car4/Cldn3 cells are displaced from the basement membrane to an apical position with respect to the p63-expressing cells. Remarkably, whereas the Car4-expressing cells positioned on the basement membrane at the posterior end of this gradient are highly proliferative (Fig. 4C, right panel), those undermined by p63-expressing cells show reduced cell cycle activity (Fig. 4C, left panel). Car4 cells with direct access to the basement membrane at E14 had a proliferation index greater than 65%, while those undermined by p63-expressing cells showed a proliferation index of approximately 10%, suggesting a mechanism by which the p63 cells prevent the Car4 cells from evolving into a proliferative metaplasia (Fig. 4D). Taken together with the metaplasia tracing using five independent markers (cf Fig. 3A, Figure S4), these data suggest a model in which the metaplasia in the p63 null mouse arises from the Car4 cells that lie on the basement membrane of the proximal stomach. We suggest that the Car4-positive primitive epithelium develops into a metaplasia after E14 because of the absence of an undermining population of squamous epithelium from the esophagus in the p63 null embryos. In support of this concept, the Car4 cells are not undermined by epithelial cells in the p63 null mouse at E14 and instead appear to rapidly progress to a proliferative columnar epithelium (Fig. 4E). A similar loss of p63 in stem cells of the epidermis and thymus results in a defect in self-renewal capacity of those squamous tissues (Yang et al., 1999; Senoo et al., 2007) but no metaplasias. These earlier studies demonstrated that epidermal and thymic epithelial stem cells lose self-renewal capability, yet undergo complete and proper differentiation in the absence of p63. Consistent with these findings, we found no evidence of squamous differentiation at any stage of the metaplasia (Figure S4). Together, these observations reveal that the Car4 cells that comprise the simple epithelium of the E13 proximal stomach are temporally and spatially distinct from the p63 expressing cells first seen in the E13 esophagus. Therefore in the p63 null embryo this Car4 epithelium evolves to yield a Barrett's-like metaplasia independent of a “transcommitment” mechanism. As well, our data suggest an essential role of p63-positive cells in the competitive displacement of the Car4 cells from the basement membrane during development (Fig. 4B,C). Car4 cells persist in the E18 and E19 metaplasia, though as a discrete population of small cells that do not express Krt7 and appear strongly associated with the basement membrane (not shown).
Figure 4.
Undermining of Car4 cells by p63-positive cells at E14
A., B. Sections through wild type E13 and wild type E14 proximal stomachs, respectively, probed with antibodies to Car4 and p63. Green arrow depicts an apparent anterior-to-posterior gradient of p63 positive cells from esophagus to proximal stomach. C. top left panel, Section through anterior portion of wild type E14 stomach showing Car4 cells (red) atop p63 cells (green) and corresponding section stained with antibodies to Ki67 (red; lower left panel). Top right panel, Car4 cells (red) in direct contact with basement membrane in distal regions lacking p63 cells. Lower right panel, corresponding section stained with antibodies to Ki67 (red). D. Schematic depicting hypothetical undermining of Car4 cells (red) by proximal migration of p63 cells (green). E. Imaging of E14 proximal stomach epithelium in sections of p63 null embryo with antibodies to Car4 (green, left panel), Claudin 3 (red, middle panel) and Ki67 (red, right panel).
Embryonic epithelium is retained into adulthood
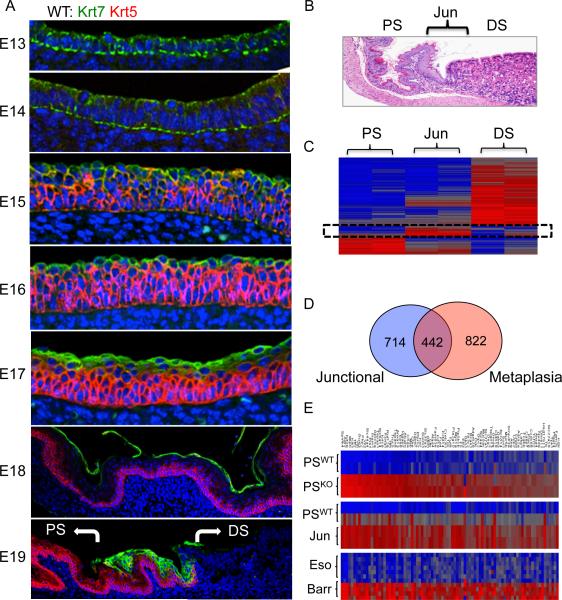
To determine the ultimate fate of the primitive epithelial cells undermined by the p63-positive cells at E14, we followed them from E13 to E19 in wild type mice using antibodies to Krt7 and Krt5 (Fig. 5A). Between E15 and E17, the primitive epithelial cells maintain their apical position above the stratifying squamous epithelia in the proximal stomach (Fig. 5, E15–E17). However, at E18, these cells undergo an abrupt and wholesale detachment in large sheets from the underlying epithelia (Fig. 5A, E18). At E19, the Krt7-expressing cells are absent from the entire proximal stomach with the exception of a discrete population of cells, numbering approximately 30 in cross-section, remaining precisely at the squamocolumnar junction (Fig. 5A, E19). Interestingly, occasional Car4-expressing cells appear at the basement membrane in association with the more numerous Krt7 cells at the junction, suggesting that this subpopulation of cells plays some functional role among these junctional cells (not shown). To probe for junction-specific genes that might mark these cells, we performed a transcriptome analysis of RNA derived by dissection of the squamocolumnar junction and the adjacent squamous and columnar epithelia of a three-week-old mouse (Fig. 5B). A three-way comparison of junctional genes and the adjacent proximal and distal stomach revealed a set of genes specific to the junction (Fig. 5C). A broader comparison of genes overrepresented in the junction compared to the wild type proximal stomach revealed a 38% overlap with those of the metaplasia of the p63 null proximal stomach (Fig. 5D). We confirmed that two of these, Muc4 and chorioembryonic antigen cell adhesion molecule (CEACAM), were present in the squamocolumnar junction of wild type adult mice and in the metaplasia of E18 p63 null mice (Figure S5). A separate comparison between genes significantly (p<0.05) high in the junction versus p63 null metaplasia versus those high in Barrett's esophagus revealed a consensus overlap of 87 genes represented as heatmaps (Fig. 5E, Table S4). These genes include some of the key markers of Barrett's esophagus including Spink4, Agr2, TFF1, TFF2, Krt8, Krt18, and Vil1, among others (Fig. 5E and Table S4).
Figure 5.
Persistence of embryonic cells at the squamocolumnar junction
A. Distribution of the Krt7 (green)- and Krt5 (red)-expressing cells in wild type embryos from E13 to E19. While originally a columnar epithelium at E13 and E14, these cells assume a supra-squamous position at E15 and ultimately disintegrate as a suprasquamous cell layer at E18 except for the squamocolumnar junction where they persist in all six embryos tested. B. Histology section through the squamocolumnar junction of a wild type three-week-old mouse bordered by the proximal stomach (PS) and the distal stomach (DS). C. Heatmap of genes differentially expressed in the proximal stomach, the junction, and the distal stomach. D. Venn diagram of intersection between genes highly expressed in the junction versus the metaplasia of the p63 null mouse. E. Heatmaps of genes overexpressed in common between the metaplasia of the p63 null mouse (KO-proximal stomach vs WT-proximal stomach, the squamocolumnar junction of the wild type adult mouse (WT-junction vs WT proximal stomach), and Barrett's esophagus (BE vs normal human esophagus). See also Figure S5 and Table S4.
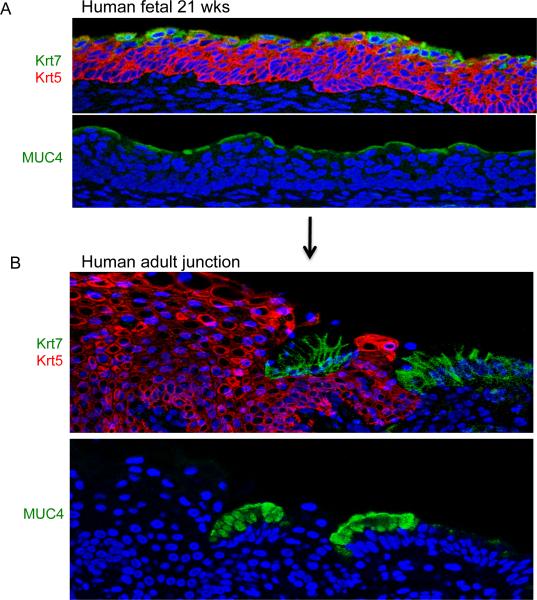
The Barrett's-like metaplasia in the p63 null mouse arose from a primitive epithelium that is ultimately displaced in the wild type animal by the stratification of underlying squamous epithelium at all sites except the squamocolumnar junction. We asked if this mouse model could predict the developmental dynamics of a similar primitive epithelium in humans. We first examined histological sections of 21-week-old human fetal esophagi for the presence of markers of the primitive epithelium and Barrett's such as Krt7 and Muc4. Remarkably these antibodies revealed a single layer of suprasquamous epithelia positive very similar to that observed in wild type E15-E17 mouse embryos (Fig. 6A). In the adult human gastroesophageal junction, however, only junctional cells stain positive for these Barrett's markers (Fig. 6B). These observations, taken together, suggest a similar developmental mechanism for the retention of embryonic epithelium at the gastroesophageal junction in adult mice and humans.
Figure 6.
Residual embryonic cells in human tissues
A. Immunofluorescence imaging of section of human 21-week-old esophagus showing suprasquamous distribution of Krt7 (green), Muc4 (green), and Krt5 (red). These results were consistent in the three independent fetuses tested. B. Expression of Krt7 (green), Krt5 (red), and Muc4 (green) cells in section of gastroesophageal junction from adult human without Barrett's esophagus. This result was consistent in the sections from three individuals tested.
Modeling early steps towards metaplasia
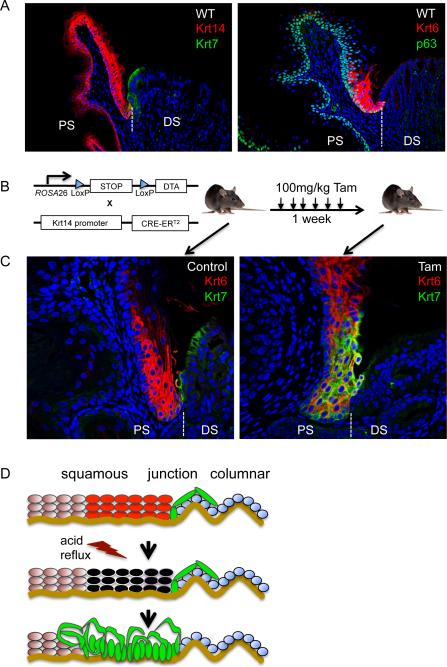
The persistence of a population of primitive epithelial cells at the squamocolumnar junction with a lineage relation to an embryonic version of a Barrett's-like metaplasia raised the possibility that they were related somehow to the clinical observations that Barrett's metaplasia always extends from the squamocolumnar junction (Fig. 7A). This possibility was further supported by our analysis of squamocolumnar junctional datasets in three-week-old mice revealed a discrete population of keratin 6 (Krt6)-expressing squamous epithelium bordering the Krt7-expressing embryonic epithelium (Fig. 7A). While the function of this Krt6-expressing squamous epithelium is unknown, it further suggests unexpected order at the junction around the primitive junctional epithelium. To test the hypothesis that these primitive junctional epithelial cells are the origins of a Barrett's-like metaplasia, we asked how they would react if we weakened the squamous epithelium as would chronic acid reflux in humans. We therefore generated mice in which diphtheria toxin A (DTA) was conditionally expressed in basal cells of stratified epithelia by crossing the ROSA26-tm-DTA mouse (Ivanova et al., 2005) with one having a Tamoxifen-dependent Cre recombinase under the control of the Krt14 promoter (Vasioukhin et al., 1999) (hereafter the DTA-Krt14Cre mouse; Fig. 7B). Treatment of three-week-old DTA-Krt14Cre mice with Tamoxifen resulted in a rapid and reproducible expansion of the Krt7-expressing cells from their ordered appearance at the squamocolumnar junction to more anterior regions of the proximal stomach among the Krt6-positive cells (Fig. 7C and Figure S6). This observation was absolute, in that all 258 junctional sections from 14 Tamoxifen-treated mice showed this anterior migration of Krt7-positive cells, while none of the 51 junctional sections from three noninjected mice showed this phenomenon. Similar staining patterns were obtained with antibodies to CEACAM to mark the same population recognized by Krt7 (Figure S6). Despite the apparent migration of the Krt7 cells, we did not observe in the short duration of these experiments a migration of the relatively rare Car4 cells from these junctions (not shown). The Krt7 cells did not stain with loricrin, a marker of differentiated squamous cells, suggesting that the migrating Krt7-positive cells do not have squamous properties (Figure S6). Significantly, as the Krt7-expressing cells migrated anteriorly, some of them came to be in intimate association with the basement membrane, which had been presumably vacated by basal cells killed as a consequence of Cre-mediated diphtheria toxin A expression. The long-term analysis of these mice was precluded by the collateral damage to all stratified epithelia due to Krt14-driven Cre recombinase, the anterior migration of the Krt7 cells is consistent with the known junctional origins of Barrett's metaplasia following chronic acid-reflux in humans.
Figure 7.
Damage-induced activation of the residual primitive epithelium in adult mice
A. left, Section through normal junction of adult mouse stained with anti-Krt14 antibodies to reveal squamous epithelia (red) and anti-Krt7 antibodies to detect residual embryonic cells (green). right, Junctional section stained with anti-p63 antibodies to reveal squamous basal cells (green) and anti-Krt6 antibodies to stain specialized junctional squamous cells (red). B. Schematic of the DTA-Krt14Cre mouse strain constructed to assess the effect of damaging the squamous epithelium on cells of the squamocolumnar junction following injections of Tamoxifen over one to three weeks. C. left, Micrograph depicting the apposition of Krt6-expressing squamous epithelium (red) and the Krt7-positive embryonic cells (green) at the squamocolumnar junction of a three-week-old mouse that has not received Tamoxifen injections. Right, Apparent migration of the Krt7-positive cells (green) to sites among and beneath the Krt6-positive squamous cells (red) at the junction following one week of Tamoxifen treatment. D. Schematic in which chronic acid-reflux damages squamous tissues and induces a hypothetical opportunistic migration of residual embryonic cells that normally reside at the junction. Krt6-expressing squamous cells are shown in red, the residual embryonic cells in green, and the gastric epithelium in green. See also Figure S6.
DISCUSSION
The work presented here models the evolution of a Barrett's-like metaplasia in both embryonic and adult mice from precursor cells that are associated by lineage. The mechanisms by which these metaplasias arise in embryos and adults are remarkably similar and suggest a fundamentally novel evolution of precursors of certain cancers in which the earliest events depend not on genetic changes but rather on competition between cell lineages for access to basement membrane (Bissell et al., 2002) essential for proliferation (Fig. 7D). The strongest support for this notion is the speed by which the metaplasias arise in our models. In the relatively short interval between embryonic day 13 and day 19, the proximal stomach epithelium of the p63 null mouse undergoes a robust transition to a Barrett's-like metaplasia. If extrapolated to the human condition this model would suggest that Barrett's too could initiate in the absence of activating mutations typically linked to chronic insults and inflammation (Schetter et al., 2010). The more salient initial role for chronic inflammation might be in altering the competitive status quo between indigenous and opportunistic cell populations as opposed to the genetic reprogramming of either of them. Once established, it is clear that Barrett's metaplasia evolves along complex pathways in which inflammation drives proliferation-induced mutations and epigenetic changes that become the basis of the observed clonal selection (Spechler and Goyal, 1986; Blot et al., 1991; Raskin et al., 1992; Antonioli and Wang, 1997; Jankowski et al., 1999; Glickman et al., 2001; Coad et al., 2005; Maley et al., 2006; Leedham et al., 2008; Badreddine and Wang, 2010). It will be important to understand what properties of the metaplastic cells render them so susceptible to dysplastic progression and malignancy.
The opportunistic cells we implicate in this rapid evolution of a precancerous metaplasia contrasts with the dominant “transdifferentiation” model that holds that acid reflux triggers the inappropriate activation of genes governing intestinal differentiation such as Cdx2 in the stem cells of the esophageal squamous epithelium (reviewed in Souza et al., 2008). The Cdx2 transdifferentiation model for Barrett's was adapted from a murine model of gastric intestinal metaplasia in which Cdx2 expression was ectopically driven in parietal cells from an H+/K+-ATPase promoter (Mutoh et al., 2002). However, the intestinal metaplasia in the Cdx2 mouse has adsorptive properties similar to the intestine while Barrett's esophagus is known to be a secretory metaplasia (Levine et al., 1989; Dixon et al., 2001; Tobey et al., 2007). Additionally, Cdx2 expression in Barrett's without dysplasia is variable at best and not an absolute feature of Barrett's gene expression profiles (van Baals et al., 2008; Stairs et al., 2008; Weimann et al., 2010). The current study presents several lines of evidence against a squamous stem cell transdifferentiation model irrespective of Cdx2 and in favor of an embryonic origin of the premetaplastic cell. First, we show by marker tracking that the metaplasia in the E19 p63-deficient embryos is derived from a group of Car4-expressing cells lining the proximal stomach at E13. Significantly, an apparently identical group of Car4-positive cells is seen in wild type E13 mouse embryos that is temporally and topologically distinct from p63-expressing cells arising in the esophagus that represent precursors to the squamous epithelium. Probably the most telling evidence comes from the analysis of wild type embryogenesis in which the Car4 cells and p63-expressing cells can be tracked independently and indeed simultaneously following their laminated interactions from E14 to E17 when the Car4 progeny are eliminated from the squamous surface except at the squamocolumnar junction. Finally, we show that cells in the normal squamocolumnar junction of mice share gene expression signatures with fully developed metaplasia in the p63 null mouse, suggesting these junctional cells, rather than any form of transdifferentiated squamous cells, are the origin of this Barrett's-like metaplasia.
It is probably important as well to dispel the notion that the Barrett's-like metaplasia arises from squamous stem cells that lack p63 in the p63 null mouse. p63 is a master regulator of self-renewal of stem cells of all stratified epithelia including mammary and prostate glands as well as skin but appears dispensable for lineage commitment or differentiation (Yang et al., 1999; Senoo et al., 2007). Indeed p63 null squamous tissues such as the epidermis and thymus still undergo normal differentiation as evidenced by loricrin and involucrin in the epidermis and the acquisition of functionally mature antigen presentation cells and normal T cell maturation in the thymus (Senoo et al., 2007). Thus the most relevant effect of the loss of p63 in the epidermis and thymus is the diminution of stem cell self-renewal while transdifferentiation events were not observed. A similar effect of the loss of p63 in the squamous stem cells present in the esophagus at E13 would explain the absence of squamous progenitors that normally undermine and suppress the uncontrolled growth of the Car4 cells in the proximal stomach. We believe that this developmental scenario for the evolution of a Barrett's-like metaplasia is one where the competition between squamous progenitors and the Car4 cells has been bias by limiting squamous cell self-renewal and is an extreme proxy for our adult mouse model and indeed for acid-reflux-induced Barrett's. It is anticipated that this competition between resident and opportunistic cell populations might have broader implications for the initiation of premalignant lesions linked to chronic inflammatory diseases.
Whether other cancers arise by the competitive mechanism proposed here for Barrett's is unclear. Most cases of gastric adenocarcinoma are thought to develop from an “intestine-like” metaplasia very similar to Barrett's that develops from multiple sites in the gastric epithelium secondary to H. pylori infection (Piazuelo et al. 2004; Correa and Chen, 1994; Goldenring et al., 2010), though it still remains to be examined whether they too are derived from residual embryonic cells. It is also becoming apparent that pancreatic intraepithelial neoplasia, an intestine-like metaplastic precursor of pancreatic adenocarcinoma, arises from a set of ductal glands expressing embryonic features (Strobel et al., 2010). Other candidates for a competitive or opportunistic mechanism include the unusual neoplasms associated with inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis. IBD patients have a high incidence of aggressive tumors from inflamed regions of both the large and small intestine with clinical and genetic features distinct from sporadic colorectal carcinoma (Kiran et al., 2010; Farraye et al., 2010).
In addition to highlighting a role for embryonic cell populations in the rise of precancerous lesions, the work presented here suggests adaptive host mechanisms to suppress the expansion of such populations. While speculative, the apparent mustering of keratin 6-expressing squamous cells, a marker of keratinocytes involved in wound healing (Wojcik et al., 2001; Wong and Coulombe, 2003) to sites adjacent to the residual embryonic epithelium is unlikely to be coincidental. Given that only a small fraction of individuals with gastroesophageal reflux actually develop Barrett's metaplasia, further understanding of host defenses might reveal new insights into risk profiles.
Finally, the dim prognosis for esophageal adenocarcinoma argues for therapies directed at preventing even the initiation of the precancerous but tenacious Barrett's metaplasia, especially if the risk status of patients can be stratified by genetics. It is unclear at present whether the residual embryonic cells observed at the squamocolumnar junction serve any function in situ or indeed are essential to nucleate Barrett's metaplasia in response to GERD. Further studies will be necessary to determine whether they represent targets for preemptive therapeutic strategies in the normal population to prevent the onset of Barrett's.
EXPERIMENTAL PROCEDURES
Animal Models
p63−/− mice used in this study have been described (Yang et al., 1999). The heterozygous DTA-Krt14-Cre strain was generated by crossing the homozygous Gt(ROSA)26Sor<tm1(DTA)Jpmb>/J strain (Ivanova et al., 2005; Jackson Laboratory) with the homozygous Tg(KRT14-cre/Esr1)20Efu/J (Vasioukhin et al., 1999; Jackson Laboratory). Diphtheria toxin A expression was transcriptionally activated in basal cells of stratified epithelia by intraperitoneal injections of Tamoxifen in corn oil (100mg/kg) for one to three weeks prior to analysis. Animals were handled in accordance with guidelines of the Harvard Medical School and the Biomedical Resource Center A*STAR, Singapore. Paraffin sections of human gastrointestinal junctions and Barrett's metaplasia were obtained from archives at the Brigham and Women's Hospital and the National University of Singapore Health Services under their respective IRB approvals.
Expression Microarrays and Bioinformatics
RNA processing and hybridization were performed on Affymetrix Mouse Genome 430 2.0 Array chips at the Microarray Core at the Dana Farber Cancer Institute. All Cel files were processed using GeneChip Operating Software to calculate probe set intensity values, and probe hybridization ratios were calculated using Affymetrix Expression Console Software to validate sample quality. These intensity values were log2-transformed and then imported into the Partek Genomics Suite 6.5 (beta). A 1-way ANOVA was performed to identify differentially expressed genes. Fold-changes and p-values for probe sets were calculated for each analysis. Principal component analysis (PCA) was carried out using all probe sets, and heatmaps were generated using sorted datasets based on Euclidean distance and average linkage methods. Gene expression datasets from normal and Barrett's esophagus (Stairs et al., 2008; Kimchi et al., 2005) were downloaded from the Gene Expression Omnibus (GEO) genesets of the NCBI. Barrett's metaplasia datasets showing squamous cell gene expression were excluded from the analysis.
Histology and Immunofluorescence
Histology, immunohistochemistry, and immunofluorescence were performed using standard techniques, processed at the Rodent Histopathology Core of the Dana Farber Harvard Cancer Center and imaged at the Nikon Imaging Facility at the Harvard Medical School.
Supplementary Material
HIGHLIGHTS
Barrett's metaplasia is recapitulated in p63 null mouse embryos.
The metaplasia arises from an embryonic epithelium rather than transdifferentiation.
A similar population of residual embryonic cells persists in adult mice and humans.
Modeling tissue damage that triggers Barrett's causes migration of embryonic cells.
Acknowledgements
We are grateful to Prof. Rod Bronson and Rodent Pathology Core at Harvard Medical School for help with histology, the staff of the Nikon Imaging Center of the Harvard Medical School for their help with imaging, and Prof. Ed Cox of the Dana Farber Cancer Institute Microarray Facility. We thank Da-Kai Mu for assistance with genotyping. We thank Prof. Birgit Lane for helpful discussions. We thank all the members in the Xian-McKeon laboratory for helpful discussions and support, and Profs. Edison Liu and Barbara Knowles for their critical review of the manuscript. This work was supported by the National Institutes of Health (RO1-GM083348 and R21CA124688), the European Research Council, Agence de Nationale, and intramural grants from the Institute of Medical Biology and the Genome Institute of Singapore of the Agency for Science, Technology, and Research, Singapore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antonioli DA, Wang HH. Morphology of Barrett's esophagus and Barrett's-associated dysplasia and adenocarcinoma. Gastroenterol. Clin. North Am. 1997;26:495–506. doi: 10.1016/s0889-8553(05)70309-5. [DOI] [PubMed] [Google Scholar]
- Badreddine RJ, Wang KK. Barrett's esophagus: an update. Nat. Rev. Gastroent. Hepatolff. 2010;7:369–378. doi: 10.1038/nrgastro.2010.78. [DOI] [PubMed] [Google Scholar]
- Barr H, Shepherd NA, Dix A, Roberts DJ, Tan WC, Krasner N. Eradication of high-grade dysplasia in columnar-lined (Barrett's) oesophagus by photodynamic therapy with endogenously generated protoporphyrin IX. Lancet. 1996;348:584–585. doi: 10.1016/s0140-6736(96)03054-1. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. J. Am. Med. Assoc. 1991;265:1287–1289. [PubMed] [Google Scholar]
- Botelho NK, Schneiders FI, Lord SJ, Freeman AK, Tyagi S, Nancarrow DJ, Hayward NK, Whiteman DC, Lord RV. Gene expression alterations in formalin-fixed, paraffin-embedded Barrett's esophagus and esophageal adenocarcinoma tissues. Cancer Biol. Ther. 2010;10:172–179. doi: 10.4161/cbt.10.2.12166. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, James R, Hammond VE, Köntgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- Coad RA, Woodman AC, Warner PJ, Barr H, Wright NA, Shepherd NA. On the histogenesis of Barrett's oesophagus and its associated squamous islands: a three-dimensional study of their morphological relationship with native oesophageal gland ducts. J. Pathol. 2005;206:388–394. doi: 10.1002/path.1804. [DOI] [PubMed] [Google Scholar]
- Correa P, Chen VW. Gastric cancer. Cancer Surv. 1994:19–20. 55–76. [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- Dixon J, Strugala V, Griffin SM, Welfare MR, Dettmar PW, Allen A, Pearson JP. Esophageal mucin: an adherent mucus gel barrier is absent in the normal esophagus but present in columnar-lined Barrett's esophagus. Am J Gastroenterol. 2001;96:2575–2583. doi: 10.1111/j.1572-0241.2001.04159.x. [DOI] [PubMed] [Google Scholar]
- Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 138:746–774. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Glickman JN, Chen YY, Wang HH, Antonioli DA, Odze RD. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus. Am. J. Surg. Pathol. 2010;25:569–578. doi: 10.1097/00000478-200105000-00002. 2001. [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggitt RC. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum. Pathol. 1994;25:982–993. doi: 10.1016/0046-8177(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 2005;43:129–135. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski JA, Wright NA, Meltzer SJ, Triadafilopoulos G, Geboes K, Casson AG, Kerr D, Young LS. Molecular evolution of the metaplasia-dysplasiaadenocarcinoma sequence in the esophagus. Am. J. Pathol. 1999;154:965–973. doi: 10.1016/S0002-9440(10)65346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi ET, Posner MC, Park JO, Darga TE, Kocherginsky M, Karrison T, Hart J, Smith KD, Mezhir JJ, Weichselbaum RR, Khodarev NN. Progression of Barrett's metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res. 2005;65:3146–3154. doi: 10.1158/0008-5472.CAN-04-2490. [DOI] [PubMed] [Google Scholar]
- Kiran RP, Khoury W, Church JM, Lavery IC, Fazio VW, Remzi FH. Colorectal cancer complicating inflammatory bowel disease: similarities and differences between Crohn's and ulcerative colitis based on three decades of experience. Ann. Surg. 2010;252:330–335. doi: 10.1097/SLA.0b013e3181e61e69. [DOI] [PubMed] [Google Scholar]
- Leedham SJ, Preston SL, McDonald SA, Elia G, Bhandari P, Poller D, Harrison R, Novelli MR, Jankowski JA, Wright NA. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett's oesophagus. Gut. 2008;57:1041–1048. doi: 10.1136/gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley CC, Galipeau PC, Finley JC, Wongsurawat VJ, Li X, Sanchez CA, Paulson TG, Blount PL, Risques RA, Rabinovitch PS, Reid BJ. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat. Genet. 2006;38:468–473. doi: 10.1038/ng1768. [DOI] [PubMed] [Google Scholar]
- Levine DS, Rubin CE, Reid BJ, Haggitt RC. Specialized metaplastic columnar epithelium in Barrett's esophagus. A comparative transmission electron microscopic study. Lab Invest. 1989;60:418–432. [PubMed] [Google Scholar]
- Mutoh H, Hakamata Y, Sato K, Eda A, Yanaka I, Honda S, Osawa H, Kaneko Y, Sugano K. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- Naef AP, Savary M, Ozzello L. Columnar-lined lower esophagus: an acquired lesion with malignant predisposition. Report on 140 cases of Barrett's esophagus with 12 adenocarcinomas. J. Thorac. Cardiovasc. Surg. 1975;70:826–835. [PubMed] [Google Scholar]
- Piazuelo MB, Haque S, Delgado A, Du JX, Rodriguez F, Correa P. Phenotypic differences between esophageal and gastric intestinal metaplasia. Mod. Pathol. 2004;17:62–74. doi: 10.1038/sj.modpathol.3800016. [DOI] [PubMed] [Google Scholar]
- Raskin WH, Norwood T, Levine DS, Haggitt RC, Rabinovitch PS, Reid BJ. Persistent clonal areas and clonal expansion in Barrett's esophagus. Cancer Res. 1992;52:2946–2950. [PubMed] [Google Scholar]
- Reid BJ. Barrett's esophagus and esophageal adenocarcinoma. Gastroenterol. Clin. North Am. 1991;20:817–834. [PubMed] [Google Scholar]
- Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat. Rev. Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar PM, Ackroyd R, Hosie KB, Patterson JE, Stoddard CJ, Kingsnorth AN. Regression and progression of Barrett's oesophagus after antireflux surgery. Br. J. Surg. 1995;82:806–810. doi: 10.1002/bjs.1800820628. [DOI] [PubMed] [Google Scholar]
- Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G211–218. doi: 10.1152/ajpgi.90250.2008. 2008. [DOI] [PubMed] [Google Scholar]
- Spechler SJ, Goyal RK. Barrett's esophagus. N. Engl. J. Med. 1986;315:362–371. doi: 10.1056/NEJM198608073150605. [DOI] [PubMed] [Google Scholar]
- Stairs DB, Nakagawa H, Klein-Szanto A, Mitchell SD, Silberg DG, Tobias JW, Lynch JP, Rustgi AK. Cdx1 and c-Myc foster the initiation of transdifferentiation of the normal esophageal squamous epithelium toward Barrett's esophagus. PLoS One. 2008;3:e3534. doi: 10.1371/journal.pone.0003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, Fernández-Del Castillo C, Warshaw AL, Thayer SP. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology. 2010;138:1166–1177. doi: 10.1053/j.gastro.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey NA, Argote CM, Vanegas XC, Barlow W, Orlando RC. Electrical parameters and ion species for active transport in human esophageal stratified squamous epithelium and Barrett's specialized columnar epithelium. Am J Physiol Gastrointest Liver Physiol. 2007;293:G264–270. doi: 10.1152/ajpgi.00047.2007. [DOI] [PubMed] [Google Scholar]
- van Baal JW, Bozikas A, Pronk R, Ten Kate FJ, Milano F, Rygiel AM, Rosmolen WD, Peppelenbosch MP, Bergman JJ, Krishnadath KK. Cytokeratin and CDX-2 expression in Barrett's esophagus. Scand. J. Gastroenterol. 2008;43:132–140. doi: 10.1080/00365520701676575. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qin R, Ma Y, Wu H, Peters H, Tyska M, Shaheen NJ, Chen X. Differential gene expression in normal esophagus and Barrett's esophagus. J. Gastroenterol. 2009;44:897–911. doi: 10.1007/s00535-009-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann A, Rieger A, Zimmermann M, Gross M, Hoffmann P, Slevogt H, Morawietz L. Comparison of six immunohistochemical markers for the histologic diagnosis of neoplasia in Barrett's esophagus. Virchows Arch. 2010;457:537–545. doi: 10.1007/s00428-010-0972-y. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Longley MA, Roop DR. Discovery of a novel murine keratin 6 (K6) isoform explains the absence of hair and nail defects in mice deficient for K6a and K6b. J Cell Biol. 2001;154:619–630. doi: 10.1083/jcb.200102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J. Cell Biol. 2003;163:327–337. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.