Abstract
Phospholipase A2 releases the fatty acid arachidonic acid from membrane phospholipids. We used the purported phospholipase A2 stimulator, melittin, to examine the effects of endogenous arachidonic acid signaling on dopamine transporter function and trafficking. In HEK-293 cells stably transfected with the dopamine transporter, melittin reduced uptake of [3H]dopamine. Additionally, measurements of fatty acid content demonstrated a melittin-induced release of membrane-incorporated arachidonic acid, but inhibitors of phospholipase C, phospholipase D, and phospholipase A2 did not prevent the release. Subsequent experiments measuring [125I]RTI-55 binding to the dopamine transporter demonstrated a direct interaction of melittin, or a melittin-activated endogenous compound, with the transporter to inhibit antagonist binding. This effect was not specific to the dopamine transporter, as [3H]spiperone binding to the recombinant dopamine D2 receptor was also inhibited by melittin treatment. Finally, melittin stimulated an increase in internalization of the dopamine transporter, and this effect was blocked by pretreatment with cocaine. Thus, melittin acts through multiple mechanisms to regulate cellular activity, including release of membrane-incorporated fatty acids and interaction with the dopamine transporter.
Keywords: endocytosis, arachidonic acid, phospholipase, dopamine transporter, dopamine receptor
1. Introduction
The arachidonic acid cascade is a complex biological signaling pathway that is found in most cell types. Free arachidonic acid is generated by phospholipase A2-mediated hydrolysis of membrane phospholipids. Altered phospholipase A2 activity is implicated in many neurological disorders such as dyslexia (MacDonell et al., 2000), Parkinson’s disease (Ross et al., 2001) and schizophrenia (Horrobin et al., 1994). Additionally phospholipase A2 activators can induce (Reid et al., 1996) while inhibitors of phospholipase A2 (Reid et al., 1996) and of arachidonic acid metabolism (Reid et al., 2002) can inhibit, psychostimulant sensitization. Considering that hyperactivity of the dopamine system may be a component of the etiology of schizophrenia, and the dopamine transporter is the primary mechanism of clearing released dopamine from the extra-synaptic space (Parsons and Justice, Jr., 1994; Wightman and Zimmerman, 1990) and is the site of action of psychostimulants such as cocaine and amphetamines (Ritz and Kuhar, 1989), understanding the effects of the arachidonic acid signaling cascade on the dopamine transporter could be crucial to understanding symptoms of addiction and other neuropsychiatric disorders.
In purified synaptosomes from rat striatum, arachidonic acid dose-dependently increases spontaneous release of dopamine and decreases dopamine synthesis and uptake. This effect is mediated by arachidonic acid itself rather than by a metabolite (L'hirondel et al., 1995). Arachidonic acid also inhibits uptake of glutamate (Volterra et al., 1992, 1994) and alters proton conductance by the rat glutamate transporter, EAAT4 (Fairman et al., 1998; Tzingounis et al., 1998). Arachidonic acid also directly stimulates a cocaine-sensitive cation conductance in the dopamine transporter that is distinct from substrate associated current (Ingram and Amara, 2000).
Phospholipase A2, which can be stimulated by G-protein coupled dopamine D2 receptors, hydrolyzes membrane phospholipids to cause a release of platelet-activating factor and arachidonic acid (Piomelli et al., 1991; Vial and Piomelli, 1995). In bovine cerebellar cells arachidonic acid can activate protein kinase Cγ (Shearman et al., 1989), yet Zhang and Reith (1996) have shown that arachidonic acid effects on dopamine transporter function are not mediated by protein kinase C. Thus, the mechanisms mediating arachidonic acid’s effects on the dopamine transporter are not clear.
Melittin is a 26 amino acid amphiphilic peptide isolated from the venom of the honeybee, Apis mellifera. Melittin activates arachidonic acid signaling pathways in neurons (Geddis et al., 2004; Muzzio al., 2001) and in multiple cell lines including rat PC12 and mouse L1210 cells (Lee et al., 2001; Palomba et al., 2004), possibly through phospholipase A2 stimulation. Additionally melittin acts through non-phospholipase A2-mediated mechanisms to release fatty acids and alter cell function (Lee et al., 2001). While melittin has been used to examine the effects of phospholipase A2 signaling on dopamine cell function (Reid et al., 1996; Zhang and Reith, 1996), the mechanism of action has been presumed to be release of arachidonic acid. The potential contributions of extra- arachidonic acid effects of melittin to dopamine cell function have not been explored. In this study we used HEK cells stably transfected with the dopamine transporter to test the hypothesis that melittin stimulates phospholipases and affects dopamine transporter function and trafficking.
2. Material and Methods
2.1 Materials
Melittin was purchased from Sigma-Aldrich (St. Louis, MO USA). The radioligands [3H] dopamine (59 Ci/mmol), [3H] arachidonic acid (60 Ci/mmol), and [3H]spiperone (73 Ci/mmol) were purchased from Amersham Biosciences (Piscataway, NJ USA), and [125I]RTI-55 (2200 Ci/mmol) was purchased from Perkin Elmer (Wellesley, MA USA). Rat anti-human dopamine transporter antibody was purchased from Chemicon (Temecula, CA USA) and goat anti-rat secondary conjugated to alkaline phosphatase was purchased from Santa Cruz (Santa Cruz, CA USA). Other reagents were purchased from commercial sources (Sigma Aldrich, St Louis, MO USA)
2.2 Stable Cell Line Transfection
The cDNAs were cloned and human embryonic kidney-293 (HEK) cells were cotransfected with the cDNAs for the human dopamine transporter (hDAT) (in pcDNA1), the long form of the dopamine D2 receptor (in pcDNA1) and the antibiotic resistance vector pBabepuro at stoichiometries of 7:7:1 using the calcium phosphate precipitation method (Chen and Okayama, 1988). The cloning and characterization of the human dopamine transporter and D2 receptor cDNAs were described previously (Eshleman et al., 1995; Neve et al., 1989). Cells were selected for resistance to puromycin (2 µg/ml), and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 0.05 U penicillin/streptomycin, 2 µg/ml puromycin, in a humidified 10% CO2 incubator at 37°C
2.3 [125I]RTI-55 binding
Experiments were performed as previously described (Eshleman et al., 1999). Briefly, cells were grown to confluence on 15 cm tissue culture plates. The cells were rinsed with Ca2+, Mg2+ -free phosphate buffered saline (PBS; 0.1 M H2PO4, 150 mM NaCl), and lysis buffer (2 mM HEPES, 1 mM EDTA) was added. Cells were then removed from the plate, centrifuged for 20 min at 30,000g, and the pellet was homogenized in 5 ml of 0.32 M sucrose with a Polytron homogenizer for 5 s. Membrane aliquots (10–30 µg of protein) were added to each assay, which resulted in binding of <10% of the total radioactivity added. Assays were performed in duplicate in Krebs-Hepes buffer (25 mM HEPES, 122 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 2.5 mM CaCl2, 1 µM pargyline, 100 µM tropolone, 2 mg glucose/ml, 1 mM ascorbic acid, pH 7.4), in a final volume of 250 µl. [125I]RTI-55 (40 pM) was added and membranes were incubated for 90 min at room temperature in the dark. Nonspecific binding was determined in the presence of 10 µM mazindol. The assay was terminated by filtration through Perkin Elmer filtermat A filters (Boston, MA USA) using a 96-well Tomtec cell harvester (Hamden, CT USA). Scintillation fluid was added to the filters and remaining radioactivity was determined using a Perkin Elmer 1205 betaplate scintillation counter. For the saturation binding experiments [125I]RTI-55 was diluted with unlabeled ligand at concentrations ranging from 0.04–10 nM.
2.4 [3H]spiperone binding
Cells were grown to confluence on 10 cm tissue culture plates then lysed with lysis buffer, centrifuged at 31,000g for 20 min, re-suspended and homogenized with a polytron (setting 6 for 10 seconds) in Tris-buffered saline (TBS; 20 mM Tris, 137 mM NaCl, pH 7.6). [3H]Spiperone binding assays were performed as previously described (Neve et al., 1989). Briefly, cell homogenates were incubated with the radioligand (0.0125 nM to 0.4 nM) for 60 min at 37°C. The assay was performed in TBS plus bovine serum albumin (BSA; 1 mg/100 mL) in a total volume of 1mL. Nonspecific binding was determined in the presence of 2 µM butaclamol. The assay was terminated and radiation quantified as described above. Experiments were conducted with duplicate determinations at each radioligand concentration.
2.5 [3H]Dopamine uptake
For attached cell assays, cells were plated in 24-well tissue culture plates coated with poly-D-lysine (1 mg/ml in H2O). Cells were incubated at 37°C with drugs in Krebs-Hepes buffer in a total volume of 500 µL. The uptake assay was initiated by addition of 50 nM [3H]dopamine at 37°C. Nonspecific uptake was determined in the presence of 10 µM mazindol, added 5 min before the radioligand. Uptake continued for 10 min and was terminated by washing the cells twice with ice-cold buffer. Radioactivity was extracted by rupturing the cellular membranes with 3% trichloroacetic acid for 30 min. Extracted radioactivity was measured using a Beckman LS 3801 scintillation counter (Fullerton, CA USA). The average values of all experiments for the maximal amount of radioactivity transported into the cells was 14,700 ± 1700 cpms and for the nonspecific cpms was 890 ± 120. Cells from one well of each assay plate were used to determine protein concentrations using the BCA protein assay kit (Pierce, Rockford, IL USA).
2.6 Fatty acid analysis by gas-liquid chromatography (GLC)
Cells grown on tissue culture plates were treated with drug in PBS at 37°C and the reaction was stopped by rapidly cooling the cells on ice. Cells were then rinsed with fatty-acid-free bovine serum albumin (0.1mg/ml in PBS) followed by a rinse in PBS. The cells were scraped from the plates and centrifuged at 200×g for 10 min. The pellets were retained for analysis. Fatty acid extraction and quantification was performed by the Lipid Atherosclerosis Laboratory at Oregon Health & Science University using the chloroform-methanol procedure described previously (Rapp et al., 1983) with some modifications. The cell extracts were mixed with chloroform:methanol (2:1), centrifuged, and the top layer removed. Free cholesteryl ester was saponified with ethanol and KOH at 37°C for 60 min. The cholesterol was extracted with hexane. Acidifying the aqueous phase and re-extracting with hexane recovered the cholesteryl ester fatty acids. The fatty acids were methylated with boron trifluoride-methanol and percents of total fatty acid were determined by gas-liquid chromatography using a 30 meter Supelco SP 2330 fused silica capillary column (run at 190°) attached to a Perkin-Elmer AutoSystem chromatograph and a Perkin-Elmer Turbochrome 41 integrator. Fatty acid standards (Supelco, Inc., Bellefonte, PA USA) were analyzed in each experiment. The 17-carbon fatty acid (C17:0) was added to each sample to determine µg amounts of each fatty acid analyzed based on relative abundance to this standard. Levels of C17:0 are extremely low in naïve cells.
2.7 [3H]Arachidonic acid release
Cells were grown on poly-D-lysine coated, 24-well tissue culture plates and incubated in DMEM with 1nM [3H]arachidonic acid (106,000 dpm / well) for 18–24 h. DMEM supplemented with 0.2% fatty acid free BSA was used for all rinses and assay conditions. Following [3H]arachidonic acid incubation the cells were rinsed twice to remove unincorporated [3H]arachidonic acid. The treatments were performed in a total volume of 500 µL. Following treatments the medium was removed and centrifuged at 12,000× g for 1 min. Released [3H]arachidonic acid in 400 µl of supernatant was measured using a Beckman LS 3801 scintillation counter. Trypsin-EDTA was added to each well to remove cells remaining on the plates. This sample was also counted to measure incorporated (non-released) [3H]arachidonic acid. The value for non-released [3H]arachidonic acid was combined with the value for released [3H]arachidonic acid to determine the total amount of [3H]arachidonic acid incorporated into cellular membranes in each well. Cells from one well of each assay plate were used to determine protein concentrations using the BCA protein assay kit (Pierce). Experiments were conducted in triplicate for each time point or drug concentration.
To address the contribution of the dopamine D2 receptor to [3H]arachidonic acid release, additional assays were conducted using a modification of a method optimized to detect [3H]arachidonic acid release following activation of the G-protein coupled 5-hydroxytryptamine 2A receptor (Kurrasch-Orbaugh et al., 2003). Briefly, cells were plated as above and incubated with [3H]arachidonic acid (0.5 µCi) for 4 h in DMEM. Medium was removed, the wells were washed 3 times with supplemented DMEM, and cells were treated with vehicle or the dopamine D2 agonist quinpirole for 1 h. The reaction was terminated and radioactivity measured as described above.
2.8 Phospholipase activity measurements
To determine the ability of melittin to activate phospholipase A2 activity in HEK- D2 -hDAT cells, phospholipase A2 activity was determined using the cytoplasmic-phospholipase A2 Assay Kit (Cayman Chemical, Ann Arbor, MI). In cell preparations that contain more than one type of phospholipase A2, the kit measures total phospholipase A2 activity. Cells were plated on 10 cm cell culture plates and tested 4 days later. Cells were treated with vehicle or melittin (10 µM, for 30 min) with or without quinacrine (10 µM). Cells were then rinsed with phosphate-buffered saline, removed from plate and centrifuged for 10 min at 4°C. The pellet was homogenized in 0.5 ml cold buffer (50 mM Hepes pH 7.4, 1 mM EDTA) and centrifuged at 14,300×g for 15 min at 4°C. The resulting supernatant was stored at −80°C until assayed. On the day of assay, the supernatant was thawed, and centrifuged as above. The cytoplasmic-phospholipase A2 kit was used as directed with 30–60 µg protein, results were expressed as µmol product/min/mg protein using bee venom cytoplasmic-phospholipase A2 (2–16 ng) as the internal standard.
Activity of phospholipase D was measured using the Amplex Red Phospholipase D assay kit (Molecular Probes). Plated cells were treated with melittin (1 µM) for 30 min either alone, or following a 30 min pretreatment with propanolol (1–100 uM). Cells were scraped from the plates and suspended in kit assay buffer supplemented with 1% triton-X-100, protease inhibitors, and PMSF. Cells were homogenized by rotating for 30 min at 4°C and lysates were collected following centrifugation (15,000 rcf, 10 min). Lysates were added directly to the assay according to company directions. The assay product was detected using a fluorescence plate reader (Molecular Devices).
To determine the ability of melittin to activate phospholipase C activity in HEK- D2 -hDAT cells, inositol phosphate formation was measured using the inositol phosphate-One Elisa Kit (CisBio, Bedford, MA). Cells were plated in 24 well plates at a density of 400,000 cells per well in DMEM supplemented with charcoal-stripped FetalClone. The next day, cells were rinsed and incubated for 1 h with unsupplemented DMEM without or with 30 µM neomycin. After aspiration of medium, cells were treated with stimulation buffer from the kit with or without 30 µM neomycin, and either buffer, 1.3 µM melittin, 1 µM quinpirole, or 1 µM dopamine. Following 1 hour incubation, the cells were lysed and processed according to kit protocols.
2.9 Biotinylation of membrane proteins
To measure dopamine transporter that was internalized following drug treatment, cells were incubated with 0.3 mg/ml NHS-SS-biotin (Pierce) in PBS at 4°C with gentle agitation for 40 min. Unbound biotin was removed by rinsing with 0.1 M glycine in PBS. Cells were then incubated with drug or vehicle in DMEM at 37°C and the reaction was stopped by removal of the media and rapid cooling of the plates on ice. Remaining cell surface biotin was stripped with glutathione (150 mM glutathione, 150 mM NaCl, pH 8.75). Cells were then rinsed with 50 mM iodoactemide in PBS to neutralize the glutathione, and solubilized in CHAPS buffer (1% CHAPS, 25mM Tris, pH 7.4, 150 mM NaCl), and lysed using a glass–glass homogenizer. The samples were centrifuged at 14,000×g, and the supernatants were reserved. The protein concentration of each sample was determined using the BCA Assay kit (Pierce). Biotinylated proteins were isolated from nonbiotinylated proteins by incubation with ImmunoPure™ Immobilized streptavidin (Pierce) for 40 min at 4°C with gentle agitation. Proteins were eluted from the streptavidin beads with 30 µl of Laemmli sample buffer (Sigma) and constant mixing for 20 min. Samples were separated by SDS-PAGE and transferred to PVDF membranes for western blotting and dopamine transporter detection with anti-dopamine transporter antibody (Chemicon). Dopamine transporter immunoreactivity was quantified by densitometry on a Typhoon phosphorimager using ImageQuant software (Molecular Dynamics; Sunnyvale CA USA).
2.10 Data Analysis
Dose-response curves were analyzed by nonlinear regression using Prism 3.0 (GraphPad Software, San Diego, CA USA). Results were analyzed using Student’s t-test or one-way ANOVA followed by Tukey’s multiple comparison test, as appropriate.
3. RESULTS
3.1 Characterization of transfected HEK cells
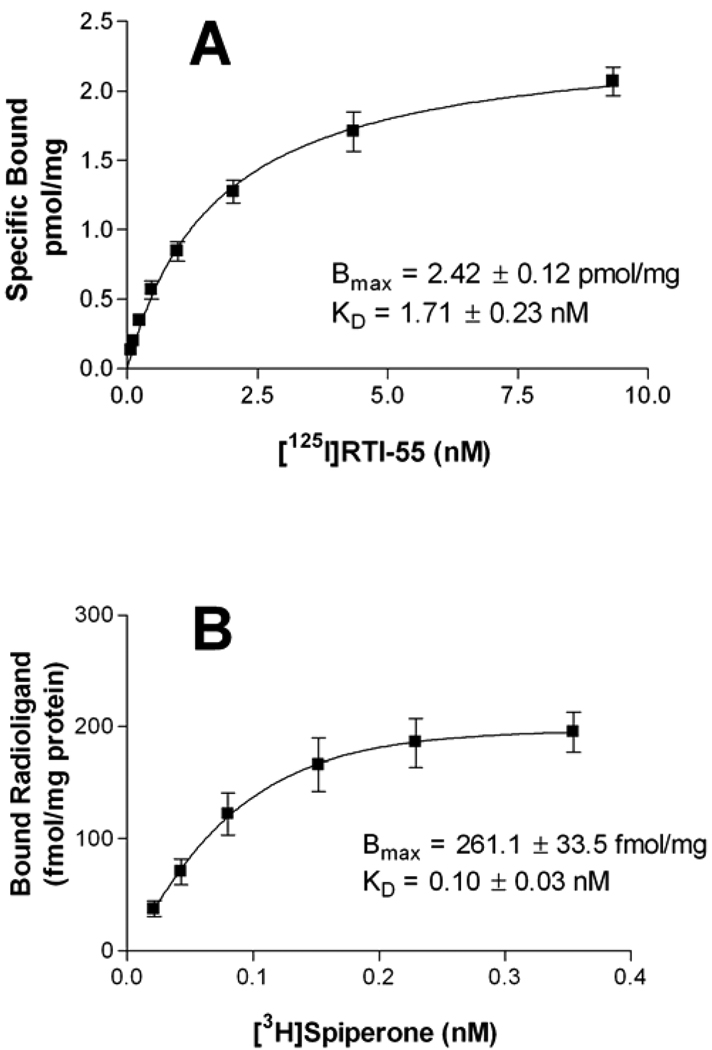
To produce a cell model for examining the effects of melittin on dopamine transporter trafficking and regulation, HEK cells were stably transfected with the human dopamine transporter and the dopamine D2 receptor. Saturation binding of the recombinant dopamine transporter was determined in the presence of 40 pM [125I]RTI-55 diluted with unlabeled ligand. The Bmax of the binding site was 2.42 ± 0.12 pmol/mg and the KD was 1.71 ± 0.23 nM (Fig 1A). Saturation binding experiments to characterize recombinant D2 receptor expression were conducted using [3H]spiperone concentrations of 0.0125nM to 0.4nM. The Bmax for radioligand binding was 261.1 ± 33.5 fmol/mg with a KD of 0.10 ± 0.03 nM (Fig 1B).
Fig 1. Characterization of stable transfections in HEK cells.
Saturation binding experiments were performed as described in the text. [125I]RTI-55 binding to the dopamine transporter, 1A, is a composite of 6 independent experiments and, [3H]spiperone binding to the D2 receptor, 1B, is a composite of 5 independent experiments each conducted with duplicate determinations.
3.2 Melittin treatment decreases dopamine transporter function
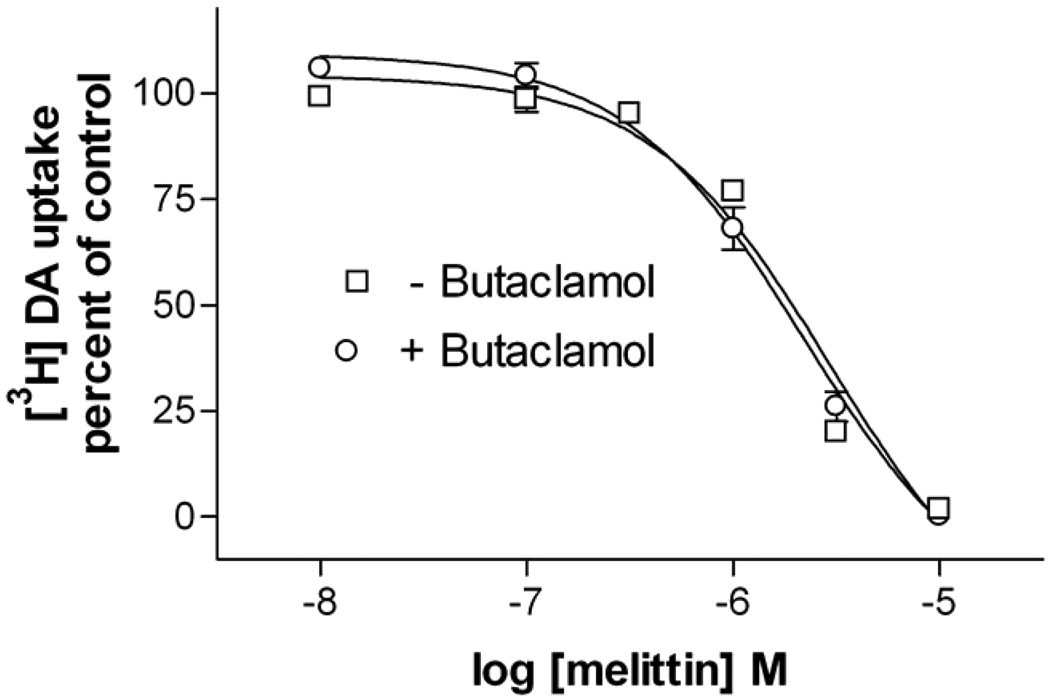
To determine the effects of phospholipase A2 activation on dopamine transporter function, HEK-D2-hDAT cells were incubated with melittin for 10–120 min (Table 1) followed by incubation with 50 nM [3H]dopamine for 10 min. Functional activity of the dopamine transporter was quantified by measuring the [3H]dopamine taken up by the cells. Melittin treatment times longer than 5 min reduced uptake almost completely. The IC50 concentration for melittin’s effects was 1.3 µM at the 30 min time point. This concentration and treatment time were used in subsequent assays. Melittin was significantly less potent following a 5 min treatment, as compared to any other time point (one-way ANOVA, P<0.001). To eliminate possible effects of feedback by the D2 receptor on dopamine uptake, butaclamol (100 nM) was added to the cells 10 min before uptake was initiated. There was no difference in [3H]dopamine uptake between butaclamol-treated and untreated cells during this time period (Fig 2).
Table 1.
Potency of melittin on inhibition of dopamine transporter function
| Treatment Time |
[3H]Dopamine uptake Melittin IC50 (µM) |
|---|---|
| 5 min | 4.2 ± 0.1 |
| 10 min | 0.82 ± 0.01 |
| 15 min | 1.76 ± 0.1 |
| 30 min | 1.3 ± 0.14 |
| 60 min | 0.8 ± 0.1 |
| 120 min | 1.79 ± 0.12 |
Cells were treated with melittin for the times indicated, followed by the addition of 50 nM [3H]dopamine. Uptake continued for 10 min. Data represent mean ± S.E.M. of three independent experiments, each conducted with triplicate determinations. The IC50 value for melittin at 5 min is significantly different than all other times (P<0.001, one-way ANOVA and Tukey’s Multiple Comparison Test), but IC50 values for melittin treatment times from 10–120 min do not differ significantly (P>0.05, one-way ANOVA).
Fig 2. Melittin causes a dose dependent decrease in dopamine transporter-mediated uptake.
HEK-D2-hDAT cells were treated with varying doses of melittin for 30 min, rinsed, and the uptake assay was initiated by the addition of 50 nM [3H]dopamine at 37°C. Experiments to block the D2 receptor were conducted in parallel using identical melittin treatments followed by the inclusion of butaclamol (100 nM) 10 min before the addition of [3H]dopamine. Results are presented as a percent of vehicle treated control cells. The curves are mean ± S.E.M. of three independent experiments, each conducted with triplicate determinations.
These findings agree with previous reports demonstrating a melittin-induced decrease in dopamine transporter-mediated uptake of dopamine following 15 min of treatment of rat C6 glioma cells transfected with the dopamine transporter. The reported decrease in uptake coincided with a decrease in binding of the cocaine analogue WIN 35,428 to the dopamine transporter in whole, attached cells (Zhang and Reith, 1996). The goal of the present study was to further characterize this effect and determine the precise mechanism of melittin’s action.
3.3 Melittin releases arachidonic acid from cellular membranes
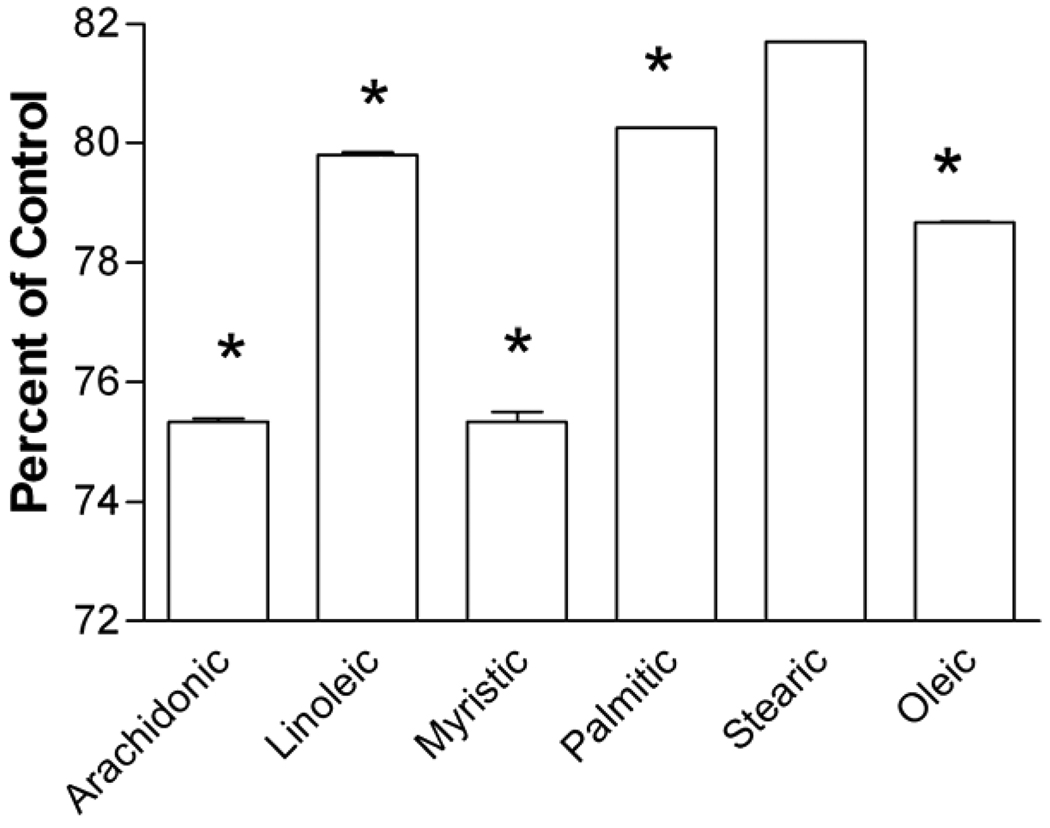
To examine the ability of melittin to activate phospholipase A2, the levels of fatty acids remaining in cellular membranes following treatments were quantified by GLC. Cells were treated with melittin (1.3 µM) for 30 min then rinsed with fatty acid-free BSA in PBS to bind and remove any free, released arachidonic acid. Following treatment, the membrane bound arachidonic acid was 75.33 ± 0.06% of vehicle-treated levels (Fig 3). There was also a 20% reduction in the arachidonic acid precursor linoleic acid. Both values are significant (P<0.001) reductions. In addition to these expected reductions, there were also reductions in the saturated fatty acids myristic, palmitic, and stearic acid of 24%, 20%, and 18% respectively, and a 21% reduction in the unsaturated fatty acid oleic acid. These results demonstrate a melittin-induced release of arachidonic acid. Release of the other fatty acids suggests additional actions of melittin on membrane lipids, not specific to phospholipase A2 stimulation.
Fig 3. Melittin releases arachidonic acid from cell membranes.
Cells were treated with melittin (1.3 µM) for 30 min and fatty acids remaining in the membrane after treatment were isolated for GLC analysis as described in the text. Levels of fatty acids remaining after treatment are presented as a percent of vehicle treated controls. Data are mean ± S.E.M. of three independent experiments (***, P<0.001 by Student’s t test).
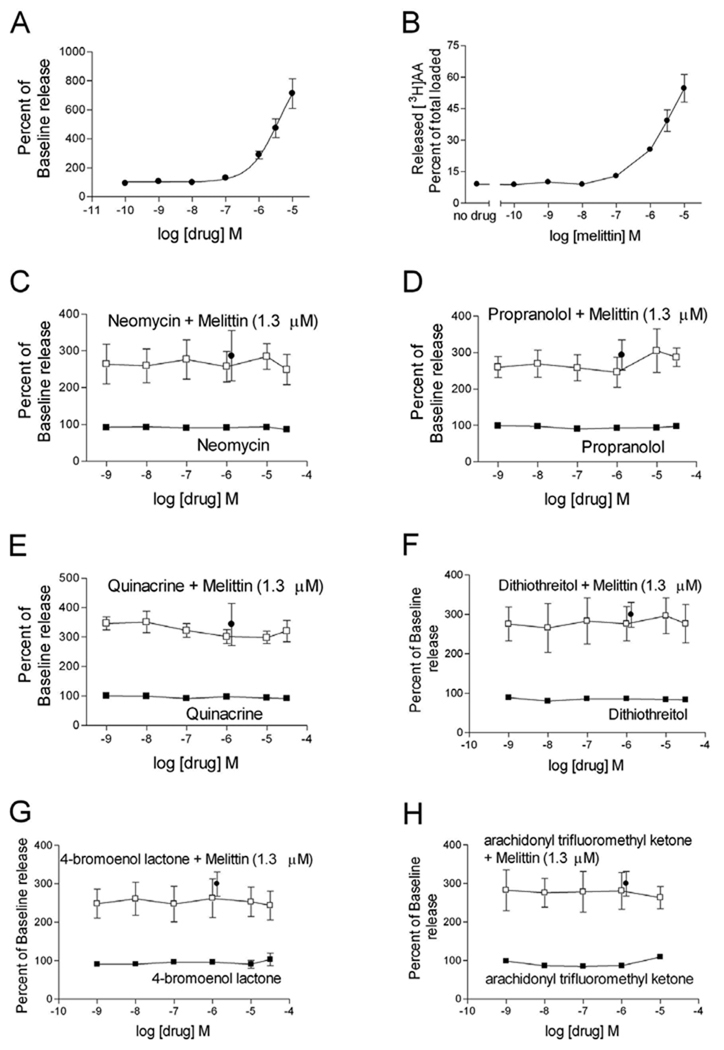
While melittin is typically utilized to stimulate phospholipase A2, recent studies have revealed that melittin simultaneously stimulates multiple phospholipases in mouse L1210 cells (Lee et al., 2001). To determine the specific phospholipase stimulated by melittin in transfected HEK cells, [3H]arachidonic acid was incorporated into lipid membranes, the cells were treated with melittin and inhibitors of phospholipase C, phospholipase D, or phospholipase A2, and the amount of [3H]arachidonic acid released following treatment was quantified. Initially, cells were treated with melittin for 30 min, demonstrating a dose-dependent release of [3H]arachidonic acid. Following treatment with 1.3 µM melittin the amount of released [3H]arachidonic acid was 324.1 ± 34.2% of baseline release (Fig 4A), 28.4 ± 2.5% of total incorporated [3H]arachidonic acid (Fig 4B), and 77.4 ± 8.0 fmol [3H]arachidonic acid/mg of protein (data not shown). These data using preloaded arachidonic acid correlate with GLC quantification of endogenous arachidonic acid released following melittin treatment.
Fig 4. Melittin-stimulated release of arachidonic acid is through non-phospholipase-mediated mechanisms.
HEK-D2-hDAT cells were incubated with [3H]arachidonic acid for 18–20 h. Stimulated [3H]arachidonic acid release was analyzed as described in the text. Cells were treated with melittin for 30 min and the percent of baseline release (A) and the percent of total cell incorporated [3H]arachidonic acid released (B) were quantified. To determine the ability of melittin to stimulate phospholipases, cells were treated with neomycin (C), propanolol (D), quinacrine (E), dithiothreitol (F), 4-bromoenol lactone (G), or arachidonyl trifluoromethyl ketone (H) for 60 min either individually (closed squares) or with the addition of a single dose of melittin (1.3 µM, open squares) during the final 30 min of the treatment. The single closed circle in graphs C–H represents the effect of melittin alone (1.3 µM). Released [3H]arachidonic acid is presented as a percent of untreated baseline release levels. Data are the mean ± S.E.M. of three experiments, each conducted with triplicate determinations.
Arachidonic acid can be released directly in a single reaction by phospholipase A2. While not releasing arachidonic acid directly, phospholipase C and phospholipase D generate free fatty acids that contain arachidonate and action by subsequent enzymes can produce free arachidonic acid (for review see Piomelli, 1995). To determine if these phospholipases were involved in [3H]arachidonic acid release, cells were preloaded with [3H]arachidonic acid and treated for 60 min with phospholipase inhibitors alone or in combination with 1.3 µM melittin during the final 30 min of treatment. Treatment with neomycin, a phospholipase C inhibitor (Carney et al., 1985), did not prevent melittin-induced [3H]arachidonic acid release (Fig 4C). While propranolol is an antagonist of β-adrenergic receptors (Mehvar and Brocks, 2001), it has also been used to inhibit phospholipase D pathway-mediated release of arachidonic acid in a variety of cells, including stimulated aortic cells (Shinoda et al., 1997), epithelial keratinocytes (Lefkowitz and Smith, 2002), and myocytes (Albert et al., 2005). Treatment with propranolol did not inhibit melittin-induced [3H]arachidonic acid release (Fig 4D). Surprisingly the general phospholipase A2 inhibitor quinacrine (Demuth et al., 2005) was also without effect on melittin-induced [3H]arachidonic acid release (Fig 4E). Phospholipase A2 inhibitors successfully block melittin-induced release of arachidonic acid in rabbit proximal tubule cells (Han et al., 2002), but quinacrine is unable to inhibit melittin-induced release of arachidonic acid in rat Leydig cells (Ronco et al., 2002).
Since the effect of melittin may be cell type specific we additionally chose inhibitors of specific subtypes of phospholipase A2 to determine if these enzymes were stimulated by melittin to cause release of arachidonic acid. Phospholipase A2 can be divided into extracellular and intracellular types. The extracellular type is a secreted enzyme (secreted phospholipase A2) that does not exhibit preference for particular fatty acids, contains disulfide bonds, and is sensitive to reducing agents, such as dithiothreitol. The intracellular types can be further divided into Ca2+-dependent phospholipase A2, requiring 0.1–1 µM of free Ca2+ for activation, and Ca2+-independent phospholipase A2 (for review see Dennis, 1994; Glaser et al., 1993). To inhibit secreted phospholipase A2, cells were treated with the reducing agent dithiothreitol prior to the application of melittin. This agent was unable to inhibit the melittin-induced release of [3H]arachidonic acid (Fig 4F). The Ca2+-independent phospholipase A2 subtype is selectively inhibited by 4-bromoenol lactone (Yang et al., 1999), and application of this inhibitor did not inhibit the effect of melittin (Fig 4G). Arachidonyl trifluoromethyl ketone is a potent and selective inhibitor of Ca2+-dependent phospholipase A2 (Street et al., 1993), yet this compound was also unable to inhibit melittin-induced release of [3H]arachidonic acid in these cells (Fig 4H).
To determine if the dopamine D2 receptors co-expressed in these cells contributed to [3H]arachidonic acid release, cells were loaded with [3H]arachidonic acid for either 4 or 24 hours. Treatment with the dopamine D2 agonist quinpirole at concentrations ranging from 1 nM to 10 µM released less than 0.4% of loaded [3H]arachidonic acid under both conditions (data not shown), indicating minimal contribution of D2 receptor activity to [3H]arachidonic acid release.
To determine if we could directly measure melittin-induced phospholipase A2 activity, we treated cells with vehicle or melittin (10 µM) with or without the general phospholipase A2 inhibitor quinacrine (10 µM) for 30 min and then measured cellular phospholipase A2 activity. Cellular control phospholipase A2 activity ranged from 0.114–0.123 µmol product/min/mg protein, and there was no significant effect of melittin (90 ± 16%), quinacrine (134 ± 19%) or melittin + quinacrine (97 ± 8%) compared to control (n=3, one way ANOVA, P>0.05).
To further clarify the effect melittin has on phospholipase D activity we treated cells with vehicle or melittin (1.3 µM) for 30 min with or without a pretreatment of propanolol (10 µM, 30 min). Following melittin treatment phospholipase D activity was 79.8 ± 10% of the activity detected in vehicle treated cells. Phospholipase D activity following propanolol alone was 62.8±14.7% of vehicle treatment and following propanolol + melittin was 73.1 ± 22% of vehicle treatment. None of these differences were significant (n=5, ANOVA, P>0.05) indicating that melittin does not activate phospholipase D in these cells.
To determine the ability of melittin to activate phospholipase C in HEK-D2-hDAT cells, we pretreated cells with vehicle or neomycin (30 µM) and then incubated cells with melittin (1.3 µM), quinpirole (1 µM) or dopamine (1 µM) for one hour. The basal inositol phosphate-1 concentration was 8.3 ± 2.5 nM, and there was no significant effect of melittin (9.5 ± 3.7 nM), quinpirole (11.1 ± 2.0 nM) or dopamine (8.8 ± 3.0 nM, n=3, one way ANOVA, P>0.05). There was also no significant difference between phospholipase C activity in the presence of melittin without or with neomycin. These data indicate that neither melittin nor the D2 receptor activates phospholipase C in these cells.
3.4 Melittin decreases antagonist binding to the transporter
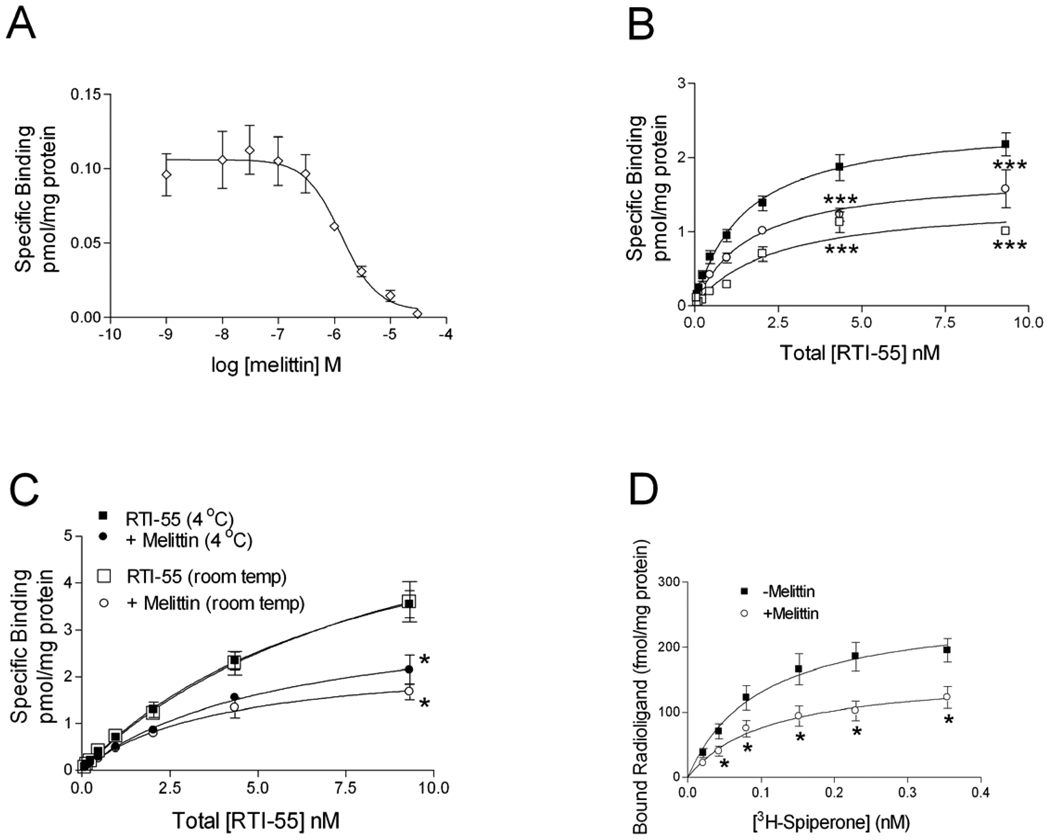
To examine possible mechanisms for melittin inhibition of dopamine transporter-mediated uptake, binding of the cocaine analogue [125I]RTI-55 to the transporter was examined. Membranes from homogenized cells were treated with varying concentrations of melittin (Fig 5A) in the presence of 40 pM [125I]RTI-55. The IC50 value for melittin inhibition of [125I]RTI-55 binding was 0.88 µM, and the Hill slope was −1.75 ± 0.21. In saturation binding assays, concurrent addition of melittin (1.3 µM; Fig 5B) to the [125I]RTI-55 binding assay significantly reduced the Bmax value (Table 2; P<0.05, one-way ANOVA, followed by Dunnett’s multiple comparison test) while there was no significant effect on the KD value (P>0.05). The decrease in Bmax suggests that melittin acts like an apparent noncompetitive inhibitor of RTI-55 binding to the dopamine transporter (Limbird, 2005; Tomlinson, 1988). A higher concentration of melittin (10 µM) further reduced the Bmax value while the KD value was not significantly altered, as compared to untreated cells (Table 2).
Fig 5. Melittin inhibits binding of RTI-55 to the dopamine transporter.
To generate dose-response binding curves for melittin (A) HEK- D2 -hDAT cells were treated with varying concentrations of melittin in the presence of 40 pM [125I] RTI-55 as described in the text. Equilibrium saturation binding experiments (B) were also conducted. The graph depicts [125I]RTI-55 binding in the absence of melittin (closed squares), and [125I] RTI-55 binding in the presence of 1.3µM (open circles) or 10µM (open squares) melittin. Data are the mean ± S.E.M. of 4–6 independent experiments (***,P<0.001 by two-way ANOVA followed by Bonferroni post test). The role of temperature in melittin’s effects on RTI-55 equilibrium saturation binding is shown in panel (C). The curves are averages of 4 independent experiments. [125I] RTI-55 binding at 4°C (closed square) and at room temperature (open square), and [125I] RTI-55 binding in the presence of melittin (1.3µM) at 4°C (closed circle) and room temperature (open circle) are depicted (*,P<0.05, by two-way ANOVA followed by Bonferroni post-test comparing treated to vehicle treated samples). The effect of melittin on [3H]spiperone binding to the D2 receptor was performed as described in the text (D) (*, P<0.05, ANOVA followed by Student’s t test)
Table 2.
Effects of melittin on RTI-55 saturation binding.
| Treatment | [125I]RTI-55 Bmax (pmol/mg) |
[125I]RTI-55 KD (nM) |
|---|---|---|
| None | 2.49 ± 0.14 | 1.48 ± 0.23 |
| Melittin 1.3 µM | 1.78 ± 0.13a | 1.62 ± 0.33 |
| Melittin 10 µM | 1.42 ± 0.16a | 2.38 ± 0.7 |
Homogenized cell membranes were incubated for 90 min with 40 pM [125I]RTI-55, varying concentrations of non-radiolabeled ligand, and melittin as indicated. Nonspecific binding was determined in the presence of 10 µM mazindol. The assay was terminated by filtration. Data are mean ± S.E.M. from at least 3 experiments.
P<0.05, one-way ANOVA followed by Dunnett’s Multiple Comparison Test.
To determine if decreased membrane fluidity played a role in melittin’s inhibition of antagonist binding to the dopamine transporter, RTI-55 binding experiments were conducted at 4°C. Parallel experiments were conducted at room temperature and at 4°C in the presence of melittin (1.3µM). The experiments continued for 16 h to allow both temperature conditions to come to equilibrium. All samples contained protease inhibitors to prevent protein degradation at room temperature. For experiments conducted at 4°C, all reagents, drugs, and membranes were chilled on ice before the assays were initiated. Melittin treatment significantly reduced the Bmax from 6.7 ± 0.8 to 2.9 ± 0.3 pmol/mg at 4°C and from 7.2 ± 1.3 to 2.4 ± 1.0 pmol/mg at room temperature (P<0.05, Fig 5C). Melittin did not significantly shift the binding affinity (KD 8.3 ± 1.8 to 4.5 ± 0.9 nM at 4°C and 9.4 ± 2.8 to 4.0 ± 1.0 at room temperature, P>0.05). Additionally, RTI-55 binding in the absence of melittin at 4°C was not significantly different from binding at room temperature, and binding in the presence of melittin at 4°C was not significantly different from RTI-55 binding in the presence of melittin at room temperature (P>0.05). Thus, membrane fluidity does not appear to play a role in melittin’s effect on the dopamine transporter.
Additional studies were conducted in these cells to determine whether melittin interacts with the dopamine D2 receptor (Fig 5D). Saturation binding experiments were conducted using [3H]spiperone concentrations of 0.0125 nM to 0.4 nM alone, or in the presence of 1.3 µM melittin. Melittin caused a significant reduction in the Bmax from 261.1 ± 33.5 fmol/mg to 158.5 ± 26.0 fmol/mg (P<0.05). Melittin did not significantly shift the binding affinity (KD 0.10 ± 0.03 to 0.11 ± 0.05 nM, P>0.05). Thus melittin also acted like an apparent noncompetitive inhibitor of spiperone binding to the dopamine D2 receptor, and so the effect of melittin on radioligand binding is not specific to the dopamine transporter.
3.5 Melittin increases dopamine transporter internalization
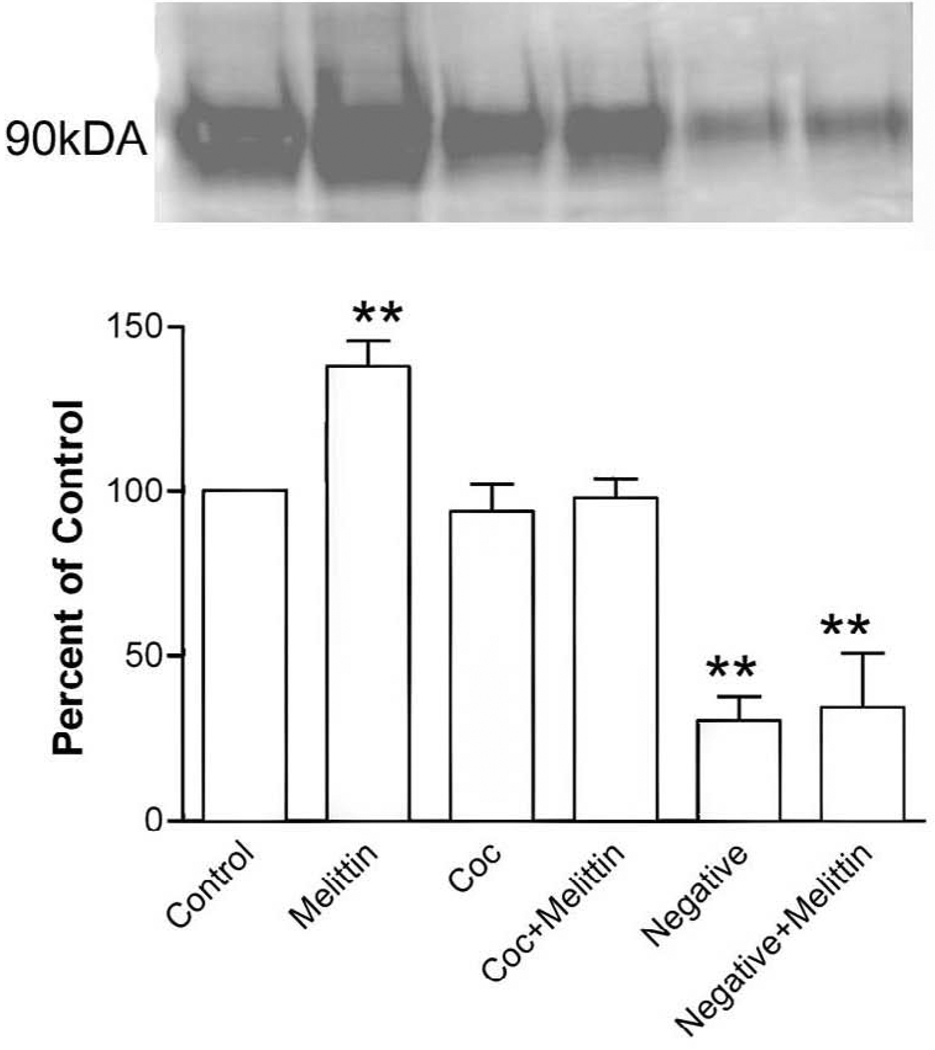
To determine whether the decrease in dopamine transporter function following melittin treatment was due to an increase in transporter internalization, biotinylation of cell surface proteins was performed. Extracellular proteins were labeled with a reducible form of biotin prior to treatments. Cell surface biotin remaining after treatment was cleaved. Thus biotin-labeled proteins internalized by treatment were protected from the reducing agent. Following treatment with melittin (1.3 µM) or vehicle for 30 min at 37°C melittin-induced internalization was significantly (P<0.01) increased to 137.9 ± 7.6% of baseline levels (Fig 6). Interestingly, this effect was blocked by 15 min pretreatment with 1 µM cocaine (97.8 ± 6.2% of control, P<0.05 compared to melittin alone), while cocaine treatment alone did not alter dopamine transporter trafficking (93.9 ± 8.4% of control). As a control, cells were biotinylated at 4°C, rinsed with cold glycine, and placed on ice while in DMEM. These cells demonstrate a non-permissive trafficking condition. Additionally, these cells demonstrate the removal of the cell surface biotin with the glutathione buffer. This condition reduced dopamine transporter internalization to 30.4 ± 7.2% of control and 34.3 ± 16.5% of control in the presence of melittin.
Fig 6. Melittin increases dopamine transporter internalization.
HEK- D2 -hDAT cells were biotinylated and then treated with vehicle or melittin (1.3 µM) for 30 min, cocaine (1 µM) for 45 min, or cocaine (1 µM) 15 min prior and during 30 min melittin (1.3 µM) treatment. Untreated, biotinylated negative control cells remained at 4°C for 30 min (cold). Following treatment the cells were rapidly chilled and the media removed. The remaining cell surface biotin was stripped and the internalized dopamine transporter was isolated by avidin purification and separated by SDS-PAGE. The immunoblot was detected with anti-dopamine transporter antibody. The representative blot shows biotinylated, internalized product for control and treated cells. Total biotinylated sample is also shown. Band optical density was quantified and expressed as a percent of vehicle treated control. Graph: dopamine transporter internalization expressed as means ± S.E.M. from 3–6 experiments (*, P<0.05; **, P < 0.01, one way ANOVA followed by Tukey’s posthoc test).
4. DISCUSSION
We generated a stable HEK cell line that expresses the recombinant human dopamine transporter and dopamine D2 receptor. While dopamine transporter function and trafficking have been examined following phorbol ester stimulation of protein kinase C, few studies have explored the role of phospholipase A2 pathways in dopamine transporter regulation. We used the phospholipase A2 stimulator melittin throughout these experiments, and found that it not only stimulated the release of membrane-incorporated arachidonic acid, but also acted directly on the dopamine transporter. While these experiments are instrumental in the initial investigation of melittin’s effects, further examination of the actions of melittin in an appropriate physiological context, such as cultured dopamine neurons, will be beneficial to understanding melittin-induced phenomena.
Melittin incorporates into outer cell membranes and alters the characteristics of the membranes and of the proteins embedded within (for review see Bernheimer and Rudy, 1986). Based on structural information and the melittin-induced shift in the Bmax and Hill slope for [125I]RTI-55 binding without a change in KD (Fig 5B & C), it is likely that melittin did not bind directly to the [125I]RTI-55 recognition site. Instead, it acted as an apparent non-competitive inhibitor, probably binding to a region of the transporter that alters the conformation of the dopamine transporter, making it unable to bind RTI-55. This designation of melittin as a noncompetitive inhibitor is in a phenomenological sense analogous to enzyme kinetics (Limbird, 2005; Tomlinson, 1988). We cannot exclude the possibility that an endogenous compound activated or released by melittin could be interacting with the dopamine transporter to decrease binding.
Alternatively, melittin’s inhibition of [125I]RTI-55 binding to the dopamine transporter could be due to nonspecific effects such as altered membrane structure. The effects of melittin on sarcoplasmic reticulum membrane fluidity are comparable to decreasing the temperature by 5°C, thus decreasing the rotational mobility of fluid lipids and reducing the rotational ability of integral Ca2+ ATPase (Mahaney et al., 1992). We observed that maximal binding of RTI-55 to the dopamine transporter, and the effects of melittin on RTI-55 binding, were not significantly different at 4°C as compared to room temperature (Fig 5C). Thus fluidity of the membrane does not alter the binding of the direct blocker RTI-55 to the dopamine transporter, and melittin’s effect on RTI-55 binding is probably not due to disruption of membrane structure.
In addition to decreasing ligand binding to the dopamine transporter, melittin was an inhibitor of [3H]spiperone binding to the dopamine D2 receptor (Fig 5D), suggesting that melittin may interact more generally with membrane-incorporated proteins. Thus melittin appears to act in a previously unreported pharmacological manner, which may serve as a confounding variable for experiments that use melittin to stimulate phospholipase A2. Furthermore, melittin binds to a variety of cellular proteins including calmodulin (Maulet and Cox, 1983), bovine mitochondrial F1-ATPase (Gledhill and Walker, 2005) and to the ubiquitous transcription factor NF-κB (Park et al., 2004). While melittin has been used as a tool to examine the peptide binding properties of the previously mentioned proteins, this is the first examination of melittin’s effects on trafficking and antagonist binding to cell surface membrane-incorporated proteins.
Melittin treatment caused a strong reduction in [3H]dopamine uptake that occurred rapidly, within 5–10 min of treatment (Table 1) and biotinylation experiments revealed an increased internalization that contributes to this effect. Melittin induces pore formation in erythrocytes (reviewed in Raghuraman and Chattopadhyay, 2007) and inhibits NaKATPase activity (Cuppoletti and Abbott, 1990), activities that could be factors in decreased transport. Additional experiments, such as electrophysiological recordings, could be done to determine whether melittin affects Na+ or Cl− gradients across cellular membranes.
As discussed above, the observed decrease in cellular [3H]dopamine uptake via the dopamine transporter following melittin treatment may be due to the multiple effects of melittin on the cells, including transporter internalization, inhibition of the binding of dopamine, and possible pore formation. These mechanisms may explain the disparities between the effects of direct application of arachidonic acid and of melittin (Zhang and Reith, 1996). Studies using rotating disk electrode voltammetry demonstrate an arachidonic acid-induced decrease in dopamine uptake. Additionally the cis-unsaturated fatty acids oleic and linoleic acid inhibit dopamine transporter-mediated uptake, although to a lesser degree (Chen et al., 2003), which suggests that there may be a property common to several fatty acids that alters dopamine transporter function. Consistent with these results, the other fatty acids released by melittin (Fig 3) could have contributed to the decrease in dopamine transporter-mediated uptake.
While no studies have specifically explored the effects of arachidonic acid on dopamine transporter trafficking, C6 cells expressing the dopamine transporter demonstrate a decrease in Bmax with no change in KD following exposure to arachidonic acid in attached, whole cell preparations (Zhang and Reith, 1996), suggesting transporter internalization. It is possible that direct actions of arachidonic acid on the dopamine transporter initiate trafficking pathways. Arachidonic acid ethyl ester, a non-metabolizable arachidonic acid analogue, incorporates into the cell membrane, but does not mimic the effects of arachidonic acid on dopamine transporter function (Chen et al., 2003; Ingram and Amara, 2000). This result suggests that arachidonic acid acts from within the lipid phase of the membrane, not by simply altering the lipid microenvironment surrounding membrane-spanning proteins and may involve a specific fatty acid binding domain on the dopamine transporter. Further studies will need to be performed to distinguish between effects mediated by direct actions of melittin and indirect effects of melittin-induced fatty acid release.
Melittin stimulates phospholipase A2, phospholipase C and phospholipase D activity in L1210 cells. The effects of melittin on fatty acid release are completely blocked only with simultaneous pretreatment with inhibitors of all three phospholipases, with phospholipase A2 activity accounting for about 10% (Lee et al., 2001). We found that melittin stimulated the release of arachidonic acid from cellular membranes (Fig 3 & 4), but this effect was not blocked by treatment with inhibitors of phospholipase C, phospholipase D, or phospholipase A2, although we did not test the effect of simultaneous treatment with the three inhibitors. Thus, melittin did not act as a specific pharmacological stimulator of phospholipases. This effect may be particular to the chosen cell type, as phospholipase A2 inhibitors successfully block melittin-induced release of arachidonic acid in rabbit proximal tubule cells (Han et al., 2002), but quinacrine is unable to inhibit melittin-induced release of arachidonic acid in rat Leydig cells (Ronco et al., 2002). Further, it is suggested that melittin has no inherent phospholipase activity, and that fatty acids are released subsequent to the membrane perturbations following melittin incorporation and pore formation (Raghuraman and Chattopadhyay, 2007). At the very least, while some effects of melittin on cellular activity may be mediated through the arachidonic acid signaling pathway, they may not be directly or exclusively produced by phospholipase A2. If melittin is found to directly interact with the transporter, then any effects of melittin-liberated fatty acids on the transporter may be masked by this interaction, and thus prove difficult to distinguish.
Since phospholipase inhibitors did not block melittin-stimulated arachidonic acid release, there may be some aspect of the peptide that interacts with the membrane to cause release of fatty acids. The 14 amino acid peptide mastoparan, found in wasp venom, is used to stimulate GTP binding regulatory proteins indirectly (Higashijima et al., 1988). Mastoparan disrupts the membrane lipid rafts where many receptors and second messenger proteins are localized, thus releasing these proteins, lipids, and arachidonic acid to the cytoplasm (Sugama et al., 2005; Nakamura et al., 2004). Experiments using nuclear magnetic resonance measurements demonstrate an interaction between melittin and ganglioside components of lipid rafts (Chatterjee and Mukhopadhyay, 2002). Thus, it is possible that melittin stimulates fatty acid release and activates signaling pathways by direct interactions with the membrane.
Cocaine (1 µM, 45 min) pretreatment had no effect by itself but prevented melittin-induced internalization (Fig 6), which may be due to a steric hindrance of the dopamine transporter by cocaine that prevented melittin’s interaction with the transporter. In contrast, cocaine (10 µM for 10 min and 1 µM for 24 h) causes an increase in dopamine transporter surface expression in human dopamine transporter-expressing HEK and N2A cells, respectively (Daws et al., 2002; Little et al., 2002), differences that may be due to drug concentration or cells used. Regardless, melittin does not affect dopamine transporter surface expression the way that cocaine does. It is not known whether cocaine also blocks the effects of arachidonic acid applied to the dopamine transporter. In addition, melittin is likely operating through a different mechanism than phorbol esters, since cocaine treatment does not alter phorbol ester-induced dopamine transporter internalization (Daniels and Amara, 1999).
Interestingly, phospholipase A2 activity may play a role in psychostimulant sensitization. Direct injections of melittin into rat ventral tegmental area cause sensitization, seen as increased locomotor activity, stereotypy, and dopamine release in the nucleus accumbens, to subsequent cocaine administration. This sensitization is blocked by pretreatment with the phospholipase A2 inhibitor quinacrine (Reid et al., 1996). Thus, the influence of phospholipase activity and arachidonic acid signaling on dopamine system functioning may be important for understanding stimulant addiction.
Acknowledgements
We thank Dr. Kim Neve for providing the dopamine D2 receptor cDNA. This project was supported by NRSA grant 5F31DA016499-02 and Tartar Trust Fellowship (DJK), and NIH/VA Interagency agreement Y1-DA5007, the VA Merit Review, and VA Research Career Scientist Programs (AJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Albert AP, Piper AS, Large WA. Role of phospholipase D and diacylglycerol in activating constitutive TRPC-like cation channels in rabbit ear artery myocytes. J Physiol. 2005;566:769–780. doi: 10.1113/jphysiol.2005.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer AW, Rudy B. Interactions between membranes and cytolytic peptides. Biochim.Biophys.Acta. 1986;864:123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Carney DH, Scott DL, Gordon EA, LaBelle EF. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985;42:479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee C, Mukhopadhyay C. Melittin-GM1 interaction: a model for a side-by-side complex. Biochem.Biophys.Res.Commun. 2002;292:579–585. doi: 10.1006/bbrc.2002.6684. [DOI] [PubMed] [Google Scholar]
- Chen CA, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- Chen N, Appell M, Berfield JL, Reith ME. Inhibition by arachidonic acid and other fatty acids of dopamine uptake at the human dopamine transporter. Eur.J Pharmacol. 2003;478:89–95. doi: 10.1016/j.ejphar.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J, Abbott AJ. Interaction of melittin with the (Na+ + K+)ATPase: evidence for a melittin-induced conformational change. Arch.Biochem.Biophys. 1990;283:249–257. doi: 10.1016/0003-9861(90)90639-g. [DOI] [PubMed] [Google Scholar]
- Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol.Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem.Biophys.Res.Commun. 2000;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Gkoumassi E, Droge MJ, Dekkers BG, Esselink HJ, van Ree RM, Parsons ME, Zaagsma J, Molleman A, Nelemans SA. Arachidonic acid mediates non-capacitative calcium entry evoked by CB1-cannabinoid receptor activation in DDT1 MF-2 smooth muscle cells. J Cell Physiol. 2005;205:58–67. doi: 10.1002/jcp.20390. [DOI] [PubMed] [Google Scholar]
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol.Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J.Pharmacol.Exp.Ther. 1999;289:877–885. [PubMed] [Google Scholar]
- Eshleman AJ, Neve RL, Janowsky A, Neve KA. Characterization of a recombinant human dopamine transporter in multiple cell lines. J.Pharmacol.Exp.Ther. 1995;274:276–283. [PubMed] [Google Scholar]
- Fairman WA, Sonders MS, Murdoch GH, Amara SG. Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nat.Neurosci. 1998;1:105–113. doi: 10.1038/355. [DOI] [PubMed] [Google Scholar]
- Geddis MS, Tornieri K, Giesecke A, Rehder V. PLA2 and secondary metabolites of arachidonic acid control filopodial behavior in neuronal growth cones. Cell Motil.Cytoskeleton. 2004;57:53–67. doi: 10.1002/cm.10156. [DOI] [PubMed] [Google Scholar]
- Glaser KB, Mobilio D, Chang JY, Senko N. Phospholipase A2 enzymes: regulation and inhibition. Trends Pharmacol Sci. 1993;14:92–98. doi: 10.1016/0165-6147(93)90071-q. [DOI] [PubMed] [Google Scholar]
- Gledhill JR, Walker JE. Inhibition sites in F1-ATPase from bovine heart mitochondria. Biochem.J. 2005;386:591–598. doi: 10.1042/BJ20041513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HJ, Park SH, Lee JH, Yoon BC, Park KM, Mar WC, Lee HJ, Kang SK. Involvement of oxidative stress in bee venom-induced inhibition of Na+/glucose cotransporter in renal proximal tubule cells. Clin.Exp Pharmacol Physiol. 2002;29:564–568. doi: 10.1046/j.1440-1681.2002.03685.x. [DOI] [PubMed] [Google Scholar]
- Higashijima T, Uzu S, Nakajima T, Ross EM. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins) J Biol.Chem. 1988;263:6491–6494. [PubMed] [Google Scholar]
- Horrobin DF, Glen AI, Vaddadi K. The membrane hypothesis of schizophrenia. Schizophr.Res. 1994;13:195–207. doi: 10.1016/0920-9964(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Amara SG. Arachidonic acid stimulates a novel cocaine-sensitive cation conductance associated with the human dopamine transporter. J Neurosci. 2000;20:550–557. doi: 10.1523/JNEUROSCI.20-02-00550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- L'hirondel M, Cheramy A, Godeheu G, Glowinski J. Effects of arachidonic acid on dopamine synthesis, spontaneous release, and uptake in striatal synaptosomes from the rat. J Neurochem. 1995;64:1406–1409. doi: 10.1046/j.1471-4159.1995.64031406.x. [DOI] [PubMed] [Google Scholar]
- Lee SY, Park HS, Lee SJ, Choi MU. Melittin exerts multiple effects on the release of free fatty acids from L1210 cells: lack of selective activation of phospholipase A2 by melittin. Arch.Biochem.Biophys. 2001;389:57–67. doi: 10.1006/abbi.2001.2314. [DOI] [PubMed] [Google Scholar]
- Lefkowitz LJ, Smith WJ. Sulfur mustard-induced arachidonic acid release is mediated by phospholipase D in human keratinocytes. Biochem.Biophys.Res.Commun. 2002;295:1062–1067. doi: 10.1016/s0006-291x(02)00811-2. [DOI] [PubMed] [Google Scholar]
- Limbird LE. Cell Surface Receptors: a short course on theory and methods. Springer Science + Business Media, Inc.; 2005. [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol. 2002;61:436–445. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- MacDonell LE, Skinner FK, Ward PE, Glen AI, Glen AC, Macdonald DJ, Boyle RM, Horrobin DF. Increased levels of cytosolic phospholipase A2 in dyslexics. Prostaglandins Leukot.Essent.Fatty Acids. 2000;63:37–39. doi: 10.1054/plef.2000.0189. [DOI] [PubMed] [Google Scholar]
- Mahaney JE, Kleinschmidt J, Marsh D, Thomas DD. Effects of melittin on lipid-protein interactions in sarcoplasmic reticulum membranes. Biophys.J. 1992;63:1513–1522. doi: 10.1016/S0006-3495(92)81736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulet Y, Cox JA. Structural changes in melittin and calmodulin upon complex formation and their modulation by calcium. Biochemistry. 1983;22:5680–5686. doi: 10.1021/bi00293a035. [DOI] [PubMed] [Google Scholar]
- Mehvar R, Brocks DR. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J Pharm.Pharm.Sci. 2001;4:185–200. [PubMed] [Google Scholar]
- Muzzio IA, Gandhi CC, Manyam U, Pesnell A, Matzel LD. Receptor-stimulated phospholipase A(2) liberates arachidonic acid and regulates neuronal excitability through protein kinase C. J Neurophysiol. 2001;85:1639–1647. doi: 10.1152/jn.2001.85.4.1639. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Hirabayashi T, Someya A, Shimizu M, Murayama T. Inhibition of arachidonic acid release and cytosolic phospholipase A2 alpha activity by D-erythrosphingosine. Eur.J Pharmacol. 2004;484:9–17. doi: 10.1016/j.ejphar.2003.10.053. [DOI] [PubMed] [Google Scholar]
- Neve KA, Henningsen RA, Bunzow JR, Civelli O. Functional characterization of a rat dopamine D-2 receptor cDNA expressed in a mammalian cell line. Mol Pharmacol. 1989;36:446–451. [PubMed] [Google Scholar]
- Palomba L, Bianchi M, Persichini T, Magnani M, Colasanti M, Cantoni O. Downregulation of nitric oxide formation by cytosolic phospholipase A2-released arachidonic acid. Free Radic.Biol.Med. 2004;36:319–329. doi: 10.1016/j.freeradbiomed.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee SH, Son DJ, Oh KW, Kim KH, Song HS, Kim GJ, Oh GT, Yoon DY, Hong JT. Antiarthritic effect of bee venom: inhibition of inflammation mediator generation by suppression of NF-kappaB through interaction with the p50 subunit. Arthritis Rheum. 2004;50:3504–3515. doi: 10.1002/art.20626. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr. Quantitative approaches to in vivo brain microdialysis. Crit Rev.Neurobiol. 1994;8:189–220. [PubMed] [Google Scholar]
- Piomelli D. Psychopharmacology: the fourth generation of progress: An official publication of the American College of Neuropsychopharmacology. Lippincott Williams & Wilkins; 1995. Arachidonic Acid. [Google Scholar]
- Piomelli D, Pilon C, Giros B, Sokoloff P, Martres MP, Schwartz JC. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991;353:164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Biosci.Rep. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- Rapp JH, Connor WE, Lin DS, Inahara T, Porter JM. Lipids of human atherosclerotic plaques and xanthomas: clues to the mechanism of plaque progression. J Lipid Res. 1983;24:1329–1335. [PubMed] [Google Scholar]
- Reid MS, Ho LB, Hsu K, Fox L, Tolliver BK, Adams JU, Franco A, Berger SP. Evidence for the involvement of cyclooxygenase activity in the development of cocaine sensitization. Pharmacol Biochem.Behav. 2002;71:37–54. doi: 10.1016/s0091-3057(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Hsu K, Tolliver BK, Crawford CA, Berger SP. Evidence for the involvement of phospholipase A2 mechanisms in the development of stimulant sensitization. J Pharmacol Exp Ther. 1996;276:1244–1256. [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Ronco AM, Moraga PF, Llanos MN. Arachidonic acid release from rat Leydig cells: the involvement of G protein, phospholipase A2 and regulation of cAMP production. J Endocrinol. 2002;172:95–104. doi: 10.1677/joe.0.1720095. [DOI] [PubMed] [Google Scholar]
- Ross BM, Mamalias N, Moszczynska A, Rajput AH, Kish SJ. Elevated activity of phospholipid biosynthetic enzymes in substantia nigra of patients with Parkinson's disease. Neuroscience. 2001;102:899–904. doi: 10.1016/s0306-4522(00)00501-7. [DOI] [PubMed] [Google Scholar]
- Shearman MS, Naor Z, Sekiguchi K, Kishimoto A, Nishizuka Y. Selective activation of the gamma-subspecies of protein kinase C from bovine cerebellum by arachidonic acid and its lipoxygenase metabolites. FEBS Lett. 1989;243:177–182. doi: 10.1016/0014-5793(89)80125-5. [DOI] [PubMed] [Google Scholar]
- Shinoda J, Suzuki A, Oiso Y, Kozawa O. Involvement of phosphatidylcholine hydrolysis by phospholipase D in extracellular ATP-induced arachidonic acid release in aortic smooth muscle cells. Arterioscler.Thromb.Vasc.Biol. 1997;17:295–299. doi: 10.1161/01.atv.17.2.295. [DOI] [PubMed] [Google Scholar]
- Street IP, Lin HK, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- Sugama J, Ohkubo S, Atsumi M, Nakahata N. Mastoparan changes the cellular localization of Galphaq/11 and Gbeta through its binding to ganglioside in lipid rafts. Mol Pharmacol. 2005;68:1466–1474. doi: 10.1124/mol.105.013524. [DOI] [PubMed] [Google Scholar]
- Tomlinson G. Potential misconceptions arising from the application of enzyme kinetic equations to ligand-receptor systems at equilibrium. Can J Physiol Pharmacol. 1988;66:342–349. doi: 10.1139/y88-059. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Lin CL, Rothstein JD, Kavanaugh MP. Arachidonic acid activates a proton current in the rat glutamate transporter EAAT4. J Biol.Chem. 1998;273:17315–17317. doi: 10.1074/jbc.273.28.17315. [DOI] [PubMed] [Google Scholar]
- Vial D, Piomelli D. Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonate-specific phospholipase A2. J Neurochem. 1995;64:2765–2772. doi: 10.1046/j.1471-4159.1995.64062765.x. [DOI] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Cassutti P, Tromba C, Galimberti R, Lecchi P, Racagni G. A role for the arachidonic acid cascade in fast synaptic modulation: ion channels and transmitter uptake systems as target proteins. Adv.Exp Med.Biol. 1992;318:147–158. doi: 10.1007/978-1-4615-3426-6_13. [DOI] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Racagni G. Glutamate uptake is inhibited by arachidonic acid and oxygen radicals via two distinct and additive mechanisms. Mol Pharmacol. 1994;46:986–992. [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res.Brain Res.Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Yang HC, Mosior M, Johnson CA, Chen Y, Dennis EA. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal.Biochem. 1999;269:278–288. doi: 10.1006/abio.1999.4053. [DOI] [PubMed] [Google Scholar]
- Zhang L, Reith ME. Regulation of the functional activity of the human dopamine transporter by the arachidonic acid pathway. Eur.J Pharmacol. 1996;315:345–354. doi: 10.1016/s0014-2999(96)00646-2. [DOI] [PubMed] [Google Scholar]