Abstract
Background
Racial and ethnic disparities are well-documented in many areas of health care but have not been comprehensively evaluated among recipients of heart transplantation.
Methods and results
We performed a retrospective cohort study of 39,075 adult primary heart transplant recipients from 1987-2009 using national data from the United Network of Organ Sharing, and compared mortality for non-white and white patients using the Cox proportional hazards model. During the study period, 8,082 non-white and 30,993 white patients underwent heart transplantation. Non-white heart transplant recipients increased over time, comprising nearly 30% of transplants since 2005. Non-white recipients had a higher clinical risk profile than white recipients at the time of transplantation but had significantly higher post-transplant mortality even after adjustment for baseline risk. Among the non-white group, only black recipients had an increased risk of death when compared with white recipients after multivariable adjustment for recipient, transplant, and socioeconomic factors (hazard ratio [HR] 1.34; 95% confidence interval [CI], 1.21-1.47; p<0.001). Five-year mortality was 35.7% (CI, 35.2%-38.3%) among black and 26.5% (CI, 26.0%-27.0%) among white recipients. Black patients were more likely to die from graft failure or a cardiovascular cause than white patients, but less likely to die from infection or malignancy. Although mortality decreased over time for all transplant recipients, the disparity in mortality between blacks and whites remained essentially unchanged.
Conclusions
Black heart transplant recipients have had persistently higher mortality than whites recipients over the past two decades, perhaps due to a higher rate of graft failure.
Keywords: Transplantation, Survival, Race, Treatment disparities, Trends
Introduction
Racial and ethnic disparities in health care have been well-documented1-3 and may lead to thousands of excess deaths each year4, 5. Members of minority groups are less likely to undergo appropriate diagnostic procedures for heart disease and less likely to undergo coronary revascularization than whites6, 7, independent of differences in clinical factors, disease burden, and socioeconomic status7. Despite significant attention from the medical community8, the elimination of racial disparities has been slow and inconsistent1, 9, 10.
Racial minority patients with end-stage heart failure who undergo heart transplantation appeared in several studies to have worse long-term survival compared with white recipients,11-15 but these studies were relatively small, conducted in selected centers, and examined short time intervals. We therefore sought to examine the association between race and mortality among all adults who underwent primary heart transplant in the United States since 1987. We also sought to evaluate additional outcomes measures—including recipient cause of death and events during two-year follow-up—in racial and ethnic subgroups to determine their association with disparities in mortality.
Methods
Design overview, setting, and participants
We performed a retrospective cohort study of American adults (age ≥18 years) registered on the United Network of Organ Sharing (UNOS) heart transplant waiting list (67,588 ‘listed’ patients) since September 1985. We selected the 39,078 patients who underwent primary heart transplantation (‘transplanted’) between October 1987 and February 2009. Patients were excluded who lacked data on race or ethnicity (three patients), or did not have any follow-up data (197 patients). The median follow-up time was 1,815 days (interquartile range, 478 to 3,304). The primary endpoint of this study was all cause mortality after transplantation.
Patient data collection and reporting were performed using standard UNOS data worksheets16, 17. The data for this study were obtained as a Standard Transplant Analysis and Research (STAR) file based on Organ Procurement and Transplantation Network (OPTN) data as of May 2009. Each STAR file contains patient-level data on all heart transplant candidates who have been listed and all transplants performed since October 1, 198718.
Clinical staff at each transplant center determined and coded race/ethnicity using the standard Office of Management and Budget categories as white, black or African American, Hispanic or Latino, Asian, American Indian or Alaskan native, native Hawaiian or Pacific Islander, or multiracial19. All patients not coded as ‘white’ were included in the ‘non-white’ cohort; survival analysis was also performed on non-white racial/ethnic subgroups (coded as black, Hispanic or Latino, and other). Non-black and non-Hispanic minority transplant recipients were categorized as ‘other’.
Statistical analysis
Continuous variables were summarized as mean ± standard deviation and compared using Student t-tests or analysis of variance (ANOVA). Categorical variables were summarized using percentages and compared using χ2 tests. Time-to-event data were summarized using Kaplan-Meier survival analysis and compared using log-rank tests. Dates of transplantation, length of follow-up, and patient outcomes were obtained from the STAR data file. Follow-up on surviving patients was censored on the last date of follow-up reported to UNOS. Patients who underwent a second heart transplant were censored as alive on the date of re-transplantation. In secondary analysis, we evaluated the composite outcome of death or re-transplantation.
Multivariable survival analysis was performed using Cox proportional hazards models. Covariates used in the analysis were selected from prior literature based on their known potential to affect mortality20; these included recipient, transplant, and socioeconomic factors. In the final adjusted Cox proportional hazards model, all listed factors were included as covariates. Proportional hazards assumptions were tested using scaled Schoenfeld residuals; variables that failed to meet the proportional hazards assumption were used to stratify the multivariable Cox regressions.
Recipient factors included age, gender, body mass index, history of diabetes, pulmonary artery diastolic pressure, creatinine, total bilirubin, medical condition (outpatient, inpatient intensive care unit [ICU], inpatient non-ICU), and need for support therapy at transplant (including dialysis, ventricular assist device, intraaortic balloon pump, mechanical ventilator, or intravenous antibiotics). Recipient heart disease diagnoses were characterized as ischemic cardiomyopathy, non-ischemic cardiomyopathy, valvular heart disease, congenital heart disease, or unknown.
Transplant factors included donor age, graft ischemic time (≥6 hrs versus <6 hrs), donor-recipient race mismatch, ABO blood group incompatibility (identical versus non-identical), level of human leukocyte antigen (HLA) mismatches (≤2, 3-4, or ≥5), transplant era, transplant center volume, and geographic region using OPTN definitions. Transplant centers were categorized into four tiers based on their total number of transplants performed during the study period to account for center volume effects on mortality21. Transplants recipients were evenly divided among the following tiers: Tier 1 (10,229 patients; 26.4%); Tier 2 (9,525 patients; 24.6%); Tier 3 (9,474 patients; 24.4%); and Tier 4 (9,549 patients; 24.6%). Transplant eras were defined as 1987-1995, 1996-2000, 2001-2004, and 2005-200920.
Socioeconomic factors included education (college or higher versus high school or lower), US citizenship, insurance type (private versus non-private), and median household income. Patient residential zip codes were used to evaluate neighborhood-level median household income based on 2000 US Census data22.
Differences in the frequency of missing data between racial/ethnic subgroups, when present, were minimal and appeared to be randomly distributed. An increased frequency of missing data was present for patients receiving transplants prior to 1995; thus, multivariable hazard ratios excluding these patients were also estimated.
Unadjusted survival estimates were generated using Kaplan-Meier survival analysis. Adjusted one- and five-year survival estimates for white and black transplant recipients, in three-year increments from 1987 to 2005, were based on the ‘corrected group prognosis method’ described by Ghali et al23; covariates included recipient age, gender, diagnosis, and medical condition; and donor age.
Causes of death were described by racial subgroup and compared using a χ2 test. Deaths resulting from a cardiovascular cause or graft failure were considered potentially associated with ‘under-immunosuppression’; those resulting from infection, post-transplant lymphoproliferative disorder (PTLD), or other malignancy were associated with ‘over-immunosuppression’ according to previous literature24-26.
The frequency of hospitalization, hospitalization for a rejection episode, and non-compliance—defined in the UNOS worksheet as ‘evidence of noncompliance with immunosuppression medication that compromised the patient’s recovery’—within two years of transplantation were compared.
Statistical analyses were performed using Stata/IC 10.1 for Macintosh (Stata Corporation, College Station, TX).
Results
During the study period, 14,732 non-white (21.9%) and 52,486 white (78.1%) adults were registered on the heart transplant waiting list. Among the 39,075 patients who underwent heart transplant, 30,993 were white (79.3%), 4,997 black (12.8%), 2,118 Latino (5.4%), 649 Asian (1.7%), 140 American Indian or Alaskan native (0.4%), 69 native Hawaiian or Pacific Islander (0.2%), and 109 multiracial (0.3%). Median time on the waiting list was shorter for non-white recipients (66 days) than for white recipients (92 days, p<0.001).
The total number of heart transplants performed declined gradually since 1990 (Figure 1), with fewer white patients placed on the waiting list over time. For example, 14,322 whites were placed on the waiting list during 1990-1994, compared with 10,526 whites during 2000-2004. The number of non-white patients who were listed and underwent transplant rose during the same time periods. As a result, the proportion of non-white transplant recipients increased steadily from 15.8% (1990-1994) to 29.6% (2005-2009, p<0.001).
Figure 1.
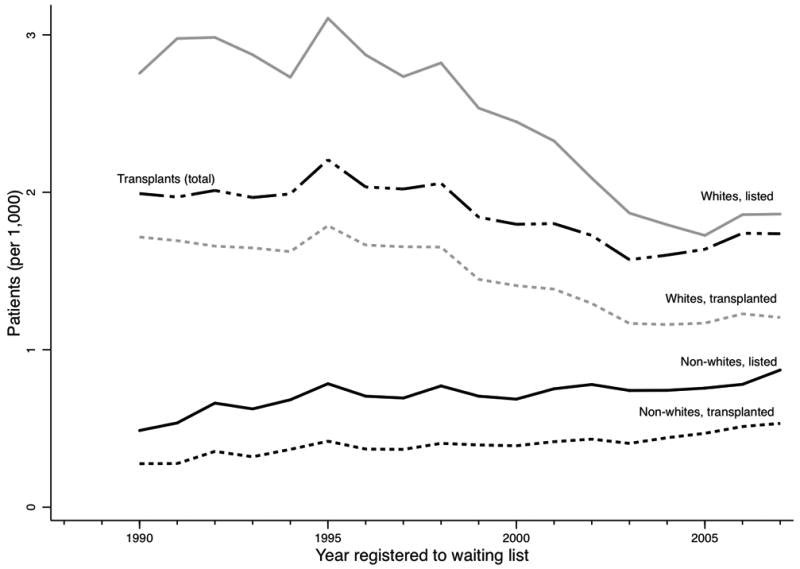
Number of non-white and white patients placed on the heart transplantation waiting list and undergoing heart transplantation by year.
Baseline characteristics
Non-white recipients were younger and more frequently female, and had higher rates of co-morbid conditions including diabetes and renal failure requiring dialysis (Table 1). Underlying conditions leading to heart transplantation differed between groups. For example, black patients most frequently underwent transplant for non-ischemic cardiomyopathy (71.0%), while white patients most frequently received transplant for ischemic cardiomyopathy (54.4%, p<0.001). Black recipients had the highest rates of HLA mismatches; all non-white recipients had high rates of donor-recipient race mismatches when compared with white recipients. Black and Hispanic recipients had lower rates of college education and private insurance; they were also from neighborhoods with lower median income.
Table 1.
Baseline characteristics of heart transplant recipients.
| Variable | White (n = 30,993) | Black (n = 4,997) | Hispanic (n = 2,121) | Other (n = 967) | P value | n |
|---|---|---|---|---|---|---|
| Recipient factors | ||||||
| Age, years | 53 (11) | 46 (12) | 49 (12) | 48 (13) | < 0.001 | 39,068 |
| Male | 24,828 (80.1) | 3,256 (65.2) | 1,607 (75.8) | 740 (76.5) | < 0.001 | 39,078 |
| Body mass index | 25.8 (4.6) | 26.0 (5.3) | 25.6 (6.0) | 23.8 (4.8) | <0.001 | 38,506 |
| Health Status, at transplant | ||||||
| Diabetes | 4,405 (14.2) | 763 (20.1) | 404 (24.8) | 162 (21.9) | <0.001 | 26,289 |
| Pulmonary artery diastolic pressure, mmHg | 21 (9) | 23 (9) | 22 (10) | 22 (9) | < 0.001 | 24,759 |
| Creatinine, mg/dL | 1.4 (1.2) | 1.5 (1.2) | 1.4 (1.1) | 1.4 (0.9) | <0.001 | 27,903 |
| Total bilirubin, mg/dL | 1.3 (3.1) | 1.3 (2.6) | 1.6 (3.8) | 1.5 (3.4) | <0.01 | 25,747 |
| Medical condition | < 0.001 | 38,787 | ||||
| Not hospitalized | 13,889 (45.1) | 1,949 (39.4) | 901 (42.9) | 418 (43.5) | ||
| Hospitalized, non ICU | 4,125 (13.4) | 846 (17.1) | 352 (16.8) | 136 (14.2) | ||
| Hospitalized, ICU | 12,759 (41.5) | 2,157 (43.6) | 848 (40.4) | 407 (42.4) | ||
| Support therapy requirements | ||||||
| Dialysis, required after listing | 505 (2.5) | 133 (3.5) | 59 (3.7) | 27 (3.6) | < 0.001 | 26,753 |
| Ventricular assist device | 3,894 (38.8) | 804 (35.8) | 267 (28.0) | 128 (30.1) | < 0.001 | 13,651 |
| Intraaortic balloon pump | 1,801 (5.8) | 260 (5.2) | 114 (5.4) | 58 (6.0) | 0.31 | 39,078 |
| Mechanical ventilator | 864 (2.8) | 104 (2.1) | 69 (3.3) | 27 (2.8) | 0.02 | 39,078 |
| On intravenous antibiotics for infection | 2,047 (10.1) | 493 (13.2) | 167 (10.5) | 84 (11.4) | < 0.001 | 26,329 |
| Diagnosis Group | < 0.001 | 39,078 | ||||
| Ischemic cardiomyopathy | 16,862 (54.4) | 1,208 (24.2) | 846 (39.9) | 379 (39.2) | ||
| Non-ischemic cardiomyopathy | 12,099 (39.0) | 3,547 (71.0) | 1,121 (52.9) | 510 (52.7) | ||
| Valvular disease | 994 (3.2) | 139 (2.8) | 87 (4.1) | 42 (4.3) | ||
| Congenital heart disease | 755 (2.4) | 62 (1.2) | 52 (2.5) | 26 (2.7) | ||
| Other | 283 (0.9) | 41 (0.8) | 15 (0.7) | 10 (1.0) | ||
| Transplantation factors | ||||||
| Donor age, years | 30 (12) | 30 (12) | 30 (12) | 29 (12) | 0.02 | 39,076 |
| Graft ischemic time, hours | 2.9 (1.0) | 2.9 (1.0) | 3.0 (1.1) | 3.0 (1.1) | 0.06 | 36,506 |
| Donor-recipient race mismatch | 6,979 (22.6) | 4,162 (83.5) | 1,538 (72.8) | 932 (96.7) | <0.001 | 38,991 |
| Blood group (ABO) compatibility, identical | 26,666 (86.1) | 4,163 (83.3) | 1,844 (86.9) | 753 (77.9) | < 0.001 | 39,073 |
| Level of HLA mismatch | < 0.001 | 32,084 | ||||
| 0 – 2 | 1,237 (4.9) | 84 (2.0) | 64 (3.6) | 13 (1.7) | ||
| 3 – 4 | 10,559 (41.6) | 1,390 (33.6) | 683 (38.2) | 272 (34.8) | ||
| 5 – 6 | 13,576 (53.5) | 2,667 (64.4) | 1,042 (58.2) | 497 (63.6) | ||
| Center Volume Tier | <0.001 | 38,774 | ||||
| 1 | 8,002 (26.1) | 1,229 (24.7) | 677 (32.0) | 321 (33.4) | ||
| 2 | 7,711 (25.1) | 1,146 (23.0) | 493 (23.3) | 174 (18.1) | ||
| 3 | 7,646 (24.9) | 1,272 (25.5) | 376 (17.8) | 180 (18.7) | ||
| 4 | 7,357 (24.0) | 1,336 (26.8) | 568 (26.9) | 286 (29.8) | ||
| Socioeconomic factors | ||||||
| College education or higher | 7,768 (51.0) | 1,175 (40.1) | 408 (33.4) | 333 (62.1) | < 0.001 | 19,933 |
| US citizenship | 30,700 (99.2) | 4,935 (98.9) | 1,935 (91.2) | 807 (83.7) | < 0.001 | 39,020 |
| Private insurance | 13,108 (63.1) | 1,826 (47.2) | 719 (43.1) | 421 (55.2) | < 0.001 | 27,091 |
| Median household income by zip code, $ | 46,412 (16,745) | 37,376 (14,057) | 38,602 (15,895) | 49,088 (19,735) | < 0.001 | 33,158 |
Data are mean(SD) or frequency (percentage). P values are from ANOVA or χ2 test, as appropriate. ICU: intensive care unit; HLA: human leukocyte antigen.
Mortality
During the study period, 16,880 patients died after transplantation. Black recipients had significantly higher unadjusted mortality than white, Hispanic, or other transplant recipients (Figure 2, log rank p<0.01); mortality did not differ significantly between whites and non-black minority recipients. One-year mortality among black recipients was 15.8% (CI, 14.8%-16.9%), while it was 13.2% (CI, 12.9%-13.6%) among white, 13.6% (CI, 12.2%-15.2%) among Hispanic, and 12.4% (CI, 10.4%-14.7%) among other recipients. Five-year mortality was 36.7% (CI, 35.2%-38.3%) among black recipients; it was 26.5% (CI, 26.0%-27.0%) among white, 29.4% (CI, 27.3%-31.7%) among Hispanic, and 26.2% (CI, 23.0%-29.7%) among other recipients.
Figure 2.
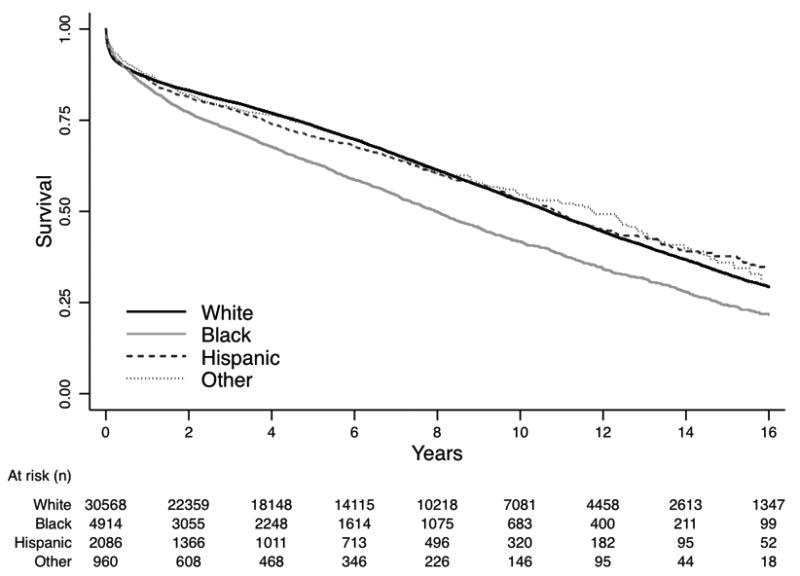
Unadjusted Kaplan-Meier survival curve for heart transplant recipients by racial or ethnic subgroup. Log-rank p-value < 0.0001
Only black transplant recipients were at an increased risk of death after multivariable adjustment for recipient, transplant, and socioeconomic variables (Table 2, HR 1.34; CI, 1.21 – 1.47; p<0.001). Hispanic and other recipients were not at an increased risk of death compared with white recipients, although comparisons were limited by fewer numbers of non-black minority recipients (Table 2). The increased risk of death among black, compared with white, recipients was of similar magnitude after adjusting for individual transplant centers (HR 1.29; CI, 1.17-1.43; p<0.001).
Table 2.
Univariate and multivariable hazard ratio estimates for the risk of post-transplant death among racial and ethnic minority subgroups compared with whites.
| Hazard Ratio (95% CI) | P value | n | |
|---|---|---|---|
| Black | |||
| Unadjusted | 1.35 (1.29 – 1.41) | < 0.001 | 35,482 |
| Model 1* | 1.50 (1.43 – 1.58) | < 0.001 | 32,463 |
| Model 2† | 1.34 (1.21 – 1.47) | < 0.001 | 13,513 |
| Hispanic | |||
| Unadjusted | 1.01 (0.94 – 1.09) | 0.75 | 32,651 |
| Model 1* | 1.07 (0.99 – 1.16) | 0.11 | 29,897 |
| Model 2† | 0.97 (0.84 – 1.12) | 0.71 | 12,234 |
| Other | |||
| Unadjusted | 0.94 (0.84 – 1.06) | 0.32 | 31,417 |
| Model 1* | 1.03 (0.90 – 1.17) | 0.65 | 28,767 |
| Model 2† | 1.16 (0.92 – 1.46) | 0.20 | 11,658 |
adjusted for age, gender, diagnosis group, transplant era, OPTN region, ECMO use, IABP use, mechanical ventilation, medical condition, ABO mismatch, HLA mismatch, BMI, donor CMV status , ischemic time, list status
Model 1 plus community median income, insurance type, citizenship, education, diabetes, dialysis, creatinine, intravenous antibiotics at transplant, pulmonary artery diastolic pressure, graft race mismatch, center volume
While one- and five-year mortality decreased among all recipients during the study period, adjusted mortality estimates among blacks remained consistently higher than among whites (Figure 3). The frequency of re-transplantation did not differ between groups; it was performed in 106 black (2.1%), 627 white (2.0%), 45 Hispanic (2.1%), and 24 (2.5%, p=0.76). Hazard ratios for the composite outcome of death or re-transplantation were similar to the hazard ratios for mortality; compared with white recipients, black recipients had a multivariable hazard ratio for death or re-transplantation of 1.35 (CI, 1.22-1.49; p<0.01). Multivariable hazard ratios excluding patients recieiving transplantation prior to 1995 were also similar (1.36; CI, 1.23-1.52; p<0.01).
Figure 3.
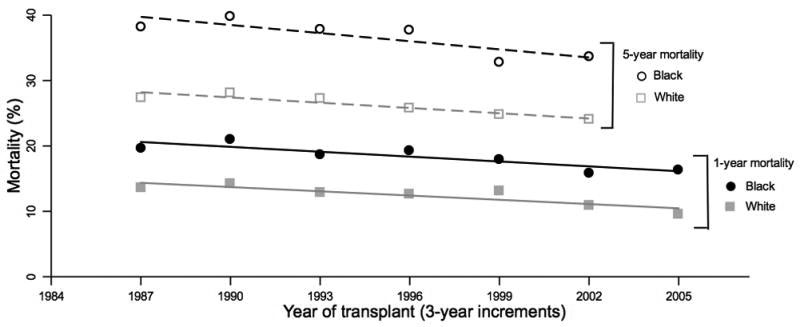
One- and five-year mortality estimates for black and white heart transplant recipients by 3-year increments adjusted for recipient age, gender, and diagnosis; donor age; and medical condition. Lines represent weighted linear regression fitted values of one-year mortality among blacks(—) and whites(
 ) and five-year mortality estimates among blacks(---) and whites(
) and five-year mortality estimates among blacks(---) and whites(
 ).
).
Cause of death
Blacks were more likely to die of graft failure or a cardiovascular cause (57.9%) than were whites (37.8%) or other non-whites (44.1%, p<0.001; Table 3), but less likely to die from an infection or malignancy (19.9%) than were whites (33.0%) or other non-whites (28.2%, p<0.001). Similar, but less pronounced, findings were present among recipients surviving more than five years after transplant. For example, 48.7% of blacks dying five or more years after transplant died from graft failure or a cardiovascular cause, compared with 31.8% of whites (p<0.001), while 25.8% of blacks died from infection or malignancy compared with 36.3% of whites (p<0.001). Non-compliance was the cause of death among 2.2% of black, 0.6% of white, 2.4% of Hispanic, and 0.4% of other recipients (p<0.001).
Table 3.
Deaths by cause and race/ethnicity.
| Number died during study | White (n=30,993) | Black (n=4,997) | Hispanic (n=2,121) | Other (n=967) |
|---|---|---|---|---|
| 11,264 (36.3) | 1,857 (37.2) | 630 (29.7) | 267 (29.2) | |
| Cause of death | ||||
| Cardiovascular | 2,269 (20.1) | 566 (30.5) | 139 (17.8) | 73 (25.9) |
| Graft failure | 1,998 (17.7) | 509 (27.4) | 139 (22.1) | 51 (18.1) |
| Infection | 1,930 (17.1) | 255 (13.7) | 112 (17.8) | 61 (21.6) |
| Non-PTLD malignancy | 1,403 (12.5) | 92 (5.0) | 42 (6.7) | 12 (4.3) |
| Pulmonary | 626 (5.6) | 70 (3.8) | 34 (5.4) | 12 (4.3) |
| Renal failure | 510 (4.5) | 66 (3.6) | 14 (2.2) | 6 (2.1) |
| Cerebrovascular | 493 (4.4) | 67 (3.6) | 24 (3.8) | 11 (3.9) |
| PTLD or lymphoma | 388 (3.4) | 22 (1.2) | 18 (2.9) | 12 (4.3) |
| Hemorrhage | 241 (2.1) | 38 (2.1) | 13 (2.1) | 6 (2.1) |
| Accident, trauma, or suicide | 144 (1.3) | 13 (0.7) | 6 (1.0) | 1 (0.4) |
| Non-compliance | 71 (0.6) | 41 (2.2) | 15 (2.4) | 1 (0.4) |
| Other | 1,189 (10.6) | 118 (6.4) | 74 (11.8) | 36 (12.8) |
Chi-squared for comparison between racial subgroups < 0.001. PTLD: post-transplant lymphoproliferative disorder
Five-year mortality among blacks was worse than that of whites for all diagnostic groups apart from valvular heart disease. For example, five-year mortality for blacks with ischemic cardiomyopathy was 35.2% (CI, 32.3%-38.3%) compared with 28.0% (CI, 27.3%-28.8%) for whites. Among those with non-ischemic cardiomyopathy, five-year mortality in blacks was 37.3% (CI, 35.5%-39.1%), compared with 24.1% (CI, 23.3%-25.0%) for whites. The adjusted risk of death for blacks, compared with whites, for non-ischemic cardiomyopathy was 1.38 (CI, 1.21-1.58; p<0.001) and for ischemic cardiomyopathy was 1.18 (CI, 1.00-1.39; p=0.054).
Events during follow-up
Within the first two years after transplant, black recipients were hospitalized at a higher rate (40.2%) than other recipients (white, 34.3%; Hispanic, 35.5%; other, 33.2%; p<0.001); however, hospitalization data were unavailable in 32% of recipients. Blacks also required hospitalization for rejection (16.1%) more frequently than white (10.0%), Hispanic (11.1%), and other (9.8%, p<0.001) recipients; data on the reason for hospitalization were missing for 67% of recipients. Rates of non-compliance with immunosuppressive medication were also higher among black recipients (15.0%) compared with white (8.9%), Hispanic (10.1%), and other (9.1, p<0.001) recipients; data was missing for 30% of recipients.
Discussion
This study found that black adults who received a heart transplant were at an increased risk of death compared with otherwise similar white patients; their long-term mortality was also worse than non-black minority recipients. While black patients had a higher risk clinical profile at baseline than white patients, the difference in mortality remained significant after adjustment for recipient, transplant, and socioeconomic factors. After transplant, black recipients were hospitalized at higher rates—overall as well as specifically for rejection—and more frequently died of causes associated with less effective immunosuppression than other recipients. The increased risk of death among black patients has not changed appreciably over the past two decades.
Our findings confirm and extend previous smaller studies that suggested an increased mortality among black heart transplant recipients11-15. Park et al evaluated 336 patients at a single center between 1983 and 1994 and found that 10-year post-transplant survival was worse among blacks than whites13. Kirklin et al found a two-fold increase in the risk of death for blacks, compared with whites, several years after transplant among 7,290 recipients in 42 institutions11. Among 4,227 pediatric patients between 1987-2004, Mahle et al reported that median graft survival was 5.3 years among black recipients and 11.0 years among non-black recipients12. Even though other studies have found no increased risk of death among black heart transplant recipients, these studies were single center studies of between 103 and 525 patients27-30. This present study covers a larger period of time than previous studies, and includes substantially more patients.
While mortality after heart transplantation has progressively decreased over time, the gap in mortality between blacks and whites has persisted. This disparity contrasts with outcomes among recipients of lung transplantation, in whom the increased risk of death prior to 2001 among non-whites has been eliminated because mortality among non-white lung transplant recipients fell faster than that among white recipients31. This improved survival among non-white lung transplant recipients may have resulted from changes in immunosuppressive therapy—specifically, the decreased use of cyclosporine and the increased use of tacrolimus. Tacrolimus, a calcineurin inhibitor associated with improved outcomes among black organ transplant patients32, 33, has become the predominant therapy for maintenance immunosuppression among lung transplant recipients since 200034, 35. Despite a similar trend of increasing use of tacrolimus among heart transplant recipients—after one-year, nearly 60% of recipients received tacrolimus in 2007 compared with less than 20% in 2000—the gap in mortality between black and white patients has remained20, 35.
Blacks were more likely to die of causes potentially associated with under-immunosuppression—graft failure and cardiovascular causes—whereas whites and other non-whites were more likely to die from causes associated with over-immunosuppression—infection and malignancy. Blacks were also more frequently hospitalized for rejection within two years of transplantation. Together, these findings suggest that lower intensity immunosuppression among blacks recipients may have contributed to the observed disparities in long-term survival. After kidney transplantation, inadequate immunosuppression has been found to contribute to worse allograft survival among blacks36. Under-immunosuppression among blacks may result from biologic factors, such as higher rates of mismatches between donor and recipient race and HLA status, need for higher levels of immunosuppression, differential drug absorption, and systemic complications of therapy. Under-immunosuppression among blacks may also result from socioeconomic factors, such as inadequate health insurance, poorer access to care, barriers to effective communication between patients and physicians, and lower adherence to treatment36.
The persistent disparity in mortality might be eliminated if the reason for less adequate immunosuppression among black recipients could be identified. Our study cannot distinguish between biologic or socioeconomic factors as the primary reason for lower rates of effective immunosuppression among black recipients. It is notable that previous research suggests that barriers to appropriate cardiovascular care exist for both black and Hispanic patients with heart disease, and that these limitations in access to care may adversely affect mortality1, 7, 37-41. Interestingly, Hispanic transplant recipients in this study had lower rates of college education, U.S. citizenship, and private insurance than black recipients. Nonetheless, Hispanic recipients had a risk of death similar to that of white transplant recipients, and lower than that of black recipients.
During the study period, we also found marked changes in the racial composition of heart transplant recipients. Non-white patients have increased in both absolute number of heart transplants and as a percentage of all heart transplant recipients; since 2005, non-whites have accounted for nearly one-third of transplants. Conversely, there has been a steady decline in the number and percentage of white patients placed on the waiting list and ultimately undergoing transplantation. This decline may reflect substantial improvements in preventive and acute care for ischemic cardiomyopathy since 198042, 43—a more common diagnosis among white heart transplant recipients. Notably, however, blacks with either ischemic or non-ischemic cardiomyopathy had higher mortality compared with whites. The overall increase in black transplant recipients, however, has not translated into improved outcomes.
Given the persistently worse mortality experienced by black heart transplant recipients over two decades, further studies are necessary to investigate the causes, which might focus in two areas. First, while non-white heart transplant recipients enrolled in recent randomized controlled trials of immunosuppressive therapy account for up to 14% of the study population44-46, differences in rates of rejection and death by racial subgroups have not been reported. Within the framework of clinical trials—which involve rigorous follow-up and invasive procedures like endomyocardial biopsy or intravascular ultrasound—differences in access to care are likely to be minimized. If present, race-based differences in outcomes may more accurately reflect differences in biologic or genetic responses to immunosuppressive therapy. Pooled samples, from meta-analyses, may offer increased statistical power for evaluating differences in outcomes but have not been analyzed47. Second, given the complex relationship between racial and ethnic factors with disparities in outcomes48, additional study to investigate unmeasured factors contributing to immunosuppression adequacy are necessary. Novel tools—both biologic markers of immunosuppression adequacy49 as well as survey instruments for non-compliance50, for example—are likely to be essential.
This study has a number of important limitations. First, we cannot conclusively identify the reasons for the observed differences in mortality because of the retrospective observational study design. Second, data on patient race and ethnicity from the UNOS dataset were determined by staff at transplant centers rather than by patient self-report. Although discrepancies in race/ethnicity coding could result in misclassification, however, it is unclear whether these would attenuate or magnify the observed effect size. Third, the smaller number of non-black transplant recipients may have limited the statistical power to determine hazard ratios among minority subgroups. However, we believe that the regression analyses among Hispanic recipients remain robust even after multivariable adjustment and provide insight about the potential mechanisms that drive mortality differences. Fourth, we were not able to evaluate detailed measures of access to care; instead we employed socioeconomic variables as surrogate measures. It remains possible that unmeasured variables, especially those that favor Hispanic over black recipients, could result in residual confounding. Finally, missing data not only limited our ability to completely assess outcomes, but also complicated the ability to identify the causes of observed differences.
In conclusion, non-white and white patients differed significantly in clinical characteristics prior to transplantation. However, only black adults had higher mortality following heart transplantation when compared with whites; this increase was independent of recipient, transplant, and socioeconomic factors. While long-term survival has improved for all recipients, the racial gap in survival among blacks has persisted over two decades and may be related in inadequate immunosuppression. Additional research aimed at identifying the source of this disparity is necessary.
Acknowledgments
Funding sources Vincent Liu was supported by NIH T32-HL008948-07 and Agency for Healthcare Research and Quality F32-HS019181-01 grants. This work was also supported in part by Health Resources and Services Administration contract 231-00-0115.
The sponsor was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflict of interests disclosures The authors have no conflicts of interest relevant to the subject of this manuscript.
References
- 1.National Healthcare Disparities Report, 2007. Full Report. Agency for Healthcare Research and Quality. 2010 Available at: http://www.ahrq.gov/qual/qrdr07.htm#toc.
- 2.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283:2579–2584. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 3.Smedly B, Stith A, Nelson A, editors. Unequal treatment: confronting racial and ethnic disparities in health care. Washington DC: Institute of Medicine; 2002. [PMC free article] [PubMed] [Google Scholar]
- 4.Satcher D, Fryer GE, Jr, McCann J, Troutman A, Woolf SH, Rust G. What if we were equal? A comparison of the black-white mortality gap in 1960 and 2000. Health Aff (Millwood) 2005;24:459–464. doi: 10.1377/hlthaff.24.2.459. [DOI] [PubMed] [Google Scholar]
- 5.Woolf SH, Johnson RE, Fryer GE, Jr, Rust G, Satcher D. The health impact of resolving racial disparities: an analysis of US mortality data. Am J Public Health. 2004;94:2078–2081. doi: 10.2105/ajph.94.12.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. 2001;135:352–366. doi: 10.7326/0003-4819-135-5-200109040-00012. [DOI] [PubMed] [Google Scholar]
- 7.The Henry J. Kaiser Family Foundation. Racial/ethnic differences in cardiac care: the weight of the evidence. 2010 Available at: http://www.kff.org/uninsured/20021009c-index.cfm.
- 8.Lurie N. Health disparities--less talk, more action. N Engl J Med. 2005;353:727–729. doi: 10.1056/NEJMe058143. [DOI] [PubMed] [Google Scholar]
- 9.Jha AK, Fisher ES, Li Z, Orav EJ, Epstein AM. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;353:683–691. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- 10.Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, Manhapra A, Mallik S, Krumholz HM. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353:671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Bourge RC, McGiffin DC, Hill JA, Rodeheffer RJ, Jaski BE, Hauptman PJ, Weston M, White-Williams C. Evolving trends in risk profiles and causes of death after heart transplantation: a ten-year multi-institutional study. J Thorac Cardiovasc Surg. 2003;125:881–890. doi: 10.1067/mtc.2003.168. [DOI] [PubMed] [Google Scholar]
- 12.Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr. 2005;147:739–743. doi: 10.1016/j.jpeds.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc. 1997;29:1460–1463. doi: 10.1016/s0041-1345(96)00567-2. [DOI] [PubMed] [Google Scholar]
- 14.Costanzo MR, Eisen HJ, Brown RN, Mehra M, Benza R, Torre G, Yancy CW, Davis S, McCloud M, Kirklin J. Are there specific risk factors for fatal allograft vasculopathy? An analysis of over 7,000 cardiac transplant patients. J Heart Lung Transplant. 2001;20:152. doi: 10.1016/s1053-2498(00)00261-8. [DOI] [PubMed] [Google Scholar]
- 15.Higgins RS, Fishman JA. Disparities in solid organ transplantation for ethnic minorities: facts and solutions. Am J Transplant. 2006;6:2556–2562. doi: 10.1111/j.1600-6143.2006.01514.x. [DOI] [PubMed] [Google Scholar]
- 16.UNOS Adult Thoracic - Heart Transplant Recipient Registration Worksheet. 2010 Available at: http://www.unos.org/SharedContentDocuments/Thoracic-Heart-Adult_Transplant_Recipient_Registration.pdf.
- 17.UNOS Adult Thoracic Transplant Recipient Follow-Up Worksheet. 2010 Available at: http://www.unos.org/SharedContentDocuments/Thoracic-HeartLung-Adult_Transplant_Recipient_Follow-Up.pdf.
- 18.Standard Transplant Analysis and Research (STAR) Dataset Files. 2010 Available at: http://optn.transplant.hrsa.gov/data/request_main.asp?refer=true.
- 19.Revisions to the standards for the classification of federal data on race and ethnicity. Federal Register. 1997;62:36873–36946. [Google Scholar]
- 20.Taylor DO, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, Rahmel AO, Kucheryavaya AY, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report--2008. J Heart Lung Transplant. 2008;27:943–956. doi: 10.1016/j.healun.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Hosenpud JD, Breen TJ, Edwards EB, Daily OP, Hunsicker LG. The effect of transplant center volume on cardiac transplant outcome. A report of the United Network for Organ Sharing Scientific Registry. JAMA. 1994;271:1844–1849. [PubMed] [Google Scholar]
- 22.U.S. Bureau of the Census. Census of population and housing, 2000: summary file 3, national. 2002 [Google Scholar]
- 23.Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Galbraith PD, Knudtson ML. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 24.Hunt SA. Current status of cardiac transplantation. JAMA. 1998;280:1692–1698. doi: 10.1001/jama.280.19.1692. [DOI] [PubMed] [Google Scholar]
- 25.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 26.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 27.Radovancevic B, Konuralp C, Vrtovec B, Radovancevic R, Thomas CD, Zaqqa M, Vaughn WK, Frazier OH. Factors predicting 10-year survival after heart transplantation. J Heart Lung Transplant. 2005;24:156–159. doi: 10.1016/j.healun.2003.11.399. [DOI] [PubMed] [Google Scholar]
- 28.Pamboukian SV, Costanzo MR, Meyer P, Bartlett L, McLeod M, Heroux A. Influence of race in heart failure and cardiac transplantation: mortality differences are eliminated by specialized, comprehensive care. J Card Fail. 2003;9:80–86. doi: 10.1054/jcaf.2003.11. [DOI] [PubMed] [Google Scholar]
- 29.Cohen O, De La Zerda D, Beygui RE, Hekmat D, Laks H. Ethnicity as a predictor of graft longevity and recipient mortality in heart transplantation. Transplant Proc. 2007;39:3297–3302. doi: 10.1016/j.transproceed.2007.06.086. [DOI] [PubMed] [Google Scholar]
- 30.Moore DE, Feurer ID, Rodgers S, Jr, Shaffer D, Nylander W, Gorden DL, Chari RS, Wright JK, Pinson CW. Is there racial disparity in outcomes after solid organ transplantation? Am J Surg. 2004;188:571–574. doi: 10.1016/j.amjsurg.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Liu V, Weill D, Bhattacharya J. Racial differences in survival following lung transplantation. Arch Surg. 2010 doi: 10.1001/archsurg.2011.4. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehra MR, Uber PA, Scott RL, Park MH. Ethnic disparity in clinical outcome after heart transplantation is abrogated using tacrolimus and mycophenolate mofetil-based immunosuppression. Transplantation. 2002;74:1568–1573. doi: 10.1097/00007890-200212150-00014. [DOI] [PubMed] [Google Scholar]
- 33.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 Kidney Transplant Study Group. Transplantation. 1998;65:515–523. doi: 10.1097/00007890-199802270-00011. [DOI] [PubMed] [Google Scholar]
- 34.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Taylor DO, Kucheryavaya AY, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report--2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Hosenpud JD, Bennett LE, Keck BM, Fiol B, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official report--1999. J Heart Lung Transplant. 1999;18:611–626. doi: 10.1016/s1053-2498(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 36.Young CJ, Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. Med Clin North Am. 2005;89:1003–1031. ix. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Carlisle DM, Leake BD, Shapiro MF. Racial and ethnic disparities in the use of cardiovascular procedures: associations with type of health insurance. Am J Public Health. 1997;87:263–267. doi: 10.2105/ajph.87.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander M, Grumbach K, Remy L, Rowell R, Massie BM. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. Am Heart J. 1999;137:919–927. doi: 10.1016/s0002-8703(99)70417-5. [DOI] [PubMed] [Google Scholar]
- 39.Barnhart JM, Wassertheil-Smoller S, Monrad ES. Clinical and nonclinical correlates of racial and ethnic differences in recommendation patterns for coronary revascularization. Clin Cardiol. 2000;23:580–586. doi: 10.1002/clc.4960230807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canto JG, Taylor HA, Jr, Rogers WJ, Sanderson B, Hilbe J, Barron HV. Presenting characteristics, treatment patterns, and clinical outcomes of non-black minorities in the National Registry of Myocardial Infarction 2. Am J Cardiol. 1998;82:1013–1018. doi: 10.1016/s0002-9149(98)00590-6. [DOI] [PubMed] [Google Scholar]
- 41.Oka RK, Fortmann SP, Varady AN. Differences in treatment of acute myocardial infarction by sex, age, and other factors (the Stanford Five-City Project) Am J Cardiol. 1996;78:861–865. doi: 10.1016/s0002-9149(96)00457-2. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 43.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 44.Hershberger RE, Starling RC, Eisen HJ, Bergh CH, Kormos RL, Love RB, Van Bakel A, Gordon RD, Popat R, Cockey L, Mamelok RD. Daclizumab to prevent rejection after cardiac transplantation. N Engl J Med. 2005;352:2705–2713. doi: 10.1056/NEJMoa032953. [DOI] [PubMed] [Google Scholar]
- 45.Kobashigawa J, Miller L, Renlund D, Mentzer R, Alderman E, Bourge R, Costanzo M, Eisen H, Dureau G, Ratkovec R, Hummel M, Ipe D, Johnson J, Keogh A, Mamelok R, Mancini D, Smart F, Valantine H. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation. 1998;66:507–515. doi: 10.1097/00007890-199808270-00016. [DOI] [PubMed] [Google Scholar]
- 46.Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sorensen K, Hummel M, Lind JM, Abeywickrama KH, Bernhardt P. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 47.Penninga L, Moller CH, Gustafsson F, Steinbruchel DA, Gluud C. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol. 2010;66:1177–1187. doi: 10.1007/s00228-010-0902-6. [DOI] [PubMed] [Google Scholar]
- 48.Rathore SS, Krumholz HM. Differences, disparities, and biases: clarifying racial variations in health care use. Ann Intern Med. 2004;141:635–638. doi: 10.7326/0003-4819-141-8-200410190-00011. [DOI] [PubMed] [Google Scholar]
- 49.Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Bogaev RC, Elashoff B, Baron H, Yee J, Valantine HA. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 50.Dobbels F, Berben L, De Geest S, Drent G, Lennerling A, Whittaker C, Kugler C. The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation. 2010;90:205–219. doi: 10.1097/TP.0b013e3181e346cd. [DOI] [PubMed] [Google Scholar]


