Abstract
The purpose of this study is to identify invariant natural killer T cells (NKT cells) in cellular infiltrate of human allergic contact dermatitis (ACD) skin challenge sites. Skin biopsy specimens were taken from positive patch test reactions from 10 different patients (9 different allergens) and studied by immunochemistry, real-time PCR, nested PCR, and in situ hybridization to identify NKT cells and the cytokines associated with this cell type. Invariant NKT cells were identified in all the 10 skin biopsy specimens studied, ranging from 1.72 to 33% of the cellular infiltrate. These NKT cells were activated in all cases, as they expressed cytokine transcripts for IFN-γ and IL-4. Invariant NKT cells are present in ACD, regardless of the allergen that triggers the reaction, and are in an activated state. We conclude that innate immunity plays a role in late phases of type IV hypersensitivity reactions and may be responding to self-lipids released during allergic inflammation. These data complement the previous work by other investigators that suggest that NKT cells are important in the early cellular response during primary immune responses to allergens. Herein, it is demonstrated that NKT cells are constantly present during the late elicitation phase of human type IV hypersensitivity reactions.
INTRODUCTION
Contact hypersensitivity (CHS) is an example of a cell-mediated immune response against hapten-self complexes that are presented to the immune system by skin-derived dendritic cells, such as Langerhans cells and dermal dendritic cells, that traffic modified self-molecules to the local lymph node. Upon interactions with skin-associated lymphoid tissue, these hapten-bearing antigen-presenting cells (APCs) educate naïve, conventional TCR α/β-bearing CD4+ and CD8+ T lymphocytes to become allergen-specific memory and effector T lymphocytes (afferent phase of CHS) (Watanabe et al., 2002). During the elicitation phase, allergen-specific T lymphocytes are reactivated in the local lymph nodes and migrate to the challenge site to produce cytokines and cytotoxic damage to the skin, resulting in the clinical signs and symptoms of CHS: erythema, edema, infiltration, and pruritus (Wakem and Gaspari, 2000).
There is a limited amount of information about the nature and the frequency of hapten-reactive lymphocytes in the skin lesions of CHS. In a guinea pig model of CHS, the frequency of allergen-specific T cells was approximately 1% of the infiltrate (McCluskey et al., 1963). Experimental CHS in human subjects with documented allergy to poison ivy indicates that the frequency of hapten-reactive conventional T lymphocytes in the peripheral blood is low (in the range of 1/10,000 clonal frequency) and that there is a relative enrichment of this subset of allergen-reactive T lymphocytes in positive patch test sites (10- to 100-fold increase above the frequency of that found in the peripheral blood) (Kalish and Morimoto, 1989; Kalish, 1990; Kalish and Johnson, 1990). Despite this relative enrichment of allergen-specific T cells in the skin-challenge sites, the frequency of the allergen-specific cells remains very low, despite a variably dense lymphocytic infiltrate that is observed in histologic sections of human CHS (Lachapelle, 1973). The nature of the lymphocytic infiltrate in the elicitation phase of CHS has been presumed to be autoreactive (Kalish, 1991), yet the antigen specificity of such an infiltrate has not been determined, nor has the nature of the self-antigens unmasked during CHS reactions been elucidated.
Natural killer T cells (NKT cells) are a unique subset of lymphocytes that express a TCR α/β, with a restricted repertoire, commonly Vα24 and Vβ11 in humans (Godfrey et al., 2004). They may be double negative for CD4/CD8 or may be single positive for CD4 or CD8. NKT cells recognize glycolipids, both microbial derived, or self glycolipids, presented in the context of CD1d, a class I major histocompatibility complex-like molecule (Sieling, 2000). After activation, NKT cells produce polarizing cytokines such as IFN-γ and/or IL-4 and therefore have the capacity to immunoregulate adaptive immunity (Sieling, 2000). Because human epidermal keratinocytes (KCs) constitutively express CD1d (Fishelevich et al., 2006), we asked the question whether NKT cells contribute to the cellular infiltrate of allergic contact dermatitis (ACD).
Herein, we report that CD1d gene expression is increased in elicitation sites of ACD relative to normal skin. Similarly, both CD161 gene expression and variable region of TCR α chain-24 (Vα24)–junctional region of TCR α chain-Q (JαQ) TCR gene expression in skin lesions of ACD are increased relative to normal skin. Digital image analysis of in situ staining of ACD skin biopsy specimens indicated a variable but significant presence of Vα24+/CD2+ or Vβ11+/CD2+ double-positive NKT cells, at a frequency that ranged from 1.72% to as high as 33% of the total of the T-lymphocyte infiltrate. The genes encoding the Th1 and Th2 polarizing cytokines such as IFN-γ and IL-4 were identified in skin biopsy specimens. Double in situ hybridization (ISH) with the invariant NKT-cell antigen receptor Vα24 or JαQ (green) to identify NKT cells and IFN-γ or IL-4 (red) indicated that NKT cells were, in part, the source of both of these cytokine transcripts. These data indicate that invariant NKT cells are commonly present in the T-lymphocyte infiltrate of ACD and contribute cytokines to the inflammatory reaction. To our knowledge, it is unreported previously that this innate immune cell type plays a role in the immune mechanisms of the height of the elicitation phase of human ACD.
RESULTS
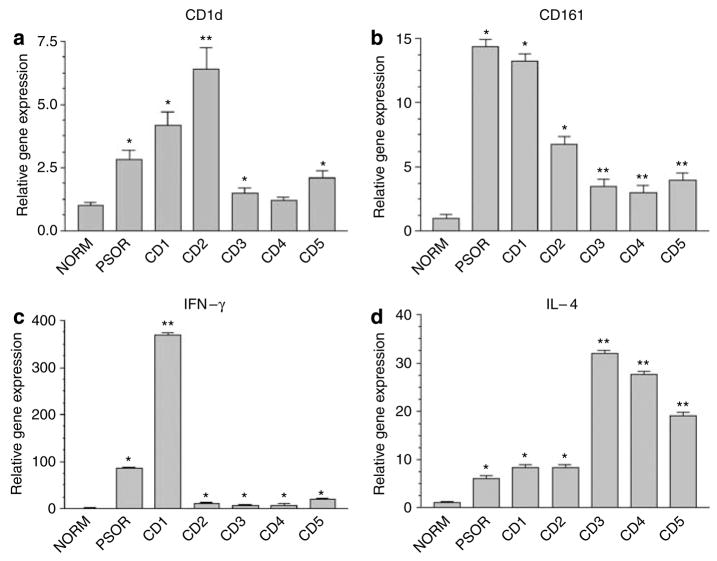
To determine whether NKT cells infiltrated into skin lesions of ACD, gene expression profiles of biopsy specimens from clinically normal skin and ACD sites (see Table 1) were studied utilizing real-time PCR. Compared to paired clinically normal skin, CD1d gene expression was significantly increased in patch test reactions, but varied in the magnitude of the increase in gene expression (ranging from 1.2- to 6.4-fold more than that in normal skin) (Figure 1a). Psoriatic plaque and paired uninvolved skin were utilized as a positive control for the detection of increased CD1d gene expression (Bonish et al., 2000). CD1d gene expression is increased in all skin biopsy specimens of ACD relative to normal skin. Frozen sections of biopsy specimens taken from ACD were stained to confirm the increased expression of CD1d and to determine the cell types that expressed this molecule (data not shown). Consistent with data previously reported on ACD secondary to Rhus antigen (Bonish et al., 2000), there was increased expression of CD1d by most epidermal KCs. These data indicate that increased CD1d expression by KCs and infiltrating bone-marrow-derived cells is a common feature of ACD.
Table 1.
Patients, allergens and studies performed
| Patient no.1 | Age (years)/race/gender | Allergen/scoring/duration2 | Studies3 |
|---|---|---|---|
| CD1 | 36/white/male | Gold/2+/96 h | PCR |
| CD2 | 49/black/female | PPD/2+/48 h | PCR/ICC/FC |
| CD3 | 40/white/male | Balsam of Peru/1+/48 h | PCR |
| CD4 | 53/white/male | Epoxy resin/1+/48 h | PCR/ICC/FC |
| CD5 | 57/white/male | Rhus antigen/3+/72 h | PCR |
| CD6 | 71/white/female | NiSO4/2+/96 h | ISH/ICC/FC |
| CD7 | 63/white/male | Thimersal/2+/96 h | ISH/ICC |
| CD8 | 55/oriental/male | Diazolidinyl urea/2+/48 h | ISH/ICC/FC |
| CD9 | 47/white/male | Epoxy resin/2+/96 h | ISH/ICC/FC |
| CD10 | 26/black/male | Neomycin/2+/48 h | ISH/ICC/FC |
Patient no. refers to contact dermatitis patients no. 1–10.
Scoring refers to North American contact dermatitis group scoring system; duration refers to duration of patch test when skin biopsy specimen was obtained.
Studies refers to PCR, ICC (immunocytochemistry), FC (flow cytometry on peripheral blood).
Figure 1. Gene expression profiling in ACD.
Real-time PCR comparing CD1d gene expression in normal skin (NORM), psoriasis (PSOR) and five cases of ACD (CD1–5). In each case, the biopsy specimen from psoriasis or contact dermatitis was compared to the paired normal control from the same patient. For the sake of simplicity, all six normal skin controls are represented as pooled data (NORM). (a) CD1d. (b) CD161; (c) IFN-γ; (d) IL-4. (*P<0.05; **P<0.001, compared to normal skin, ANOVA). The data depicted represent mean±SD for all real-time PCR experiments).
CD161 is type II membrane glycoprotein that is expressed by most NK cells and NKT cells (Metelitsa, 2004). CD161 gene expression was also studied in psoriatic skin and five ACD specimens. Robust CD161 gene expression was detected in all the five ACD specimens (2.97- to 13.20-fold more than in paired normal skin) (Figure 1b). There was also robust CD161 gene expression in a biopsy specimen from a psoriatic lesion (14.33-fold more than in uninvolved skin).
Previous studies of the cytokine profiles of ACD indicate that cytokine profiles are consistent with a Th1-lymphocyte-dominant, hapten-specific immune response (Watanabe et al., 2002). Our semi-quantitative analysis using real-time PCR analysis of the lymphokine profiles of the elicitation phase of ACD indicated very strong expression of Th1-lymphokine (IFN-γ) transcripts in the psoriasis and CD1 biopsy specimen and significantly lower Th2-lymphocyte (IL-4) transcripts with the remaining four biopsy specimens revealing equivalent Th1–Th2 lymphokine transcripts (CD2) or significantly greater Th2 lymphokine transcripts (CD3–5) (Figure 1c and d). These data suggest that Th1-lymphokine transcript expression in the elicitation phase of ACD is not uniformly a Th1-lymphocyte dominant event. These cytokine analysis profiles were derived from skin biopsy specimens from five different allergens in five different patients. It is not clear whether it is the nature of the allergens or individual patient-related factors that were responsible for the apparent Th1- or Th2-lymphocyte-dominant cytokine profiles in these biopsy specimens.
A nested PCR was used to first detect Vα24 gene expression and then to detect JαQ rearranged gene segment of the T-cell receptor (Norris et al., 1999). This specific segment of the TCR is uniquely detected in invariant human NKT cells. Because this nested PCR represented two rounds of amplifications of cDNA, appropriate control studies were initiated to confirm that the PCR products amplified appropriate cDNA sequences. First, RNA was extracted and cDNA was synthesized from a bulk NKT cell line derived from peripheral blood (Norris et al., 1999) (positive control); cultured monolayers of KCs, fibroblasts, and a transformed Jurkatt T-lymphocyte cell line (a non-NKT cell line) were synthesized as well. All of these latter cells served as a negative control. As expected, the nested PCR only amplified expected PCR products from the NKT cell line, but not from KC, fibroblasts, or transformed Jurkatt T-lymphocytes (Figure 2a). Having demonstrated the specificity of this nested PCR, cDNA from normal skin or paired ACD samples or a psoriasis lesion was amplified. In the psoriasis lesion, but not normal skin, as well as in all the five ACD specimens, the gene encoding the Vα24-JαQ segment was detected (Figure 2b).
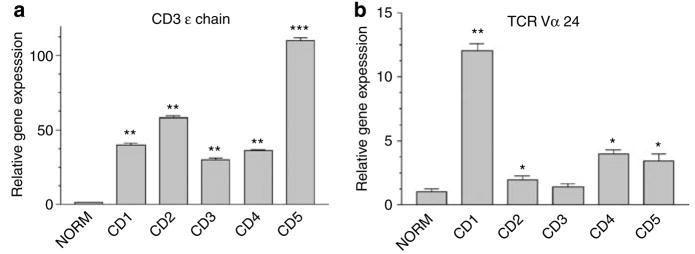
Figure 2. Unique Vα24JαQ NKT-associated TCR gene expression occurs in ACD, but not in normal skin.
Nested PCR to detect unique Vα24-JαQ TCR sequences in skin biopsy specimens. RNA was extracted from an NKT cell line, Jurkat cells, and foreskin KC (FSKC), cDNA was synthesized, subjected to the nested PCR, and run on an agarose gel as described in Methods. (a) Control lane (Ctrl) represents no cDNA added to nested PCR. (b) This same nested PCR was applied to normal skin (NORM), psoriasis (PSOR) and five cases of ACD (CD1–5). (c) Nested PCR from same specimens was subjected to real-time PCR amplification, with SYBR green as the fluorochrome (*P<0.05; **P<0.001; ***P<0.01, compared to normal skin, ANOVA).
The same tissue specimens were studied utilizing real-time PCR and SYBR green to quantify the relative increase in gene expression in psoriasis and ACD relative to the paired normal skin. There was a robust increase in the gene encoding the Vα24-JαQ segment in all cases of ACD (ranging from 2- to 92-fold more than in the paired normal control skin) (Figure 2c). Interestingly, in four out of five ACD specimens, the fold increase was greater than that of the psoriasis lesion (12.7- fold more than in control). These data again confirm that there is infiltration of Vα24-JαQ gene-expressing cells infiltrating into ACD challenge sites.
Next, the PCR data was confirmed by in situ double immunostaining. We performed double staining to confirm that Vα24 was paired with Vβ11 (classical, invariant NKT cells). In all ACD specimens studied, all Vα24-bearing T cells (green stain) co-expressed Vβ11 (red stain), indicating that they were invariant NKT cells (see overlays in Figure 3a and b). Next, we used double staining to estimate the frequency of NKT cells among all infiltrating lymphocytes. There were many more CD2 single positive cells (conventional T cells) in the infiltrate than there were double positive cells (NKT-cells). Using digital image analysis to count single positive (CD2, red) and double positive (CD2, red, and Vα24 or Vβ11, green, resulting in a yellow stain on overlays), the frequency of NKT cells among the total T lymphocyte infiltrate was estimated (Table 2). Among the six biopsy specimens studied, the overall frequency of NKT cells among the total T-cell infiltrate was low, ranging from 1.72% to as high as 33.0% (median value 5.8%). When Vβ11/CD2 double staining was utilized to estimate NKT-cell frequencies in biopsy specimens of the elicitation phase of ACD, on these same specimens, similar NKT-cell frequencies were noted (Table 2). The mean frequency of Vα24/CD2 double-positive cells was not significantly different than the frequency of the Vβ11/CD2 double-positive cells (Table 2), indicating good agreement with the estimation of NKT-cell frequency with the two TCR-specific mAbs. These immunohistochemistry data confirm the PCR data that NKT-cells are present in all cases of ACD studied. In most cases, the frequency was relatively low (CD2, 4, 6, 8, 10), except for CD9, in which the frequency was 33.0%. Flow cytometry of peripheral blood specimens taken at the time of the patch testing confirmed that a localized dermatitis did not affect the frequency of NKT cells (Vα24/CD2 double-positive lymphocytes) in the peripheral blood (Table 2). The comparison of the NKT-cell frequencies in the contact dermatitis sites and the peripheral blood confirm the enrichment of the NKT-cells at these allergic reactions.
Figure 3. Vα24+/Vβ11+ NKT cells are detected in ACD skin biopsy specimens by immunohistochemistry.
Frozen sections from skin-biopsy specimens of ACD were double-stained with anti-Vα24 and Vβ11. (a and b) Double staining of two different biopsy specimens (CD2 and 4) revealed that all Vβ11 (red, top panel)-bearing T cells also expressed Vα24 (green, middle panel); overlay (orange-yellow, bottom panel) (bar=10 μm).
Table 2.
Frequency of NKT cells in the skin and the blood of patients with CHS
| Frequency of NKT in skin1 (%) |
Frequency of NKT in blood2 (%) | ||||
|---|---|---|---|---|---|
| UPN | Allergen | NACDG score/duration (h) | Vα24 | Vβ11 | Vα24 |
| CD2 | Paraphenylene diamine | 2+/48 | 1.72 | 1.50 | <0.1 |
| CD4 | Epoxy resin | 1+/48 | 5.50 | 5.00 | <0.1 |
| CD6 | NiSO4 | 2+/96 | 3.50 | 4.20 | <0.1 |
| CD8 | Diazolidinyl urea | 2+/96 | 5.80 | 7.00 | <0.1 |
| CD9 | Epoxy resin | 2+/48 | 33.00 | 29.00 | <0.1 |
| CD10 | Neomycin | 2+/48 | 4.50 | 6.50 | <0.1 |
| Average frequency in skin lesions (±SD) | 9.00±8.86 | 11.803±10.05 | |||
NACDG, North American contact dermatitis group; UPN, unique patient number.
Frequency of double-positive cells=(frequency of Vα24/CD2 or Vβ11/CD2 double-positive cells/total no. of CD2 single-positive cells)×100.
Estimation by two-color flow cytometry of NKT cells (Vα24/CD2 double-positive lymphocytes) in peripheral blood.
P=0.36, not statistically significant on unpaired t-test.
Next, the relative gene expression of Vα24 (NKT cells) was compared to that of the total T-cell infiltrate (using epsilon chain of the CD3 antigen complex (CD3 ε) as a marker for all TCR-bearing lymphocytes) (Hassan-Zahraee et al., 1998) (Figure 4). Rather than using a nested PCR with two rounds of amplification as in Figure 2, a single round of amplification to identify Vα24 TCR gene expression was utilized, under conditions that were identical to that used to amplify CD3 ε-chain gene (see Materials and Methods). Thus, these semi-quantitative data were comparable. There was robust CD3 ε-chain gene expression in all the five CD skin biopsy specimens compared to paired normal skin (Figure 4a). Vα24 TCR gene expression was also detected (Figure 4b), but at a lower level than that of CD3 ε chain. When comparing the relative abundance of steady-state mRNA of Vα24 to that of CD3 ε in the five biopsy specimens of CD (ratios of relative gene expression Vα24/CD3×100), the range was 3.12–30%, with a median value of 11.11%. This relative abundance of Vα24 mRNA (compared to CD3 ε) is remarkably similar to that of the estimated frequency of NKT cells using double staining (Table 2). These data confirm the presence of NKT cells at varying levels in CD skin lesions at relatively low levels, compared to the total infiltrate of T cells.
Figure 4. CD3 gene expression is more robust than Vα24 expression in ACD skin biopsy specimens.
Real-time PCR was utilized to detect (a) CD3 ε gene expression (a molecular marker for all TCR-bearing T cells) or (b) Vα24 (a molecular marker for NKT cells) using identical methods (single round of PCR amplification, with SYBER green fluorescence dye for real-time PCR detection. (*P<0.05; **P<0.01; ***P<0.001, compared to normal skin, ANOVA).
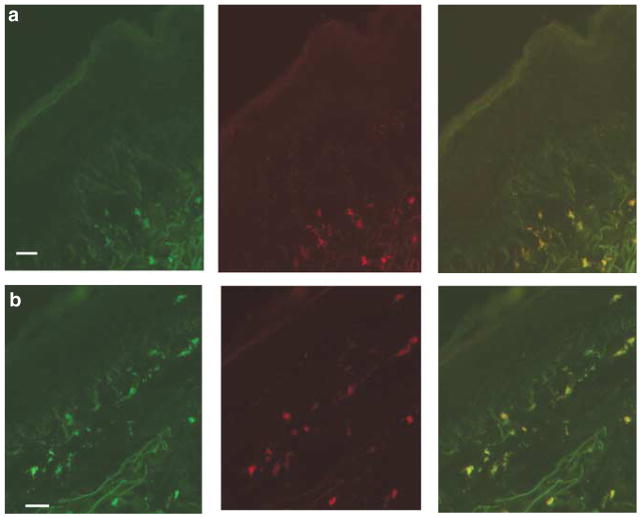
We studied the cytokine gene expression in NKT cells utilizing ISH with fluorescent-labeled oligoprobes to detect Vα24 gene expression (fluorescein labeled) and oligoprobes to detect IFN-γ (rhodamine labeled) and/or IL-4 expression (rhodamine labeled). Thus, frozen sections were double-hybridized with Vα24 (green probe) to identify NKT cells as well as IFN-γ (red probe) or IL-4 (red probe). Biopsy samples from five ACD elicitation skin sites were studied. As demonstrated in Figure 5, NKT cells (as defined by Vα24 gene expression by in situ labeling) expressed transcripts for both IFN-γ (Figure 5a) and IL-4 (Figure 5b). There was a remarkable consistency in the expression of both cytokine transcripts (IFN-γ and IL-4) in NKT cells from all the five specimens studied (data not shown). These data indicate that NKT cells are in an activated state in skin lesions of the elicitation phase of ACD.
Figure 5. Infiltrating NKT cells in ACD express both transcripts for the cytokines IFN-γ and IL-4.
ACD skin-biopsy specimens studied by ISH: double-labeled with antisense oligoprobes for Vα24 (left panel, green; middle panel, cytokine (a) (IFN-γ) or (b) IL-4 (red); right panel, overlays) (bar=10 μm).
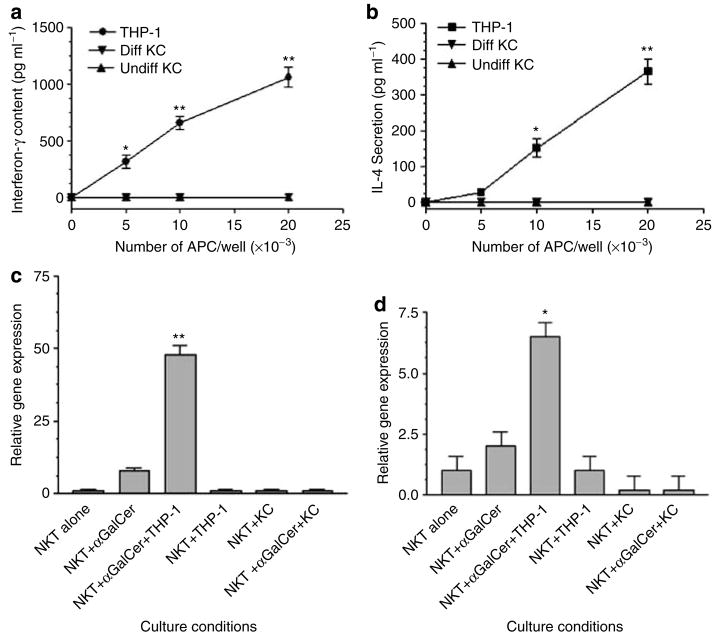
Our in situ immunostaining of CD1d expression in ACD elicitation sites indicated that epidermal KCs as well as infiltrating mononuclear cells expressed CD1d, providing the potential to present glycolipids to NKT cells in the skin and to trigger cytokine secretion. Therefore, we studied antigen presentation by CD1d-bearing KCs as well as CD1d bone-marrow- derived cells. NKT cells incubated with KCs and α-galactosylceramide did not secrete either IFN-γ or IL-4 as measured by a sensitive ELISA (Figure 6a). In contrast, THP-1, a monocyte-derived cell line, triggered robust IFN-γ secretion (Figure 6a) as well as significant IL-4 secretion (Figure 6b). To confirm the ELISA data, in parallel, we extracted total cellular RNA from NKT cells in this experiment and assayed IFN-γ and IL-4 gene expression using real-time PCR (Figure 6c and d). This sensitive PCR confirmed that NKT cells were triggered to activate IFN-γ and IL-4 gene expression in the presence of CD1d+ monocyte cell line and α-galactosylceramide, but not by CD1d-bearing KCs. Thus, our observation that NKT cells infiltrating in ACD lesions expressed both IFN-γ and IL-4 transcripts suggests that these cells are likely to be activated by professional APCs, rather than KCs, which fail to trigger cytokine production, and may result in a state of immune tolerance.
Figure 6. CD1d+ Monocytic APCs, but not CD1d+ KC, induce cytokine gene expression and secretion by NKT-cells in vitro.
A polyclonal NKT cell line was cultured with the CD1d+ cell line, THP-1 or CD1d+ KC (differentiated or undifferentiated, and treated with IFN-γ, see Methods) in the presence (a and b) or absence (data not shown) of α-galactosylceramide for 24 hours, and supernatants were harvested for specific luminex assay. (a) IFN-γ; (b) IL-4. In another experiment, the NKT cells were isolated after 6 hours of incubation with the above cell lines, and RNA was extracted, cDNA was synthesized, and gene expression was studied by (c) real-time PCR IFN-γ; (d) IL-4. (*P<0.05; **P<0.001, compared to control, unstimulated NKT cells, ANOVA).
DISCUSSION
Previous studies of the role of NKT cells in CHS have focused on experimental mouse models, using inbred mice and strong, Th1-lymphocyte-polarizing haptens such as oxazalone as the sensitizing agent (Askenase, 2001; Campos et al., 2003; Stein-Streilein, 2003; Nieuwenhuis et al., 2005). These studies indicated a role for this cell type in the early molecular events of CHS, in which NKT cells respond to hapten application and interact with B-1 lymphocytes, resulting in production of hapten-specific IgM (Campos et al., 2003). Another animal model study confirmed the role of NKT cells in murine CHS with impaired contact sensitization in Vα14 gene-targeted mice and examined the therapeutic role of antagonistic lipids that bound to CD1d but do not activate NKT cells (Nieuwenhuis et al., 2005). Such antagonistic lipids, when administered topically to mouse skin or when administered systemically, interfered with both the afferent and efferent phases of CHS (Nieuwenhuis et al., 2005), suggesting that inhibition of CD1d-antigen-presenting pathway to NKT cells interfered with both phases of CHS. A direct inhibitory effect on CD1d-dependent presentation of glycolipids to NKT cells was not demonstrated.
In this study, we demonstrate that human invariant NKT cells regularly appear in the lymphocytic infiltrate that occurs during the elicitation phase of human ACD, either at the 48 or 96 hours time frame of clinical readings of this skin reaction. Immunohistochemistry (Figure 3) as well as real-time PCR and a nested PCR confirmed the presence of NKT cells in the cellular infiltrate of ACD (Figure 2). In healthy adults, NKT cells are present at a very low frequency in the peripheral blood, approximately 0.1% of the total population of peripheral blood leukocytes (Lee et al., 2002). Considering that we demonstrated the frequency of NKT cells in the ACD lesions to range from 1.72 to as high as 33% of the total CD2+ infiltrating T lymphocytes, this represents a relative enrichment of NKT cells ranging from a 17- to 330-fold enrichment above the normally very low frequency found in the peripheral blood. This suggests that NKT cells may be specifically recruited into ACD sites, rather than a passive extravasation event, in which relative enrichment of frequency relative to other cell types would be expected not to occur. This recruitment of NKT cells into positive patch test reactions is remarkably similar to what has been reported for the recruitment of urushiol-specific conventional T lymphocytes into positive urushiol patch tests (10- 100-fold increase in the clonal frequency of allergen-reactive T cells above that found in the peripheral blood) (Kalish and Morimoto, 1989; Kalish, 1990; Kalish and Johnson, 1990). An alternative explanation for the increase in NKT cells would be a local expansion of this cell type.
Our immunohistochemical studies of normal skin did not detect Vα24-bearing cells in normal skin (data not shown). Considering that normal human skin is a rich source of peripheral T lymphocytes (Clark et al., 2006), our observation indicates that NKT cells play a special surveillance role to be mobilized into, but not to reside in, normal human skin. Our finding of NKT cells in the elicitation sites of ACD is a remarkably constant event that occurred in the skin from 10 different volunteers in response to 9 different allergens derived from naturally occurring percutaneous exposures that resulted in a symptomatic allergic state (CHS). These observations in humans demonstrate the existence of NKT cells in CHS, which complements the experimental murine model, in which a limited number of strong, experimental haptens were studied using inbred mice.
We utilized psoriasis as a disease control, because a number of studies have demonstrated the presence of NKT cells in the cellular infiltrate of this disease, mainly in the dermis, but also in the epidermis of mature skin lesions or at the leading edge of developing skin lesions (Cameron et al., 2002; Vissers et al., 2004; Bovenschen et al., 2005; Liao et al., 2006; Ottaviani et al., 2006). Estimated frequencies indicated that cells bearing NKT markers may be present in as high as 10% of total CD3+ T lymphocytes (Liao et al., 2006). Activated NKT cells can be injected into symptomless skin from psoriasis patients that had been transplanted onto severe combined immunodeficient mice, and they induce the clinical and histologic features of psoriasis (Nickoloff et al., 1999). These data suggest that NKT cells can be pathogenic in this common skin disease. A number of studies have indicated that NKT cells are present in inflammatory infiltrate of both early and mature lesions of psoriasis (Cameron et al., 2002; Vissers et al., 2004; Bovenschen et al., 2005; Liao et al., 2006; Ottaviani et al., 2006). Our studies of the elicitation phase of ACD indicate that a similar persistent population of NKT cells is commonly present. These data complement previous studies that suggested that NKT cells play an indispensable role in the early molecular events (within hours) in the afferent phase of CHS (Campos et al., 2003).
Our frequency analysis of NKT cells indicated that the frequency of NKT cells in ACD may be similar to that previously reported in psoriasis (Liao et al., 2006). Considering the proposed pathogenic role of NKT cells in psoriasis (Nickoloff et al., 1999), and the striking parallels we have identified in ACD with psoriasis (presence, distribution, and frequency of NKT cells in lesions), it is worth considering that NKT cells contribute to the pathophysiology of the elicitation phase of human CHS. Previous studies suggested that NKT cells played an indispensable role in the early molecular events of CHS (Campos et al., 2003). Our data indicate that NKT cells play an important role in the efferent phase of CHS, even at later phases of this allergic inflammation (NKT cells being present in the skin of elicitation sites at either the 48 or 96 hours readings).
Analysis of cytokine gene expression in five ACD specimens indicated the presence of both IFN-γ and IL-4 in all specimens studied, with variability in the relative increases in IFN-γ and IL-4 among the five specimens. Both of these cytokines have been demonstrated to play an important role in the pathophysiology of ACD. For IFN-γ, previous studies have focused hapten-specific Th1 and Tc1-mature, peripheral TCR α/β-bearing lymphocytes as the source of these cytokines in local lymph nodes and skin (Xu et al., 1996, 1997; Watanabe et al., 2002).
For IL-4, there have been conflicting data concerning the role of this cytokine in experimental mouse systems. In IL-4 gene-targeted mice, there is impaired ACD to certain haptens (Weigmann et al., 1997; Traidl et al., 1999). Other haptens, such as fluorescein isothiocyanate, elicit a Th2-dominant response (Tang et al., 1996). Additionally, sensitization with strong Th1-polarizing haptens followed by repeated elicitations over time results in a transition from the initial strong Th1-lymphocyte responses to that of a Th2-dominant immune response (Kitagaki et al., 1997). Studies of the gene expression profiles in the elicitation phase of human ACD to urushiol have indicated that there is both IFN-γ and IL-4 gene expression in the skin (Ryan and Gerberick, 1999).
Our cytokine profiles are consistent with these observations, in that we observed both IFN-γ and IL-4 in all the specimens studied (Figure 1). Our ISH studies (Figure 5) indicate that NKT cells contribute to this cytokine profile, in that we detected both IFN-γ and IL-4 gene expression in the infiltrating NKT cells in the elicitation phase of ACD. Our in vitro studies with NKT cells indicate that only professional APCs (CD1d+ THP-1 monocyte cell line) were able to trigger both cytokines, but IFN-γ secretion and gene expression were much more robust than the low-level IL-4 gene expression and cytokine secretion (Figure 6a–d). KCs did not trigger cytokine secretion or cytokine gene expression by NKT cells.
Correlation of in vitro studies of NKT-cell activation and their in situ gene expression suggests that NKT cells have been activated by professional APCs, because they express both IFN-γ and IL-4 transcripts, which is identical to the pattern observed when NKT cells are activated by professional APCs in vitro (Figure 6c and d). The inability of KCs to trigger cytokine production by NKT cells is remarkably similar to their inability to trigger cytokine secretion and clonal proliferation by conventional TCR α/β-bearing Th1 lymphocytes (Gaspari and Katz, 1988).
Future studies will determine whether KCs induce NKT-cell clonal anergy as has been demonstrated for CD4+ Th1 memory lymphocytes (Gaspari et al., 1988). In ACD skin biopsy specimens, the ISH studies of cytokine mRNA expressed by Vα24+ NKT cells indicated that these cells were not anergic because they expressed both IFN-γ and IL-4 transcripts. This suggests that professional CD1d+ APCs may have activated NKT cells rather than tolerogenic CD1d+ KCs.
These data indicate that NKT cells represent a significant component of the lymphocytic infiltrate of CHS, because they were detected in elicitation sites of all the 10 skin biopsy specimens. Furthermore, NKT cells contribute to the cytokine gene expression profiles generated in ACD, of both IFN-γ and IL-4. The nature of the endogenous glycolipids released/generated during the elicitation phase of ACD remains to be determined. Previous investigators have hypothesized that the lymphocytes migrating into skin lesions of ACD were autoreactive T lymphocytes (Kalish, 1991).
Our data are consistent with this hypothesis, in that NKT cells have been demonstrated to react to self-lipids, and thus they may be reacting to self-glycolipids released during ACD.
MATERIALS AND METHODS
RNA extraction, cDNA synthesis, and real-time PCR (RNeasy Mini Protocol, Qiagen, Valencia, CA)
Skin biopsy specimens from paired clinically normal skin and elicitation sites of ACD were snap-frozen in liquid nitrogen and stored at −70°C until RNA extraction. RNA was extracted and cDNA was prepared, using previously published methods (Fishelevich et al., 2006). Real-time PCR (LightCycler, Roche, Indianapolis, IN) was used to confirm differences in the levels of expression of selected mRNA. The primers, PCR protocol, and product quantification for 18S ribosomal RNA were exactly as reported previously (SYBER green detection) (Schmittgen and Zakrajsek, 2000). All other primers and hybridization probes were designed and prepared by TIB Molbiol (Adelphia, NJ). The hybridization probes were labeled with fluorescein at the 5′terminus (3FL) and with LightCycler Red at the 5′terminus of the other probe. Amplification (40–50 cycles) of a single PCR product was confirmed by gel electrophoresis and melting curve analyses. The sequences of the sense and antisense and internal hybridization probes are available as Supplementary materials. The cDNAs were assayed in duplicate, and mean±SD values are depicted in the graphics for the quantitative PCR.
PCR
Detection of CD1d mRNA was performed using RT-PCR. Sequences of the primers were as follows: exon 2—sense, 5′-CTG CAG ATC TCG TCC TTC GCC AAT-3′; exon 3—antisense, 5′-TTG AAT GGC CAA GTT TAC CCA AAG-3′. These primers amplified a 400 bp product using an annealing temperature of 55°C and 35 cycles of PCR. The PCR products were run on an agarose gel and photographed. These primers have been demonstrated to be specific for CD1d and do not amplify CD1a, CD1b, or CD1c (Bonish et al., 2000).
Analysis of Vα24-JαQ TCR gene expression
The expression of Vα24-JαQ TCR gene was performed using a nested PCR amplification technique exactly as described previously (Norris et al., 1999). In Figure 4, the second round of amplification with the Vα24-JαQ oligonucleotide primers was eliminated. The amplification proceeded for 40 cycles of amplification, which was identical to the conditions utilized to amplify CD3 ε chain (see above for the sequence of oligonucleotide primers).
ISH
ISH was utilized to detect cytokine gene expression by NKT cells. This was accomplished with a double hybridization procedure: a green probe to detect Vα24 gene expression and a red probe to identify IFN-γ or IL-4 expression. Thus, double-labeled cells (yellow color) resulted from double hybridization with the dual probe sets.
Gene expression was detected using GeneDetect (Auckland, New Zealand) oligonucleotide probes directly labeled with Green-Star* fluorescent dyes. For ISH, 16 μm frozen sections were cut on a cryostat and mounted onto poly-L-lysine-coated slides. The slide sections were fixed in 4% paraformaldehyde, then washed with 0.1 M phosphate buffer, and then the slides were incubated with 100% ethyl alcohol for 5 minutes. Hybridization buffer (20× SSC (solution of NaCl and Na citrate), dextran, and formamide) were mixed and heated to 37°C, and hybridization probes were added (at a concentration of 400 ng ml−1; except for polyDT, which was at 200 ng ml−1). The slides were then incubated with the probe (approximately 50 μl per slide section) and the tissue sections were covered with parafilm and incubated in a plastic chamber overnight at 37°C (approximately 18 hours). Post-hybridization washes were done with 1× SSC and subsequent washes with 0.5× SSC. The sections were then rinsed with distilled, deionized H2O and vectashield (Vector labs) was added as an antifading agent. The slides were examined under a fluorescent microscope and photographed. The sequences of the ISH Probes are available as Supplementary materials.
Specificity controls were as follows: (1) RNase digestion of the tissues before ISH prevented polyDT or specific probe hybridization; (2) sense probes were utilized and did not bind to the tissue; (3) 10× unlabeled antisense probe blocked the hybridization of the fluorescent labeled antisense probe. Before studying the expression of Vα24, IFN-γ, or IL-4, all the tissue sections were probed with polyDT (either FITC or Rhodamine) to assure the integrity of the RNA in the tissue specimens.
mAbs
The following mAbs were used for the immunohistochemical staining of frozen sections or for flow cytometry: CD1d (Nor 3.2, Biosource, Camarillo, CA), CD2 (RPA-2.1, BD Pharmingen, San Diego, CA), CD161 (DX12, Ancell-Alexis Biochemicals, San Diego, CA), Vα24 (C15, Immunotech, Fullerton, CA), Vβ11 (C21, Immunotech, Fullerton, CA), HLA-DR (TU36, BD Pharmingen, San Diego, CA). Cryostat sections (5 μm thick) of skin biopsy specimens were processed and stained as described previously (Fishelevich et al., 2006). For the two-color staining of the Vα24 and Vβ11 expression, the Vβ11 antibody (C21, mouse IgG2a) was followed by goat antimouse IgG rhodamine-conjugated, followed by 10% normal mouse serum as a blocking agent. This was followed by the Vα24 antibody (C15, IgG1), followed by a specific biotinylated goat anti-mouse IgG1 and then by 1:100 dilution of streptavidin-conjugated FITC. Appropriate combinations of relevant antibodies and secondary antibodies or secondary antibodies alone demonstrated that the immunostaining was specific (not shown). The stained slides were examined with a Nikon Eclipse E600 epifluorescence microscope equipped with a digital camera (RT-spot slider, Diagnostic Instruments Inc., Sterling Heights, MI) and photographed at the indicated magnifications.
Flow cytometry
For flow cytometry of peripheral blood mononuclear cells, blood was drawn and centrifuged over lymphoprep using standard methods (Fishelevich et al., 2006). The stained cell suspensions were then analyzed by a Coulter EPICS Elite ESP flow cytometer. Viable leukocytes were identified by forward and side-scatter properties, with dead cells being excluded from analysis. A total of 100,000 events from each sample were analyzed. NKT cells were defined as double-positive cells (Vα24+/CD2+).
NKT-cell assay
A highly enriched cell line (>98% Vα24 positive) was developed using previously published methods (Motsinger et al., 2002). Cultured KCs were derived from foreskins and cultured in serum-free medium containing low (0.05mM) or high (1.20mM) CaCl2. KC culture was performed as published previously (Fishelevich et al., 2006). Twenty-four hours before co-culture with NKT cells, the KC monolayers were treated with IFN-γ (500U ml−1) to increase CD1d cell-surface expression. The KCs were trypsinized into single-cell suspensions and co-cultured with NKT cells at the indicated numbers (Figure 5) in a 96-well, round-bottomed plate in a 5% CO2 atmosphere at 37°C for 24 hours in RPMI 1,640 with 10% fetal calf serum. After 24 hours, cell-free culture supernatants were collected and were frozen at −20°C until a specific cytokine immunoassay was performed. A luminex-based assay was utilized to determine IFN-γ or IL-4 concentrations in culture supernatants (Pickering et al., 2002).
Human subjects
Human subjects being evaluated for contact dermatitis were recruited from our clinical practice. The criteria for enrollment of subjects in this study was the presence of a 1–2+ score (North American contact dermatitis group scoring system) of the patch test allergic reaction at 48 or 96 hours clinical readings (Belsito, 2004). Informed consent was obtained, and skin biopsy specimens were obtained from paired clinically normal skin and the patch test reaction sites. This study was approved by the University of Maryland Baltimore Institutional review board, and adhered to the Declaration of Helsinki Principles. The patients, allergens, patch test scoring, timing of obtaining the skin biopsy specimens from the patch test skin reactions, and studies performed on such specimens are summarized in Table 1. One patient with plaque-type psoriasis was studied as a disease control. A skin biopsy specimen was taken from uninvolved, clinically normal skin as well as the edge of a psoriatic plaque.
Statistical analyses
Quantitative data were analyzed for statistically significant differences between control and treatment groups using the GraphPad Instat Software Program (GraphPad Software, San Diego, CA). Because multiple comparisons were examined, a one-way ANOVA (Tukey Kramer multiple analyses) or unpaired Students’ t-test for a simple analysis of two means were applied to the quantitative data (P<0.05 were considered significant).
Abbreviations
- ACD
allergic contact dermatitis
- APC
antigen-presenting cell
- CD no
contact dermatitis no. (unique identifier for each of the 10 patients in this study)
- CD3 ε
epsilon chain of the CD3 antigen complex
- CHS
contact hypersensitivity
- ISH
in situ hybridization
- Jα
junctional region of TCR α chain
- KC
keratinocyte
- NK cells
natural killer cells
- NKT cells
natural killer T cells
- SSC
solution of NaCl and Na citrate
- Vα
variable region of TCR α chain
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary Materials and Methods.
References
- Askenase PW. Yes T-cells, but three different T-cells (alpha/beta, gamma/delta and NKT-cells), and also B-1 cells mediate contact hypersensitivity. Clin Exp Immunol. 2001;125:345–50. doi: 10.1046/j.1365-2249.2001.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsito DV. Patch testing with a standard allergen (“screening”) tray: rewards and risks. Dermatol Ther. 2004;17:231–9. doi: 10.1111/j.1396-0296.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- Bonish B, Julien D, Dutroac Y, Huang BB, Modlin R, Spada FM, et al. Overexpression of CD1d by KC in psoriasis and CD1d-dependent IFN-γ production by NKT-cells. J Immunol. 2000;165:4076–85. doi: 10.4049/jimmunol.165.7.4076. [DOI] [PubMed] [Google Scholar]
- Bovenschen HJ, Van De Kerkhof PCM, Gerritsen WJ, Seyger MMB. The role of lesional T-cells in recalcitrant psoriasis during infliximab therapy. Eur J Dermatol. 2005;15:454–8. [PubMed] [Google Scholar]
- Cameron AL, Kirby B, Fei W, Griffiths CEM. Natural killer and natural killer-T cells in psoriasis. Arch Derm Res. 2002;294:363–9. doi: 10.1007/s00403-002-0349-4. [DOI] [PubMed] [Google Scholar]
- Campos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, et al. Cutaneous immunization rapidly activates liver invariant Va14 NKT-cells stimulating B-1 B-cells to initiate recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785–96. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–9. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Fishelevich R, Malanina A, Luzina I, Atamas S, Smyth MJ, Porcelli SA, et al. Ceramide-dependent regulation of human epidermal keratinocyte CD1d expression during terminal differentiation. J Immunol. 2006;176:2590–9. doi: 10.4049/jimmunol.176.4.2590. [DOI] [PubMed] [Google Scholar]
- Gaspari AA, Katz SI. Induction and functional characterization of class II MHC (Ia) antigens on murine keratinocytes. J Immunol. 1988;140:2956–63. [PubMed] [Google Scholar]
- Gaspari AA, Jenkins MK, Katz SI. Class II MHC-bearing keratinocytes induce antigen-specific unresponsiveness in hapten-specific TH1 clones. J Immunol. 1988;141:2216–20. [PubMed] [Google Scholar]
- Godfrey DHL, MacDonald HR, Kronenberg M, Smyth MJ, VanKaer L. NKT-cells: what’s in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Hassan-Zahraee M, Wu Jiangping W, Gordon J. Rapid synthesis of IFN-γ by T-cells in skin may play a pivotal role in the human skin immune system. Int Immunol. 1998;10:1599–612. doi: 10.1093/intimm/10.11.1599. [DOI] [PubMed] [Google Scholar]
- Kalish R, Johnson K. Enrichment and function of urushiol (poison ivy)-specific T-cells in lesions of allergic contact dermatitis to urushiol. J Immunol. 1990;145:3706–13. [PubMed] [Google Scholar]
- Kalish RS. The use of human T-lymphocyte clones to study T-cell function in allergic contact dermatitis to urushiol. J Invest Dermatol. 1990;94(Suppl 6):108S–11S. doi: 10.1111/1523-1747.ep12876061. [DOI] [PubMed] [Google Scholar]
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558–63. [PubMed] [Google Scholar]
- Kalish RS, Morimoto C. Quantitation and cloning of human urushiol specific peripheral blood T-cells: isolation of urushiol triggered suppressor T-cells. J Invest Dermatol. 1989;92:46–52. doi: 10.1111/1523-1747.ep13070998. [DOI] [PubMed] [Google Scholar]
- Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a Th1 to a Th2 cell profile. J Immunol. 1997;159:2484–91. [PubMed] [Google Scholar]
- Lachapelle JM. Comparative histopathology of allergic and irritant patch test reactions in man. Current concepts and new prospects. Arch Belg Dermatol Syphiligr. 1973;29:83–92. [PubMed] [Google Scholar]
- Lee PT, Putnam A, Benlagha K, Teyton L, Gottleib PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YH, Jee SH, Sheu BC, Huang YL, Tseng MP, Hsu SM, et al. Increased expression of the NK inhibitory receptor CD94/NKG2A and CD158b on circulating and lesional T-cells in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:318–24. doi: 10.1111/j.1365-2133.2006.07301.x. [DOI] [PubMed] [Google Scholar]
- McCluskey RT, Benacerraf B, McCluskey J. Studies on the specificity of the cellular infiltrate in delayed sensitivity reactions. J Immunol. 1963;90:466. [PubMed] [Google Scholar]
- Metelitsa LS. Flow cytometry for natural killer T cells: multi-parameter methods for multifunctional cells. Clin Immunol. 2004;110:267–76. doi: 10.1016/j.clim.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002;195:869–79. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis EES, Gillessen S, Scheper RJ, Exley MA, Taniguchi M, Balk SP, et al. CD1d and CD1d-restricted iNKT-cells play a pivotal role in contact hypersensitivity. Exp Dermatol. 2005;14:250–8. doi: 10.1111/j.0906-6705.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Wrone-Smith T, Bonish B, Porcelli SA. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes. Arch Dermatol. 1999;135:546–52. doi: 10.1001/archderm.135.5.546. [DOI] [PubMed] [Google Scholar]
- Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gamma/delta T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Nasorri F, Bedini C, De Pita O, Girolomoni G, Cavani A. CD56 bright CD16- NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–28. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- Pickering JW, Martins TB, Greer RW, Schroder MC, Astill ME, Litwin CM, et al. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am J Clin Pathol. 2002;117:589–96. doi: 10.1309/lmch-c4q2-vfl9-3t1a. [DOI] [PubMed] [Google Scholar]
- Ryan CA, Gerberick GF. Cytokine mRNA expression in human epidermis after patch treatment with rhus and sodium lauryl sulfate. Am J Contact Dermat. 1999;10:127–35. [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/s0165-022x(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Sieling PA. CD1 restricted T-cells: T-cells with a unique immunological niche. Clin Immunol. 2000;96:3–10. doi: 10.1006/clim.2000.4863. [DOI] [PubMed] [Google Scholar]
- Stein-Streilein J. Invariant NKT-cells as initiators, licensors, and facilitators of the adaptive immune response. J Exp Med. 2003;198:1779–83. doi: 10.1084/jem.20031946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Judge TA, Nickoloff BJ, Turka LA. Suppression of murine allergic contact dermatitis by CTLA-4/Ig. tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J Immunol. 1996;157:117–25. [PubMed] [Google Scholar]
- Traidl C, Jugert F, Krieg T, Merk H, Hunzelmann N. Inhibition of allergic contact dermatitis to DNCB but not to oxazolone in IL-4-deficient mice. J Invest Dermatol. 1999;112:476–82. doi: 10.1046/j.1523-1747.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- Vissers WHPM, Arndtz CHM, Muys L, Van Erp PEJ, De Jong EMG, Van De Kerkhof PCM. Memory effector and cytotoxic T-cells appear early in the margin zone of spreading psoriatic lesions in contract to cells expressing natural killer receptors, which appear late. Br J Dermatol. 2004;150:852–9. doi: 10.1111/j.1365-2133.2004.05863.x. [DOI] [PubMed] [Google Scholar]
- Wakem PW, Gaspari AA. Mechanisms of allergic and irritant contact dermatitis. In: Kydonieus AF, Wille JJ, editors. Biochemical modulation of skin reactions in transdermal and dermal drug delivery. CRC Press; Boca Raton, FL: 2000. pp. 83–106. [Google Scholar]
- Watanabe H, Unger M, Tuvel B, Wang B, Sauder DN. Contact hypersensitivity: the mechanism of immune responses and T-cell balance. J Interferon Cytokine Res. 2002;22:407–12. doi: 10.1089/10799900252952181. [DOI] [PubMed] [Google Scholar]
- Weigmann B, Schwing J, Huber H, Ross R, Mossman H, Knop J, et al. Diminished contact hypersensitivity response in IL-4 deficient mice at a late phase of the elicitation reaction. Scand J Immunol. 1997;45:308–14. doi: 10.1046/j.1365-3083.1997.d01-402.x. [DOI] [PubMed] [Google Scholar]
- Xu H, Banerjee A, Dilulio NA, Fairchild RL. Development of effector CD8+ T-cells in contact hypersensitivity occurs independently of CD4+ T-cells. J Immunol. 1997;158:4721–8. [PubMed] [Google Scholar]
- Xu H, Dilulio NA, Fairchild RL. T-cell populations primed by hapten sensitization in contact hypersensitivity are distinguished by polarized patters of cytokine production: IFN-γ producing Tc1 effector CD8+ T-cells and IL-4/10 producing (Th2) negative regulatory CD4+ T-cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]