Abstract
Twenty nine Amaryllidaceae alkaloids and their derivatives belonging to five most common groups, including lycorine-, lycorenine-, tazettine-, crinine-, and narciclasine-types, were evaluated for antiproliferative, apoptosis inducing and antiinvasive activities in vitro. The antiproliferative properties of each test compound are in agreement with those reported in the literature, while the high potency of amarbellisine is reported for the first time. It was also found that with the exception of ungeremine, amarbellisine and hippeastrine, the antiproliferative effect of the potent compounds is apoptosis-mediated. Thus, apoptosis in Jurkat cells was triggered by narciclasine, narciclasine tetraacetate, C10b-R-hydroxypancratistatin, cis-dihydronarciclasine, trans-dihydronarciclasine, lycorine, 1-O-acetyllycorine, lycorine-2-one, pseudolycorine, and haemanthamine. With the exception of narciclasine, lycorine and haemanthamine, the apoptosis inducing properties of these compounds are reported for the first time. The collagen type I invasion assay revealed potent antiinvasive properties associated with N-methyllycorine iodide, hippeastrine, clivimine, buphanamine, and narciclasine tetraacetate, all of which were tested at non-toxic concentrations. The antiinvasive activity of buphanamine is particularly promising since this alkaloid is not toxic to cells even at much higher doses. This work has resulted in identification of several novel leads for anticancer drug design.
Keywords: Amaryllidaceae, alkaloid, apoptosis, invasion, drug lead
Introduction
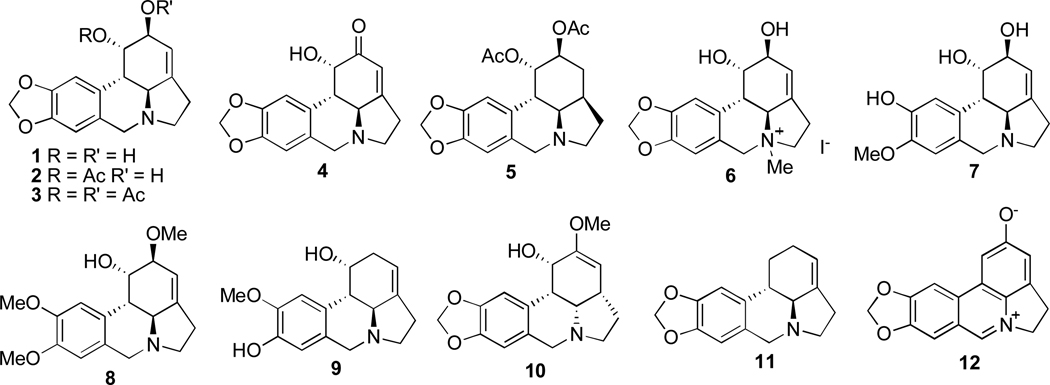
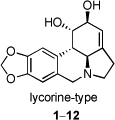
Natural products and their derivatives are an important source of new drug leads. Thus, 42% of all drugs approved from 1983 to 1994 originated from natural products [1]. This trend is particularly pronounced in the area of cancer therapy, where the fraction of the drugs derived from natural products amounts to 60%. Among various natural sources that have been investigated in search of small molecule constituents with potential use in cancer treatment, plants of the Amaryllidaceae family have been particularly fruitful [2]. The anticancer properties of these plants were already known in the fourth century B.C., when Hippocrates of Cos used oil from the daffodil Narcissus poeticus L. for the treatment of uterine tumors [3]. The topical anticancer uses of extracts from N. poeticus [4,5] as well as from Narcissus pseudonarcissus [6–8] were recorded in the first century A.D. by the Roman natural philosopher Pliny the Elder [9], and the applications of narcissus oil in cancer management continued in the middle ages in Chinese, North African, Central American and Arabian medicine [2,10]. Other genera of the Amaryllidaceae family, for example Hymenocallis caribaea, were also commonly utilized by early European medical practitioners for inflammatory tumors [11]. In more recent times, more than 100 structurally diverse alkaloids, possessing a wide spectrum of biological activities have been isolated from various Amaryllidaceae species [12]. Lycorine (1, Fig. 1), shown to possess antitumor and antiviral activities, was the first member of this family isolated in 1877 [13]. Other examples of natural and synthetically derived compounds based on this pyrrolo[de]phenanthridine scaffold include 1-O-acetyllycorine (2), 1,2-O,O-diacetyllycorine (3), lycorine-2-one (4), 1,2-O,O-diacetyl-α-dihydrolycorine (5), N-methyllycorine iodide (6), pseudolycorine (7), galanthine (8), norpluviine (9), amarbellisine (10), lycorene (11), and ungeremine (12).
Fig. 1.
Selected lycorine-type alkaloids and their synthetic derivatives
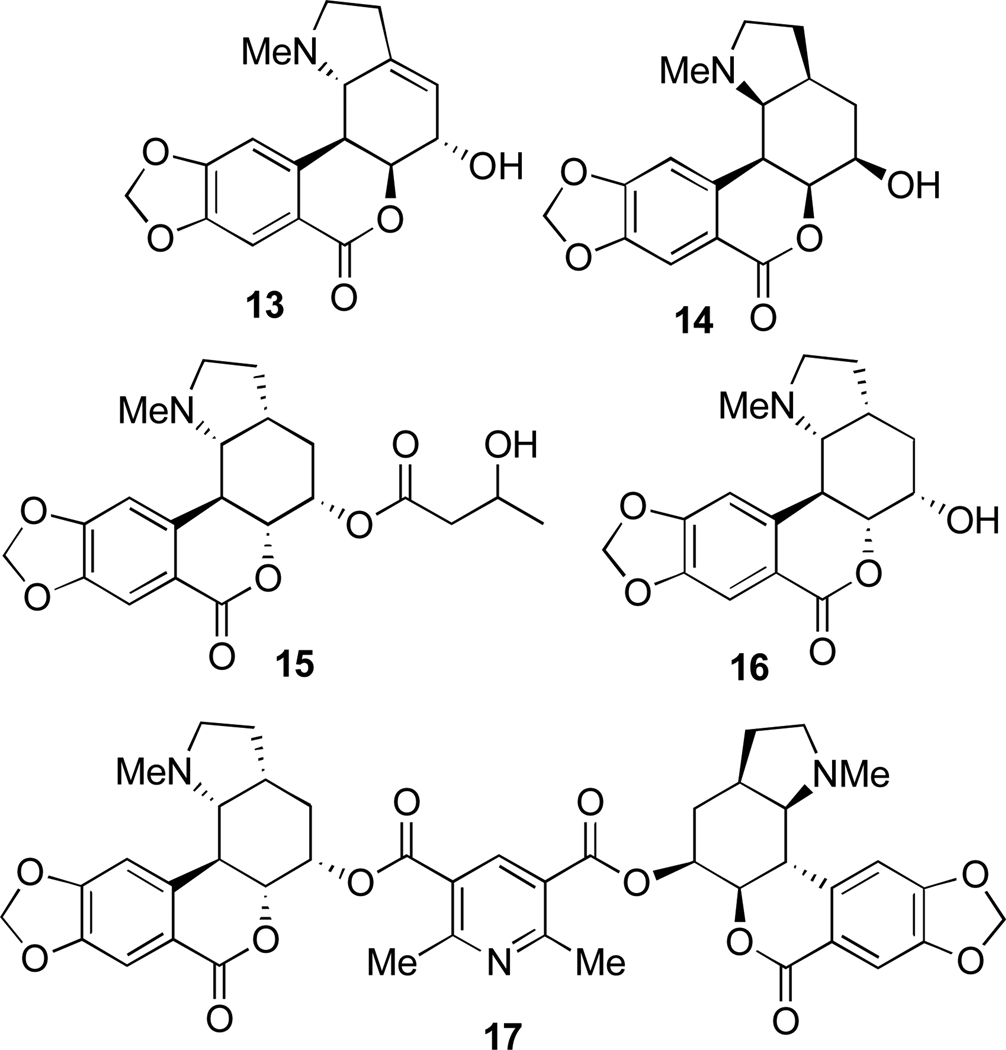
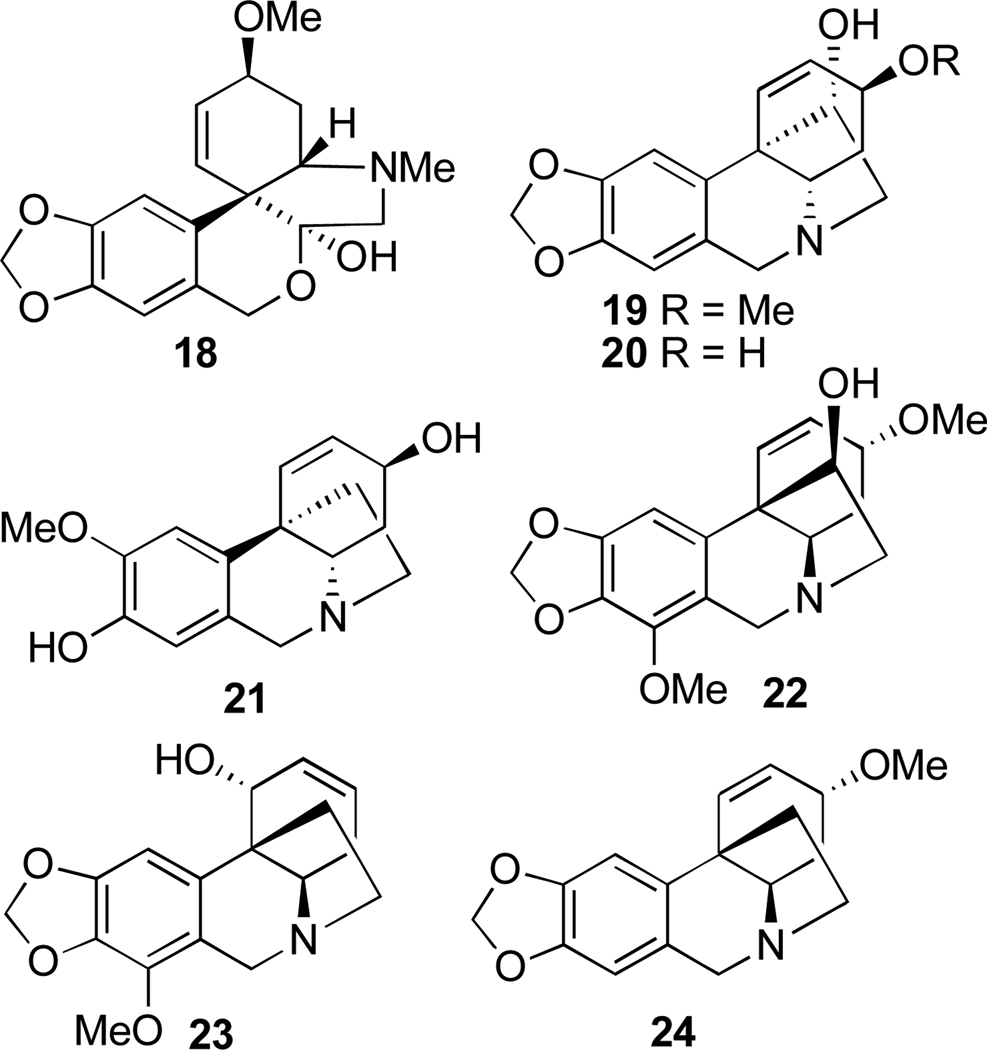
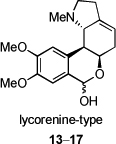
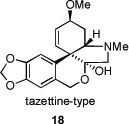
Another large alkaloid group from Amaryllidaceae is referred to as lycorenine-type and is based on [2]benzopyrano[3,4-g]indole skeleton. Examples are hippeastrine (13), nobilisitine A (14), nobilisitine B (15), clivonine (16), and clivimine (17, see Fig. 2). Fig. 3 shows the structures of tazettine (18), as an example of the tazettine group, as well as alkaloids incorporating 5,10b-ethanophenanthridine skeleton known as crinine-type, namely haemanthamine (19), 11-hydroxyvittatine (20), (+)-8-O-demethylmaritidine (21), ambelline (22), buphanamine (23), and buphanisine (24).
Fig. 2.
Selected lycorenine-type alkaloids
Fig. 3.
Selected tazettine- and crinine-type alkaloids
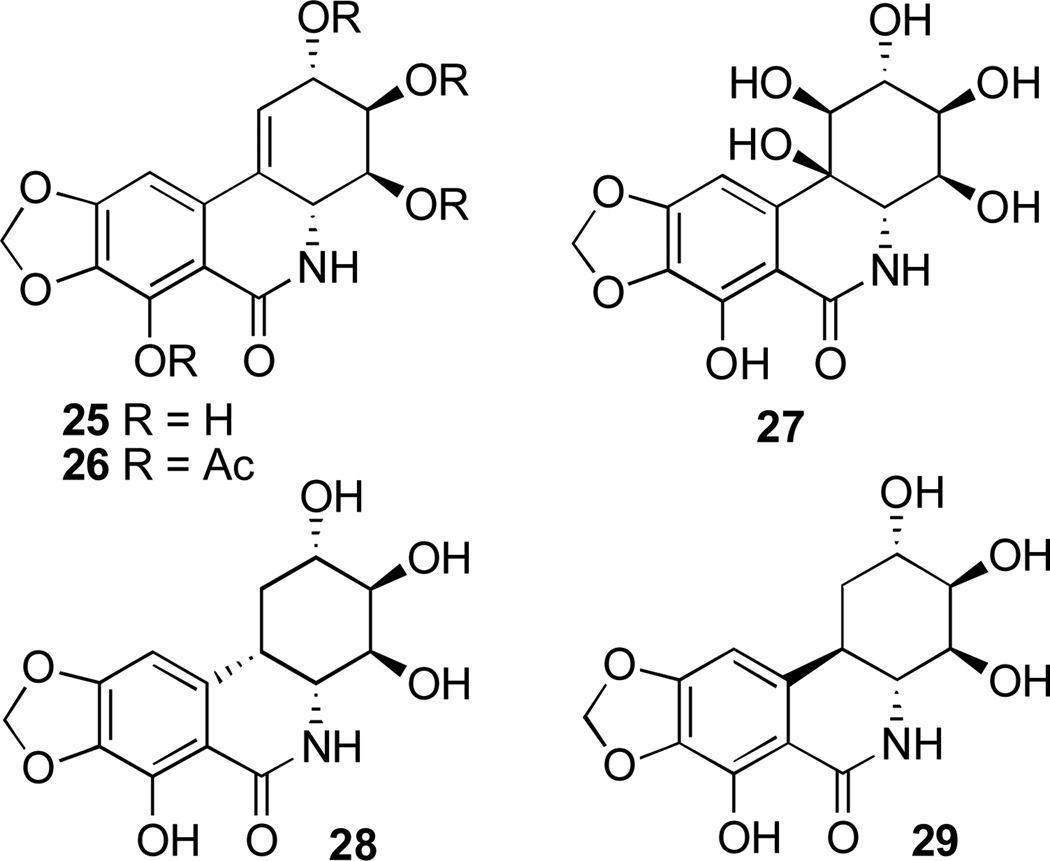
Finally, narciclasine-type metabolites based on phenanthridone skeleton are commonly referred to as alkaloids as well, in spite of the non-basic nature of the nitrogen atom (Fig. 4). Selected members of this group and their synthetic derivatives are narciclasine (25), narciclasine tetraacetate (26), C10b-R-hydroxypancratistatin (27), cis-dihydronarciclasine (28), and trans-dihydronarciclasine (29).
Fig. 4.
Selected narciclasine group members and their synthetic derivatives
Many of the Amaryllidaceae alkaloids have been reported to exhibit antiproliferative properties and it has been proposed that they exhibit this effect by disrupting eukaryotic protein biosynthesis [14–16]. Despite the undisputed potential of these plant metabolites to serve as novel anticancer drug leads, to our knowledge no single compound derived from the Amaryllidaceae constituents has advanced to human cancer clinical trials so far [17].
It has been reported that the anticancer efficacy of many currently used chemotherapeutic agents is strongly correlated with their ability to induce apoptosis in cancer cells [18] and, thus, many primary screens for novel anticancer leads are now based on identification of compounds possessing apoptosis inducing properties rather than general antiproliferative activity [19–21]. Therefore, the paucity of literature data on the apoptosis inducing potential of Amaryllidaceae alkaloids could be one important impediment to their development as anticancer agents. Furthermore, the severe adverse effects associated with conventional chemotherapy have led to the exploration of non-toxic methods of combating cancer. Some examples include the development of inhibitors of angiogenesis [22] and tumor cell invasion [23]. Both processes rely heavily on the upregulated expression of specific proteins (e.g. hypoxia-inducible factor in the former case [24] and matrix metalloproteinases in the latter one [25]) and, therefore, inhibition of ribosomal protein biosynthesis exhibited by many Amaryllidaceae alkaloids could lead to abrogation of these processes and compromised tumor survival and/or metastasis. To our knowledge investigations of this type have not been described in the literature. We report herein a systematic evaluation of the alkaloids shown in Fig. 1–4 for antiproliferative, apoptosis inducing and antiinvasive properties.
Materials and methods
Synthetic chemistry
Unless noted otherwise all reagents were purchased from commercial sources and used without purification. Triethylamine (Et3N) was distilled from calcium hydride. Tetrahydrofuran was distilled from sodium-benzophenone ketyl solution prior to use. All reactions were performed under nitrogen atmosphere and monitored by thin layer chromatography (TLC) on pre-coated (250 µm) silica gel 60F254 glass-backed plates. Visualization was accomplished with UV light and aqueous ceric ammonium molybdate solution or potassium permanganate stain followed by charring on a hot-plate. 1H and 13C NMR spectra were recorded on JEOL 300 MHz spectrometer at New Mexico Institute of Mining and Technology and on Bruker 600 MHz spectrometer at the Instituto di Chimica Biomolecolare del CNR Pozzuoli Italy. MS analyses were performed at the Mass Spectrometry Facility, University of New Mexico and at the Instituto di Chimica Biomolecolare del CNR Pozzuoli Italy. The optical rotations were measured on a JASCO P1010 digital polarimeter.
Plant material
Bulbs of Sternbergia lutea Ker Gawl and Narcissus pseudonarcissus (variety King Alfred) were collected near Bari, Italy and New Mexico, USA, respectively. S. lutea was identified by Prof. O. Arrigoni, Dipartimento di Botanica e Patologia Vegetale, Universita di Bari, Italy, where a voucher sample was deposited. Bulbs and whole plants of Amaryllis belladonna L., Clivia nobilis Regel and Pancratium maritimum L. were collected from flowering plants cultivated in Alexandria, Egypt and sandy hills on the northern coast of Egypt (Baltim). They were identified by Prof. Alam El-Din Negm and Prof. Mohammed Abd El-Maskood, University of Alexandria, and Prof. N. El Hadidy, University of Cairo, Egypt, respectively. The voucher samples of the three plants were deposited in the Collection of Department of Pharmacognosy, Faculty of Pharmacy, University of Alexandria, Egypt.
Alkaloids and their synthetic derivatives
Lycorine (1) and narciclasine (25) were isolated from dried bulbs of S. lutea and N. pseudonarcissus using the procedures reported by Evidente and co-workers [26] and Evidente [27], respectively. The purity of the samples was confirmed by TLC, 1H NMR and optical rotation analyses. Amarbellisine (10), 11-hydroxyvittatine (20), hippeastrine (13), clivimine (17), 8-demethylmaritidine (21), nobilisitines A (14) and B (15), and ungeremine (12) were isolated from A. belladonna L., C. nobilis Regel, and P. maritimun L., respectively, as previously reported by Evidente and co-workers [28–30] and Abou-Donia and co-workers [31]. 1-O-acetyllycorine (2), 1,2-O,O-diacetyllycorine (3), 1,2-O,O-diacetyl-α-dihydrolycorine (5), lycorine-2-one (4), and lycorene (11) were prepared from lycorine according to the procedures reported by Evidente and co-workers [32,33]. Ambelline (22), buphanamine (23), buphanisine (24), galanthine (8), haemanthamine (19), N-methyllycorine iodide (6), and pseudolycorine (7) were generously supplied by Prof. Fales, H. M. Department of Health, Education and Welfare, Bethesda, MD, USA. Clivonine (16) and norpluviine (9) were kindly supplied by Prof. Fuganti, C., Instituto di Chimica, Politecnico di Milano, Italy. Narciclasine tetraacetate (26), C10b-R-hydroxypancratistatin (27), cis-dihydronarciclasine (28), and trans-dihydronarciclasine (29) were prepared using the procedures reported by Mondon and Krohn [34] as well as by Pettit and co-workers [35,36].
Cell culture
The Jurkat cell line (Clone E6-1), a human T cell leukemia was purchased from the American Type Culture Collection (ATCC #TIB-152, USA) and was cultivated in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS), 100 mg/L penicillin G, 100 mg/L streptomycin, 1.0 mM sodium pyruvate (all from Gibco, Invitrogen: Life Technologies, USA), 1.5 g/L sodium bicarbonate, and 4.5 g/L glucose (Sigma) at 37 °C in a humidified atmosphere with 10% CO2.
Cell lines HeLa (ATCC #CCL-2.2, USA) and Vero (WHO Vero, National Institutes of Health, USA) were cultivated in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum (Gibco, Invitrogen: Life Technologies, USA), 100 mg/L penicillin G, 100 mg/L streptomycin, and 0.05 mg/mL gentamicin (all from Cellgro, Media Tech. Inc.) at 37 °C in a humidified atmosphere with 5% CO2.
MTT assay
Cell viability of HeLa and Vero cells was tested in accordance with Mosmann [37]. Briefly, mitochondrial dehydrogenase activities were measured by an MTT-reagent (Sigma). Cells were seeded in microtiter plates at an initial density of 4 × 104 cells in 200 µL culture medium and treated with the panel of test compounds at 5 and 25 µM for 48 h. In each experiment, eight wells were used to determine the % cell viability. The experiments were repeated at least twice for each compound per cell line.
Annexin V/propidium iodide apoptosis assay
3 × 105 Jurkat cells/mL were treated for 24 h with test compounds at 1 and 25 µM. After centrifugation, at 2200 rpm (400G) the cell pellets were resuspended in 200 µL Annexin-V FITC (Caltag Laboratories, 40 µL per mL AAB) and propidium iodide (Sigma, 2 µL (1 mg/mL) per mL AAB) solution in Annexin Binding Buffer (ABB: Heinz-Hepes Buffer (HHB: 30 mM HEPES; 110 mM NaCl; 10 mM KCl; 10 mM glucose; 1 mM MgCl2; pH 7.4) plus 5 µL CaCl2 (1.5 M) per mL HHB) and incubated at 37 °C for 20 min. Values of relative fluorescence intensity were measured with a Becton Dickinson FACscan flow cytometer and analyzed by CellQuest software.
Collagen Type I Invasion Assay
Six-well plates were filled with 1.25 ml neutralized type I collagen (0.09 %, Millipore, Billerica, MA) and incubated for 1 h at 37 °C to allow gelification. For invasion into collagen type I gel, HeLa cells were harvested using Moscona buffer and trypsin/EDTA and seeded on top of collagen type I gels. Cultures were incubated for 24 h at 37 °C in the presence or absence of test compounds at concentrations, which resulted in less than 20% antiproliferative effect as determined by a prior MTT assay. Numbers of cells penetrating into the gel or remaining at the surface were counted using an inverted microscope and expressed as the invasion index, being the percentage of invading cells over the total number of cells.
Statistics
All treatments were matched and carried out at least 2 times. Data were analyzed using Excel, for determination of mean, SD, and student t-test (95%).
3. Results and discussion
Compounds 1–29 were evaluated for their antiproliferative activity against HeLa and Vero cell lines as well as for their ability to induce apoptosis in Jurkat cells (Table 1).
Table 1.
Anticancer evaluation of Amaryllidaceae alkaloids and their synthetic derivatives
| skeleton type | alkaloid or synthetic derivative |
% cell viabilitya | % apoptosisb | ||||
|---|---|---|---|---|---|---|---|
| HeLa | Vero | Jurkat | |||||
| 5 µM | 25 µM | 5 µM | 25 µM | 1 µM | 25 µM | ||
 |
1 | 33 ± 4 | 17 ± 2 | 31 ± 3 | 23 ± 1 | 13 ± 1 | 60 ± 2 |
| 2 | 26 ± 1 | 13 ± 1 | 26 ± 2 | 18 ± 1 | 4 ± 2 | 42 ± 2 | |
| 3 | 67 ± 4 | 28 ± 3 | 96 ± 5 | 47 ± 4 | 3 ± 1 | 3 ± 4 | |
| 4 | 82 ± 5 | 38 ± 2 | 86 ± 5 | 71 ± 3 | 6 ± 1 | 23 ± 1 | |
| 5 | 100 ± 4 | 100 ± 6 | 77 ± 4 | 69 ± 5 | 3 ± 1 | 2 ± 4 | |
| 6 | 85 ± 2 | 59 ± 2 | 79 ± 2 | 65 ± 2 | 3 ± 2 | 4 ± 1 | |
 |
7 | 52 ± 6 | 18 ± 3 | 42 ± 6 | 28 ± 2 | 6 ± 4 | 54 ± 3 |
| 8 | 90 ± 9 | 84 ± 5 | 98 ± 8 | 77 ± 4 | 3 ± 1 | 7 ± 1 | |
| 9 | 89 ± 8 | 84 ± 6 | 71 ± 5 | 63 ± 3 | 5 ± 1 | 6 ± 1 | |
| 10 | 51 ± 3 | 23 ± 2 | 44 ± 3 | 28 ± 1 | 4 ± 3 | 4 ± 1 | |
| 11 | 99 ± 4 | 79 ± 4 | 100 ± 3 | 84 ± 4 | 4 ± 2 | 3 ± 1 | |
| 12 | 83 ± 5 | 46 ± 15 | 78 ± 3 | 78 ± 9 | 5 ± 1 | 7 ± 1 | |
 |
13 | 78 ± 5 | 57 ± 2 | 78 ± 7 | 48 ± 2 | 3 ± 1 | 5 ± 1 |
| 14 | 100 ± 7 | 92 ± 3 | 100 ± 5 | 71 ± 3 | 3 ± 3 | 3 ± 2 | |
| 15 | 99 ± 5 | 89 ± 2 | 64 ± 4 | 59 ± 3 | 4 ± 2 | 13 ± 1 | |
| 16 | 98 ± 14 | 97 ± 11 | 95 ± 8 | 97 ± 5 | 3 ± 1 | 4 ± 1 | |
| 17 | 94 ± 10 | 67 ± 7 | 83 ± 14 | 64 ± 6 | 3 ± 1 | 5 ± 1 | |
| 18 | 95 ± 3 | 99 ± 5 | 82 ± 2 | 83 ± 5 | 3 ± 1 | 3 ± 1 | |
 |
19 | 30 ± 2 | 21 ± 2 | 44 ± 3 | 32 ± 2 | 4 ± 1 | 22 ± 1 |
| 20 | 86 ± 3 | 72 ± 5 | 64 ± 2 | 64 ± 6 | 3 ± 1 | 4 ± 1 | |
| 21 | 90 ± 7 | 82 ± 7 | 87 ± 4 | 82 ± 5 | 5 ± 1 | 4 ± 1 | |
| 22 | 100 ± 9 | 79 ± 10 | 95± 8 | 89 ± 6 | 4 ± 4 | 4 ± 1 | |
| 23 | 98 ± 4 | 96 ± 9 | 83 ± 2 | 90 ± 10 | 3 ± 3 | 3 ± 1 | |
| 24 | 100 ± 8 | 99 ± 6 | 74 ± 7 | 79 ± 6 | 3 ± 3 | 5 ± 1 | |
 |
25 | 10 ± 1 | 6 ± 1 | 10 ± 2 | 6 ± 1 | 60 ± 1 | 76 ± 1 |
| 26 | 21 ± 2 | 6 ± 1 | 21 ± 10 | 10 ± 1 | 50 ± 2 | 57 ± 2 | |
| 27 | 83 ± 10 | 32 ± 2 | 70 ± 4 | 49 ± 3 | 3 ± 1 | 22 ± 1 | |
| 28 | 37 ± 3 | 20 ± 1 | 46 ± 2 | 14 ± 1 | 5 ± 1 | 50 ± 1 | |
| 29 | 9 ± 1 | 5 ± 1 | 14 ± 1 | 6 ± 1 | 49 ± 2 | 64 ± 1 | |
% Remaining cell viability after 48 h of treatment with indicated compounds relative to DMSO control ± SD from two independent experiments, each performed in eight replicates, determined by MTT assay.
% Apoptotic cells after 24 h of treatment with indicated compounds ± SD from two independent experiments, each performed in 3 replicates, determined by flow cytometric Annexin-V/propidium iodide assay. 0.1% DMSO control exhibited 2–3% apoptosis.
Our results indicate that the most potent antiproliferative activities are associated with the narciclasine group of Amaryllidaceae alkaloids, which is consistent with the previous literature reports [17,39]. In addition, the powerful induction of apoptosis by narciclasine (25) is in agreement with the finding of Kiss and co-workers [40], who reported that 25 induced marked apoptosis in human MCF-7 breast and PC-3 prostate carcinoma cells. Furthermore, trans-dihydronarciclasine (29), whose antiproliferative potency rivals that of 25, is also efficient at inducing apoptosis in Jurkat cells. In contrast, cis-dihydronarciclasine (28) and C10b-R-hydroxypancratistatin (27) are weaker apoptosis inducers and possess inferior antiproliferative properties. Interestingly, narciclasine tetraacetate (26) exhibits high potencies in both assays, although lower than those of narciclasine itself. In all likelihood, 26 serves as a prodrug and intracellular hydrolysis of acetate esters converts it to cytotoxic 25. These observations argue that potent cytotoxicity displayed by the narciclasine-type alkaloids and their synthetic derivatives is apoptosis-mediated. We expect that these findings will encourage further efforts to advance narciclasine-type compounds to human clinical trials [17].
Previous studies indicate that among the lycorine-, lycorenine-, tazettine-, and crinine-type alkaloids shown in Fig. 1–3, good levels of antiproliferative activity are expected for lycorine (1) [41,42], pseudolycorine (7) [41], ungeremine (12) [43], hippeastrine (13) [41], and haemanthamine (19) [41,42]. In addition, apoptosis inducing properties were reported for 1 [44,45] and 19 [46]. The results in Table 1 suggest that cytotoxic effect of 7 is also apoptosis-mediated. We also show for the first time that amarbellisine (10) has a pronounced antiproliferative effect. Interestingly, 10, 12 and 13 exhibit no apoptosis induction in Jurkat cells at concentrations as high as 25 µM. Further study is required to clarify whether this is a cell-specific property or if these alkaloids are general growth inhibitors. Oxidation of the C2-hydroxyl in lycorine gives lycorine-2-one (4), which possesses diminished, but still significant cytotoxicity. In contrast, acetylation of the C1-hydroxyl in lycorine results in equally potent 1-O-acetyllycorine (2). This derivative may also be a prodrug of 1 and its somewhat weaker apoptosis inducing potential in Jurkats cells may be explained by the 24 h treatment, which may be insufficient for the complete hydrolytic removal of the acetate ester. This hypothesis is supported by the observation of a complete lack of activity of 1,2-O,O-diacetyllycorine (3) in the 24 h apoptosis assay, compared with its reasonable potency in the 48 h MTT tests. In this case the steric encumbrance associated with the two acetyl groups may lead to their significantly slower hydrolysis. Finally, 11-hydroxyvittatine (20), a demethylated natural congener of cytotoxic haemanthamine (19), was reported to have no antiproliferative activity [47]. We also observed only a modest activity in the MTT assay and no induction of apoptosis as opposed to 19, demonstrating that the methyl ether is an important part of the cytotoxic pharmacophore.
Although metastasis is a primary cause of mortality from cancer [48], very few therapies are available that target cancer cell invasion into surrounding tissue and/or their spreading to remote locations [23,25]. The process of cancer cell invasion is predominantly controlled by the interaction of cancer cells and their surrounding extracellular matrix, in which many enzymes and adhesion proteins play an integral part [49]. Because many Amaryllidaceae alkaloids exert their biological effects by inhibiting ribosomal protein biosynthesis (vide supra), these plant metabolites and their derivatives seem ideally suited for affecting the balance of invasion promoters and suppressors. Yet, we are unaware of biological evaluation studies of Amaryllidaceae alkaloids with respect to their ability to regulate or inhibit cell invasion. Therefore, we used a collagen invasion assay [50,51], in which collagen type I serves as a model for extracellular matrix in vitro. Because invasive cells secrete matrix metalloproteinases that break collagen type I fibers, this assay adequately models the modulation of cell invasion by tissue inhibitors in vitro [52]. Furthermore, both cell-matrix and cell-cell adhesion molecules, such as integrins [53], E-cadherin [54], N-CAM [55], and MUC-1 [56] have been demonstrated to regulate invasion into type I collagen.
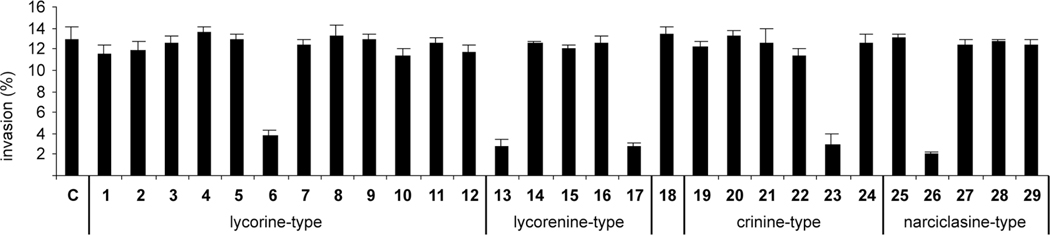
Before evaluating compounds 1–29 for antiinvasive activity, a non-toxic concentration for each compound was determined by using a MTT assay on HeLa cells. The optical density value of 80%, which corresponds to 20% of inhibition of proliferation, was used as a guide. The results of the collagen type I invasion assay indicate that a number of alkaloids and their derivatives, each at its non-toxic concentration, suppress invasion of HeLa cells (Fig. 5). Interestingly, among this small group of compounds there is a representative of each type of skeleton in Fig. 1–4, namely N-methyllycorine iodide (6, lycorine-type), hippeastrine (13), clivimine (17, both lycorenine-type), buphanamine (23, crinine-type), and narciclasine tetraacetate (26, narciclasine-type). This discovery opens the door for systematic structure-activity relationship studies utilizing each of these skeletons.
Fig. 5.
Collagen type I invasion assay. Invasion index is expressed as % invading cells into type I collagen after 24 h of treatment with indicated compounds at non-toxic concentrations. C = 0.1% DMSO control. Non-toxic concentrations are those that resulted in < 20% of inhibition of proliferation as determined with a prior MTT assay and they are as follows: 1 1 µM, 2 0.5 µM, 3 3 µM, 4 5 µM, 5 25 µM, 6 5 µM, 7 3 µM, 8 25 µM, 9 25 µM, 10 5 µM, 11 25 µM, 12 5 µM, 13 3 µM, 14 25 µM, 15 25 µM, 16 25 µM, 17 15 µM, 18 25 µM, 19 1 µM, 20 5 µM, 21 25 µM, 22 25 µM, 23 25 µM, 24 25 µM, 25 0.05 µM, 26 0.1 µM, 27 5 µM, 28 1 µM, 29 0.05 µM.
Although all these compounds were tested at non-toxic concentrations, the antiinvasive activity of buphanamine (23) is a particularly encouraging finding since this alkaloid does not show any toxicity even at much higher concentrations. In contrast, the activity of 6, 13, 17 and 26 should be treated with more caution as the test concentrations for these compounds are close to their toxic levels. Therefore, more thorough dose-dependent studies are required to assess their therapeutic indexes. If 17 is hydrolyzed within cells, it should produce 16 and the claim of the genuine antiinvasive activity of 17 might be questioned. However, such rapid hydrolysis (24 h treatment time) would be highly unlikely. In addition, 16 displays no antiinvasive properties. This is also consistent with the intriguing inhibitory effect of narciclasine tetraaacetate (26) and a lack of such in the case of narciclasine (25). The bona fide inhibitor must be 26 itself or one of the persistent hydrolytic intermediates containing fewer acetyl groups. Further studies, involving monitoring the intracellular hydrolysis kinetics, would be required to solve this puzzle. Finally, we believe that the antiinvasive activity of clivimine and buphanamine represents the first observation of any biological activity associated with either of these two alkaloids, with the exception of weak (millimolar) affinity toward the 5HT1A serotonin transporter reported for buphanamine [57].
In conclusion, a systematic evaluation of diverse Amaryllidaceae alkaloids and their synthetic derivatives for antiproliferative, apoptosis inducing and antiinvasive properties was performed and resulted in a number of interesting findings. In our view two of these are most significant. First, most compounds that show promising antiproliferative activities are also good apoptosis inducers. These are narciclasine, narciclasine tetraacetate, C10b-R-hydroxypancratistatin, cis-dihydronarciclasine, trans-dihydronarciclasine, lycorine, 1-O-acetyllycorine, lycorine-2-one, pseudolycorine, and haemanthamine. Important exceptions are ungeremine, amarbellisine and hippeastrine, which may be strictly growth inhibitory. Second, a number of structurally diverse compounds completely suppress cell invasion in vitro at non-toxic concentrations. These are N-methyllycorine iodide (lycorine-type), hippeastrine, clivimine (both lycorenine-type), buphanamine (crinine-type), and narciclasine tetraacetate (narciclasine-type). The antiinvasive activity of buphanamine is particularly promising since this alkaloid is not toxic to cells even at much higher concentrations.
Acknowledgments
We thank the Italian Ministry University and Research (MIUR, contribution DISSPAPA N. to be added after acceptance) and the US National Institutes of Health (CA-99957 and RR-16480) for financial support of this work. The staff members of “Servizio di Spettrometria di Massa del CNR” and “Istituto di Chimica Biomolecolare del CNR” are gratefully acknowledged for providing mass and NMR spectra, respectively. The Mass Spectrometry Facility at the University of New Mexico is acknowledged as well. We thank Dr. Anna Andolfi, Professor Snezna Rogelj and Professor Patrick S. Mariano for their kind assistance with technical issues, flow cytometry and preparation of this manuscript, respectively.
References
- 1.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 2.Hartwell JL. Plants used against cancer. A survey. Lloydia. 1967;30:379. [PubMed] [Google Scholar]
- 3.Gardeil JB. Traduction des Oeuvres Médicales d’Hippocrate. 4 vols. Toulouse: Fages, Meilhec et Cie; 1801. (translator) [Google Scholar]
- 4.Martin SF. The Alkaloids. New York: Academic Press; 1987. p. 251. [Google Scholar]
- 5.Gibbs RD. Chemotaxonomy of Flowering Plants. Vol. III. Montreal: McGill-Queen’s University Press; 1974. p. 1924. [Google Scholar]
- 6.Tojo E. (+)-Narcidine, a new alkaloid from Nacissus Pseudonarcissus. J Nat Prod. 1991;54:1387–1388. [Google Scholar]
- 7.Noumbissie BE, H KapnangH, Fomum ZT, Martin MT, Bodo B. The myristicaeae of Cameroon. 1. Staudtienic acid, a diterpene from Staudtia Kamerunesis. J Nat Prod. 1992;55:137–139. [Google Scholar]
- 8.Bastida J, Codina C, Viladomat F, Rubiralta M, Quirion JC, Weniger B. Narcissus alkaloids. 14. (+)-8-O-acetylhomolycorine and vasconine, 2 novel alkaloids from Narcissus Vasconicus. J Nat Prod. 1992;55:122–125. [Google Scholar]
- 9.Bostock J, Riley HT, editors. C. Plinius Secundus (Pliny the Elder) Natural History. 6 vols. London: Bohn; 1855. Translated by. [Google Scholar]
- 10.Hartwell JL. Plants Used Against Cancer. Lawrence, MA: Quarterman Publication; 1982. [Google Scholar]
- 11.Kosteletzky BF. Allgemeine medizinisch-pharmazeutische Flora. 6 vols. Prague: Borrosch and André; pp. 1831–1836. [Google Scholar]
- 12.Hoshino O. The Amarillidaceae alkaloids. In: Cordell GA, editor. The Alkaloids. Vol. 51. London: Academic Press; 1998. pp. 323–376. [Google Scholar]
- 13.Cook JW, Loudon JD. The Alkaloids. New York: Academic Press; 1952. p. 331. [Google Scholar]
- 14.Carrasco L, Fresno M, Vazquez D. Narciclasine: an antitumor alkaloid which blocks peptide bond formation by eukaryotic ribosomes. FEBS Lett. 1975;52:236–239. doi: 10.1016/0014-5793(75)80813-1. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez A, Sanchez L, Vazquez D. Location of resistance to the alkaloid narciclasine in the 60S ribosomal subunit. FEBS Lett. 1975;55:53–56. doi: 10.1016/0014-5793(75)80955-0. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez A, Santos A, Alonso G, Vasquez D. Inhibitors of protein synthesis in eukariotic cells. Comparative effects of some Amaryllidaceae alkaloids. Biochim Biophys Acta. 1976;425:342–348. doi: 10.1016/0005-2787(76)90261-6. [DOI] [PubMed] [Google Scholar]
- 17.Kornienko A, Evidente A. Chemistry, Biology, and Medicinal Potential of Narciclasine and its Congeners. Chem Rev. 2008;108:1982–2014. doi: 10.1021/cr078198u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vial JP, Belloc F, Dumain P, Besnard S, Lacombe F, Boisseau MR, et al. Study of the apoptosis induced in vitro by antitumoral drugs on leukaemic cells. Leukemia Res. 1997;21:163–172. doi: 10.1016/s0145-2126(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HZ, Kasibhatla S, Kuemmerle J, Kemnitzer W, Ollis-Mason K, Qiu L, et al. Discovery and structure-activity relationship of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J Med Chem. 2005;48:5215–5223. doi: 10.1021/jm050292k. [DOI] [PubMed] [Google Scholar]
- 20.Kemnitzer W, Kasibhatla S, Jiang S, Zhang H, Zhao J, Jia S, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure-activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg Med Chem Lett. 2005;15:4745–4751. doi: 10.1016/j.bmcl.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 21.Kemnitzer W, Drewe J, Jiang S, Zhang H, Wang Y, Zhao J, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 1. Structure-activity relationships of the 4-aryl group. J Med Chem. 2004;47:6299–6310. doi: 10.1021/jm049640t. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N, Kerbel RS. Angiogenesis as a Therapeutic Target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson RB, Johnson MD, Maemura M, Low J. Anti-invasion drugs. Breast Cancer Res Treat. 1996;38:121–132. doi: 10.1007/BF01803790. [DOI] [PubMed] [Google Scholar]
- 24.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol/Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Khasig PZ, Pdobed OV, Gracheva TS, Salbiev KD, Grachev SV, Brezov TT. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochem Moscow. 2003;68:711–717. doi: 10.1023/a:1025051214001. [DOI] [PubMed] [Google Scholar]
- 26.Evidente A, Iasiello I, Randazzo G. An improved method for the large-scale preparation of lycorine. Chem Ind. 1984:348–349. [Google Scholar]
- 27.Evidente A. Narciclasine: 1H and 13C-NMR data and a new improved method of preparation. Planta Med. 1991;57:293–295. doi: 10.1055/s-2006-960098. [DOI] [PubMed] [Google Scholar]
- 28.Evidente A, Andolfi A, Abou-Donia AH, Touema SM, Hammoda HM, Shawsky E, et al. (−)-Amarbellisine, a lycorine-type alkaloid from Amaryllis belladona L. growing in Egypt. Phytochemistry. 2004;65:2113–2118. doi: 10.1016/j.phytochem.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Evidente A, Abou-Donia AH, Darwish FA, Amer ME, Kassem FF, Hammoda HAM. Nobilisitines A and B, two masanane-type alkaloids from Clivia nobilis. Pytochemistry. 1999;51:1151–1155. [Google Scholar]
- 30.Evidente A, Andolfi A, Abou-Donia AH, Darwish FA, Hammoda HAM, Motta A. Minor alkaloids from Clivia nobilis Regel. Alex J Pharm Sci. 2005;19:49–53. [Google Scholar]
- 31.Abou-Donia AH, Abib AA, El Din AS, Evidente A, Gaber M, Scopa A. Two betaine-type alkaloids from bulbs of Pancratium maritimum. Phytochemistry. 1992;31:2139–2141. [Google Scholar]
- 32.Evidente A, Cicala MR, Randazzo G, Riccio R, Calabrese G, Liso R, et al. Lycorine structure-activity relationships. Phytochemistry. 1983;22:2192–2196. [Google Scholar]
- 33.Evidente A, Arrigoni O, Liso R, Calabrese G, Randazzo G. Further experiments on lycorine-structure-activity relationships among the lycorine alkaloids. Phytochemistry. 1986;25:2739–2743. [Google Scholar]
- 34.Mondon A, Krohn K. Chemistry of narciclasine. Chem Ber. 1975;108:445–463. [Google Scholar]
- 35.Pettit GR, Melody N, O’Sullivan M, Thompson MA, Herald DL, Coates B. Synthesis of B-10-R-hydroxypancratistatin via narciclasine. J Chem Soc Chem Commun. 1994:2725–2726. [Google Scholar]
- 36.Pettit GR, Melody N, Herald DL, Schmidt JM, Pettit RK, Chapuis JC. Synthesis of 10b(R)-hydroxypancratistatin, 10b(S)-hydroxy-1-epipancratistatin, 10b(S)-hydroxy-1,2-diepipancratistatin and related isocarbostyrils. Heterocycles. 2002;56:139–155. [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival - application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis - flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein-labeled annexin-V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 39.Pettit GR, Pettit GR, III, Backhaus RA, Boyd MR, Meerow AW. Antineoplastic agents, 256. Cell growth inhibitory isocarbostylis from Hymenocallis. J Nat Prod. 1993;56:1682–1687. doi: 10.1021/np50100a004. [DOI] [PubMed] [Google Scholar]
- 40.Dumont P, Ingrassia L, Rouzeau S, Ribaucour F, Thomas S, Roland I, et al. The amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts. Neoplasia. 2007;9:766–776. doi: 10.1593/neo.07535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weniger B, Italiano L, Beck JP, Bastida J, Bergonon S, Codina C, et al. Cytotoxic activity of Amaryllidaceae alkaloids. Planta Med. 1995;61:77–79. doi: 10.1055/s-2006-958007. [DOI] [PubMed] [Google Scholar]
- 42.Hohmann J, Forgo P, Molnar J, Wolfard K, Molnar A, Thalhammer T, et al. Antiproliferative Amaryllidaceae alkaloids isolated from the bulbs of Sprekelia formosissima and Hymenocallis x festalis. Planta Med. 2002;68:454–457. doi: 10.1055/s-2002-32068. [DOI] [PubMed] [Google Scholar]
- 43.Barthelmes HU, Niederberger E, Roth T, Schulte K, Tang WC, Boege F, et al. Lycobetaine acts as a selective topoisomerase II beta poison and inhibits the growth of human tumour cells. Br J Cancer. 2001;85:1585–1591. doi: 10.1054/bjoc.2001.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Hu WX, He LF, Ye M, Li Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004;578:245–250. doi: 10.1016/j.febslet.2004.10.095. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Liu J, Tang LJ, Shi YW, Ren W, Hu WX. Apoptosis induced by lycorine in KM3 cells is associated with the G0/G1 cell cycle arrest. Oncol Rep. 2007;17:377–384. [PubMed] [Google Scholar]
- 46.McNulty J, Nair JJ, Codina C, Bastida J, Pandey S, Gerasimoff J, et al. Selective apoptosis-inducing activity of crinum-type Amaryllidaceae alkaloids. Phytochemistry. 2007;68:1068–1074. doi: 10.1016/j.phytochem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Abou-Donia AH, Arner ME, Darwish FA, Kassem FF, Hammoda HAM, Abdel-Kader MS, et al. Two new alkaloids of the crinane series from Pancratium sickenbergeri. Planta Med. 2002;68:379–381. doi: 10.1055/s-2002-26754. [DOI] [PubMed] [Google Scholar]
- 48.Christofori G. New signals from the invasive front. Nature. 2006;25:441–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 49.Mareel MM, Bracke ME, Van Roy FM, de Baetselier P. Molecular mechanisms in cancer invasion. In: Bertino JR, editor. Encyclopedia of Cancer. Vol II. San Diego: Academic Press; 1997. pp. 1072–1083. [Google Scholar]
- 50.Schor SL, Schor AM, Winn B, Rushton G. The use of 3-dimensional collagen gels for the study of tumor-cell invasion in vitro: experimental parameters influencing cell-migration into the gel matrix. Int J Cancer. 1982;29:57–62. doi: 10.1002/ijc.2910290110. [DOI] [PubMed] [Google Scholar]
- 51.Bracke ME, Boterberg T, Bruyneel EA, Mareel MM. Collagen Invasion Assay. In: Brooks S, Schumacher U, editors. Metastasis Research Protocols. Totowa: Humana Press; 2001. pp. 81–89. [Google Scholar]
- 52.Fisher C, Gilbertson-Beadling S, Powers EA, Petzold G, Poorman R, Mitchell MA. Interstitial collagenase is required for angiogenesis in vitro. Dev Biol. 1994;162:499–510. doi: 10.1006/dbio.1994.1104. [DOI] [PubMed] [Google Scholar]
- 53.Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, et al. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vleminckxstarf K, Vakaet L, Mareel M, Fiersstarf W, Van Roystarf F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 55.Meyer MB, Bastholm L, Nielsen MH, Elling F, Rygaard J, Chen W, et al. Localisation of NCAM on MCAM-B-expressing cells with inhibited migration in collagen. APMIS. 1995;103:197–208. doi: 10.1111/j.1699-0463.1995.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 56.Suwa T, Hinoda Y, Makiguchi Y, Takahashi T, Itoh F, Adachi M, et al. Increased invasiveness of MUC1 cDNA-transfected human gastric cancer MKN74 cells. Int J Cancer. 1998;76:377–382. doi: 10.1002/(sici)1097-0215(19980504)76:3<377::aid-ijc15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 57.Sandager M, Nielsen ND, Stafford GI, van Staden J, Jäger KJ. Alkaloids from Boophane disticha with affinity to the serotonin transporter in rat brain. J Ethnopharmacol. 2005;98:367–370. doi: 10.1016/j.jep.2005.01.037. [DOI] [PubMed] [Google Scholar]