Abstract
In addition to functional disorders of paresis, paralysis, and cardiopulmonary complications, subsets of West Nile virus (WNV) patients may also experience neurocognitive deficits and memory disturbances. A previous hamster study has also demonstrated spatial memory impairment using the Morris water maze (MWM) paradigm. The discovery of an efficacious therapeutic antibody MGAWN1 from pre-clinical rodent studies raises the possibility of preventing or treating WNV-induced memory deficits. In the current study, hamsters were treated intraperitoneally (i.p.) with 32 mg/kg of MGAWN1 at 4.5 days after subcutaneously (s.c.) challenging with WNV. As expected, MGAWN1 prevented mortality, weight loss, and improved food consumption of WNV-infected hamsters. The criteria for entry of surviving hamsters into the study were that they needed to have normal motor function (forelimb grip strength, beam walking) and normal spatial reference memory in the MWM probe task. Twenty-eight days after the acute phase of the disease had passed, MGAWN1- and saline-treated infected hamsters were again trained in the MWM. Spatial memory was evaluated 48 hours after this training in which the hamsters searched for the location where a submerged escape platform had been positioned. Only 56% of infected hamsters treated with saline spent more time in the correct quadrant than the other three quadrants, as compared to 92% of MGAWN1-treated hamsters (P ≤ 0.05). Overall these studies support the possibility that WNV can cause spatial memory impairment and that therapeutic intervention may be considered.
Keywords: West Nile virus, MGAWN1, hamsters, spatial memory, monoclonal antibody
Introduction
Published results of WNV-patient records and surveys indicate that a subpopulation of patients recovering from the acute West Nile virus infection complain of problems with memory, in addition to other neurological sequelae(Carson et al., 2006; Cook et al., 2010; Gottfried et al., 2005). WNV-induced memory impairment was confirmed in a hamster model as reported in an abstract (Smeraski et al., 2009) where hamsters were trained for spatial learning and memory of a submerged platform in a Morris Water Maze (MWM). Hamsters were then infected with WNV and retrained with the MWM test at 1 month and 2 months later. At 48 hours after the acquisition training, these animals had statistically significant memory loss. Sham-injected hamsters did not develop the memory loss. These data prompted us to evaluate the ability of a potent humanized monoclonal antibody, MGAWN1, to prevent spatial memory loss using the same MWM protocol.
MGAWN1 is being developed by MacroGenics, Inc. for the possible treatment of WNV disease. A Phase 1 safety and pharmacokinetics study in human volunteers has been completed(Beigel et al., 2010) and Phase 2 and expanded access studies to assess safety and efficacy in individuals with WNV neuroinvasive disease have been initiated. These clinical trials were supported by preclinical rodent studies, in which MGAWN1 (also known as hE16) administered once by i.p. injection significantly improved survival in mice (Oliphant et al., 2005), and improved survival, weight change, and disease signs in hamsters (Morrey et al., 2006, 2007). It was effective in hamsters when administered up to day 5 after viral challenge when infected neurons were identified in the brain. It also prevented acute flaccid paralysis when administered after the virus had infected the lumbar spinal cord (Morrey et al., 2008). If treatment were delayed too long (day 6), however, MGAWN1 was not efficacious (Morrey et al., 2007).
Rodents infected with Theiler’s murine encephalomyelitis virus (Buenz et al., 2006) and Borna disease virus (Rubin et al., 1998) develop spatial memory loss and had more search errors during MWM probe trials. These deficits are associated with infection at the hippocampal formation, particularly at the CA1 and dentate gyrus that are involved in spatial learning and memory function(Kandel et al., 2000). As in these viral infections, WNV-infected cells have also been identified in the hippocampus of infected mice (Hunsperger and Roehrig, 2006) and hamsters (Siddharthan et al., 2009), which provided presumptive evidence that WNV might result in impaired memory function. Thus, the purpose of this study was to further evaluate the efficacy of MGAWN on rescuing cognitive functions following WNV challenge. In this study, we hypothesized that a single i.p. injection of WNV-infected hamsters with MGAWN1 at day 4.5 after challenge could prevent spatial memory impairment.
Materials and Methods
Animals and virus inoculation
Male Syrian hamsters, Mesocricetus auratus (Charles River Laboratories), 9-10 weeks of age were individually housed, given ad libitum access to food and water, and entrained to a 14 hour light: 10 hour dark cycle. Prior to inoculation and for approximately 4 weeks after, hamsters were weighed and handled daily for health assessment, and the amount of food consumed was measured. Consumption was measured by regularly weighing the amount of food present in the cage, including pieces of food pellets that were catched in the bedding, and calculating the daily loss.Animal studies were in compliance with Colorado State University Institutional Animal Care and Use Committee and the animals were kept in an AAALAC-accredited biosafety level 3 facility. Hamsters were s.c. injected with 104plaque-forming units (pfu) of NY99 4132 strain (crow brain) of WNV. At 4.5 days post inoculation (dpi), 13 hamsters were i.p. injected with 32 mg/kg of MGAWN1 (MacroGenics Inc., Rockville, MD) and 20 hamsters were i.p. injected with sterile physiological saline. MGAWN1 was the same as hE16 formerly identified in the literature(Morrey et al., 2006, 2007). Excess animals were included in the saline (control treatment) group so as to provide sufficient numbers of surviving animals for the studies conducted after the acute infection.
Evaluation of cognitive performance: spatial reference memory
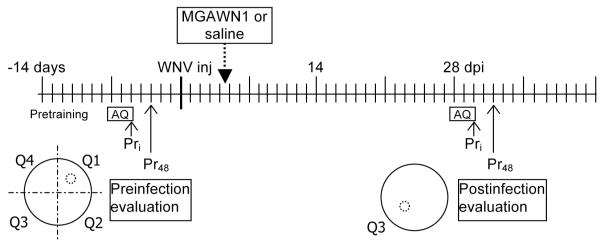
Spatial memory performance was assessed in a Morris Water Maze(MWM) probe paradigm,a task widely used to evaluate learning and memory in rodents (Morris, 1984; Morris et al., 1986).Treatment groups were not blinded since the readouts were objective, and since the health of MGAWN1-treated hamsters were obviously better than the saline-treated hamsters. To be included in this study, animals underwent pretesting and training in the MWM to meet behavioral criterion, so as to exclude animals that are inherently cognitively deficient, easily stressed, or not sufficiently motivated to complete the procedural task (i.e., poor learners or those with sensorimotor deficits). A timeline of the experimental paradigm is outlined in Figure 1. The protocol consisted of training or acquisition trials, where animals learned the position of a stationary hidden escape platform, and probe trials, where animals were allowed to search for the platform, which had been removed from the maze (see details below).
Figure 1.
Timeline of experimental paradigm for evaluating spatial memory performance in hamsters. Abbreviations: Pretraining, prior to acquisition training, animals first acclimate to the maze and learn to mount a platform to escape the water; AQ, Morris water maze acquisition trials- (15 trials= 5 trials per day for 3 days-permits animals to learn to use distal cues around the room to locate a hidden submerged escape platform); PRi , initial probe trial (platform is removed) conducted after 15th acquisition trial; PR48, probe trial conducted 48 h after last acquisition trial; Q1-Q4 divisions (quadrants) of the of the water maze (larger circle) in which a submerged (hidden) escape platform (small dotted circle) was positioned in the center (quadrant 1 during pre-infection acquisition trials and quadrant 3 during post-infection acquisition trials; WNV inj, West Nile virus (104 pfu, NY99) was administered s.c.; MGAWN1 (32 mg/kg) or saline was administered i.p. 4.5 days after WNV inoculation.
The MWM consisted of a circular basin (150 cm in diameter and 50 cm deep) filled with milk-clouded water (~26 ± 1°C) and pos itioned under a video camera. The circular maze was visually divided into 4 quadrants. The submerged, hidden “escape” platform was positioned in one of four quadrants of the maze, and prominent visual cues in the room provided distal spatial references to the platform’s fixed position. Swimming animals were trained to mount the submerged platform in order to escape the maze. Fifteen acquisition trials over 3 days (5 trials per day) permitted animals to learn the spatial location of the hidden escape platform. Each trial began by placing a hamster in the water at any of 8 randomly chosen positions and ended when the animal mounted the hidden platform or 90 s had elapsed. Animals were run in squads of 4. Following the last acquisition trial on day 3, a probe trial was performed with the platform removed, and the animal allowed to explore or probe the maze for 90 s. The percentage of time spent in the each of the quadrants in the first 60 s was measured from playback of recorded video. This initial probe trial was used to verify whether hamsters had learned the location of the hidden escape platform during the acquisition trials by exhibiting a preference for the target/training quadrant where the platform was previously positioned. A preference for the target quadrant was exhibited during the probe trial if the time the animal spent in the target quadrant wasgreater than 30% of total time (random occupancy in 1 of 4 quadrants is 25%), and was greater than in any other quadrant, thus indicating that the animal had acquired the spatial (short-term/working) memory of the escape platform’s location. Performing well in the initial probe trial (having a bias for the training quadrant) was used as an indicator that learning and working memory/short-term memory is intact. Following the initial probe trial, the platform was then replaced in its original location and animals received an additional reinstatement trial to minimize extinction of the escape platform reward. Forty-eight hours later, a single probe trial (no platform present) was repeated to measure long-term retention of the platform’s position. Animals that exhibited poor learning or memory performance (failure to occupy the target quadrant greater than 30% of the time) during acquisition and probe trials prior to WNV inoculation were excluded from the study.
Evaluation of cognitive performance following WNV inoculation & MGAWN1 therapy
At 4.5 days after injection with WNV, 13 hamsters were treated i.p. with a single dose of MGAWN1 (32 mg/kg of body mass)(Morrey et al., 2006, 2007), and 20 hamsters were treated i.p. with sterile physiological saline (placebo therapy). A second session of acquisition trials was performed to assess spatial learning and memory performance in WNV-inoculated animals with and without MGAWN1 therapy. This second session of spatial acquisition trials were performed beginning at 28 days after WNV inoculation, with the fixed hidden platform now positioned in the opposite quadrant to that in trials that were conducted prior to infection (Figure 1). The purpose was to evaluate the animals’ ability to learn a new spatial position for the platform and secondly, to determine whether long-term memory of the new spatial position is retained 48 hours later on the final retention probe trial. Twenty-eight days is a time after infection when WNV-infected animals were expected to have recovered from acute illness, in as much as sickness behavior was absent, food consumption had normalized, body mass had returned, virus had cleared from the blood, and antibodies specific for WNV had developed (Morrey et al., 2007; Xiao et al., 2001). Acquisition trials and subsequent probe tests with the platform removed were conducted as described aboveduring the pre-infection period.
To verify that animals could still perform tasks that were not based on reference memory of distal spatial cues, 5 trials using a visible platform (cueing) situated above the water were performed immediately after the 48 h probe trial at 32 dpi. Success of all animals mounting the platform within the mean of pre-infection times indicated that animals did not have sustained sensorimotor impairments that may have affected memory performance.
Motor function assessment
Animals were periodically (~1x/week, pre- and post-inoculation)evaluated on motor function by traversing a horizontal flat beam (8 cm wide, 1 meter long), and ladder (13 cm wide, 1 meter long), each suspended 60 cm off the floor by supports at either end. A non-invasive Grip Strength Meter (Columbus, Instruments) was utilized to assess the recovery of forelimb grip strength performance. These evaluations were performed primarily to ensure that motor function was intact at 28 dpi (prior to spatial memory assessment) in animals that survived WNV infection so as to not confound the cognitive assessment used in the MWM.
Results
Survivorship, body mass and food consumption
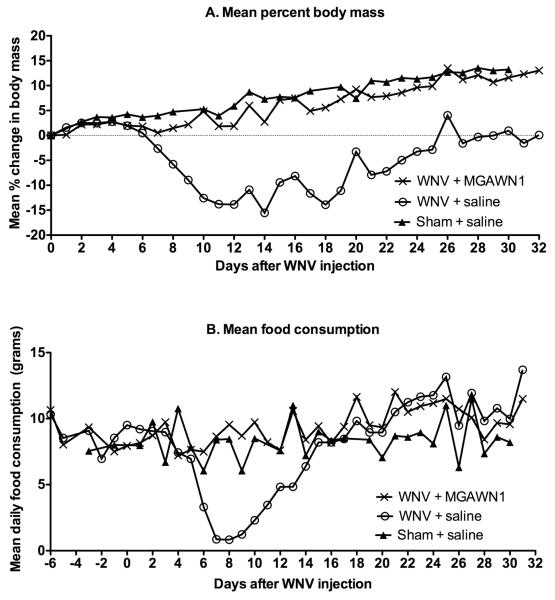
As expected from prior studies(Morrey et al., 2006, 2007), all 13 WNV-infected hamsters treated once i.p. with MGAWN1 at 32 mg/kg survived (Figure 2). Only 9 of 20 (45%) WNV-infected hamsters treated with saline survived. Likewise, MGAWN1 treatment reduced weight loss and anorexic behavior typically observed during the acute stage of illness (Morrey et al., 2004; Xiao et al., 2001) (Figure 3). MGAWN1-treated animals typically maintained or increased their body mass over the month long period following inoculation (10% average). All saline-treated infected hamsters exhibited decreases in body mass (5 – 22% loss from 0 dpi) beginning at approximately 5-7 dpi, but returned to baseline levels by about 25 dpi. Minimal or zero food consumption was observed in saline-treated hamsters by 7 dpi, typically for 3-4 days, then gradually returned, reaching baseline levels by 15 dpi (6-12 g/day) (Figure 3B). No changes in food consumption were observed in MGAWN1-treated animals overall.In a prior study(Morrey et al., 2007), a maximum dosage of 32 mg/kg and half-log dilutions were used to establish that the minimal effective concentration of MGAWN1 for improving survival was 0.32 mg/kg. Further studies will be required to identify the minimal effective concentration for improving memory.
Figure 2.
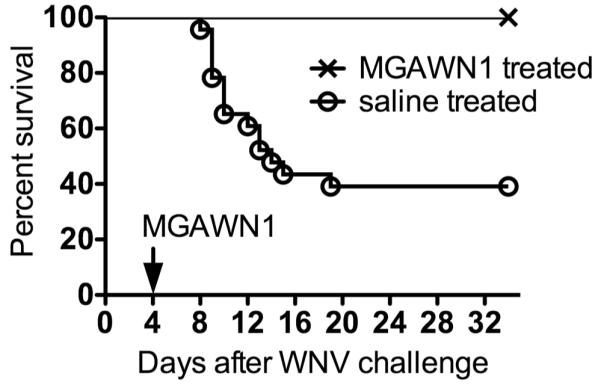
Survival of hamsters treated i.p. with MGAWN1 (32 mg/kg) or saline at 4.5 days after s.c. WNV injection.
Figure 3.
Effect of i.p. administration of MGAWN1 or saline on A) body mass, or B) food consumption of WNV-infected hamsters in Figure 2.
Evaluation of Long-term Memory following WNV inoculation and MGAWN1 therapy
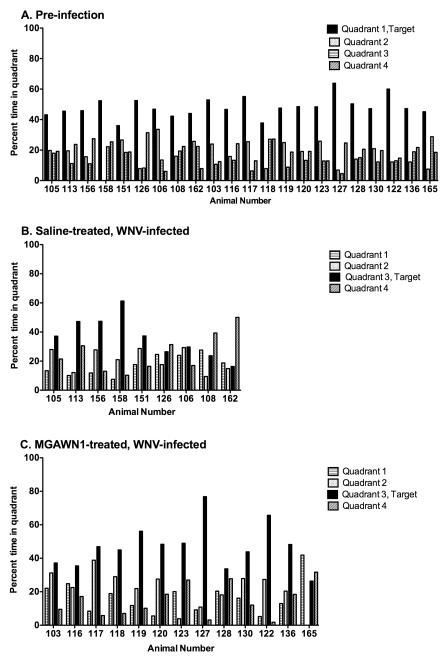
Prior to WNV inoculation, 33 animals meeting criterion were able to learn the position of the hidden escape platform and exhibited long-term spatial memory 48 h later, as evidenced by a preference for the platform area when the platform was absent (probe trial) (Figure 4A). All animals entered into the study had normal motor functions as illustrated with the grip strength test (Figure 5). Thirteen infected hamsters were treated with MGAWN1 and 20 were treated with saline at 4.5 dpi. A month after viral challenge, the MWM test was initiated with 9 surviving saline-treated hamsters and all 13 of the MGAWN1-treated hamsters. The 48 h probe trial was used as a measure to evaluate long-term spatial memory performance in WNV-inoculated hamsters in each treatment group (MGAWN1 or saline). Of the saline-treated hamsters, only 5 of the 9 (56%) were able to rememberthe spatial position of the platform 48 h later, as evidence by occupying the target quadrant more than any of the other quadrants (Table 1) (Figure 4B). The reduction from 100% successful MWM probe trial prior to viral challenge, to 56% 1 month after viral challenge was statistically significant (P ≤ 0.01), and agrees with earlier findings from an abstract (Smeraski et al., 2009) that WNV infection impairs long-term spatial memory.
Figure 4.
Morris Water Maze (MWM) 48 h probe trial for spatial learning and memory of hamsters (A) prior to WNV inoculation, or 28 days following inoculation and treated i.p. with B) saline or C) MGAWN1 (32 mg/kg) at 4.5 dpi. Timeline of experimental paradigm is outlined in Figure 1. The percentage of time spent in each of the quadrants during the first 60 s of the probe trail was measured from playback of video. The criterion for a successful trial used to construct Table 1 is that the hamster needed to occupy more time in the target quadrant than in any other quadrant, indicating that their long-term memory of the platform’s position during acquisition trials remained intact (25% dwell time = chance). (A) Pre-infection 48 hr probe trial of the 22 animals that survived WNV infection and whose 48 hr probe results following infection are shown in B and C. (B) Four of the 9 hamsters treated with saline (#126, #106, #108, #162) performed poorly on the 48 h probe trial; (C) only 1 of the 13 MGAWN1 treated hamsters (#165) did not remember the platform’s position and exhibited primarily thigmotaxic swimming during acquisition and probe trials.
Figure 5.
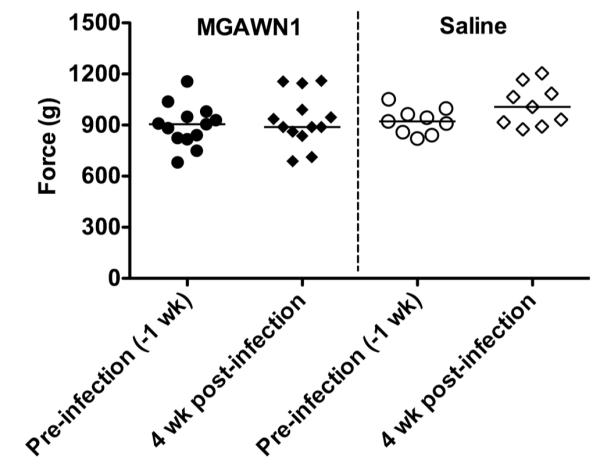
Forelimb grip strength test in hamsters before or after viral challenge treated i.p. with MGAWN1 or saline.
Table 1.
Percent of WNV-infected hamsters exhibiting successful performance in a spatial memory probe test at day 32 treated. Hamsters were treatedi.p. with the vehicle (saline) or with MGAWN1 at 4.5 days after s.c.viral challenge. The criterion for a successful probe trial was that the hamster spent more time in the target (training) quadrant than in any other quadrant as illustrated in Figure 5.
| Time of spatial memory probe trial |
WNV-infected, saline-treated |
WNV-infected, MGAWN1-treated |
|---|---|---|
| Pre-infection | 100% (9/9) | 100% (13/13) |
| 32 days post-infection | 56% (5/9)## | 92% (12/13)* |
P ≤ 0.05 compared to the 32-day probe trial of WNV-infected, saline-treated hamsters
P ≤ 0.01 compared to the same animals during the pre-infected probe trial. For statistical analysis, thetwo-sided Chi square test (Prism 5 for Mac OS X, GraphPad Software, Inc) of whether animals spent more time in the target quadrant than any other quadrant was used.
Of the MGAWN1-treated hamsters at 32 dpi, 12 of 13 (92%) remembered the position of the platform during the long-term (48 h) probe trial (Figure 4C). These data representing a 92% success rate with MGAWN1-treated hamsters compared with a 56% success with saline-treated hamsters indicated that WNV-infected hamsters treated withMGAWN1at 4.5 dpi significantly (P≤ 0.05) improved performance on the long-term memory probe trial.
Furthermore, of the 4 WNV-infected hamsters (saline treated) that did not perform well on the 48-hour probe trial (Figure 4B), 3 performed well when tested on the initial probe trial conducted immediately after acquisition training (at 30 dpi) (data not shown). It was only after 48 hours that these 3 animals failed to remember the location of the absent platform during the 48 h probe trial, indicating long-term memory impairment. Failure to perform well in the initial probe trial by the one animal indicates that learning and/or short term/working memory may be impaired, which clearly impacts long-term memory. The one animal (#165) in the MGAWN1 treated group that did not exhibit long-term (48 hour) spatial memory of the platform position exhibited primarily thigmotaxic behavior (swimming along the walls of the water basin) during many of the acquisition trials and both probe trials. This stress-related swimming behavior may have accounted for its failure to pass the memory probe trial test, rather than representing learning and memory deficits.
Discussion
The results indicate that MGAWN1, administered 4.5 days after WNV challenge in hamsters, prevented spatial memory impairment as measured by the MWM probe task 32 days after WNV challenge and is in agreement with other therapeutic benefits of MGAWN1 previously identified in mouse and hamster models. Single i.p. injections with MGAWN1 (also known as hE16), a potent humanized monoclonal antibody, significantly improves survival in mice (Oliphant et al., 2005) inoculated with WNV. As also in agreement with this study, MGAWN1 administered once in hamsters on day 5 after viral challenge when the virus has infected the brain improves survival, weight change, and disease signs (Morrey et al., 2006; Morrey et al., 2007). Moreover, it prevents acute flaccid paralysis when administered after the virus has infected the lumbar spinal cord (Morrey et al., 2008). Because WNV has been shown to initiate infection of the central nervous system of hamsters by at least day 3 (Morrey et al., 2010), we initiated treatment in this study at day 4.5.
We used hamsters in this study because they model certain aspects of WNV disease in human patients. WNV can infect, damage or destroy neurons in the brain and spinal cord both hamsters and humans(Agamanolis et al., 2003; Morrey et al., 2006; Morrey et al., 2007; Omalu et al., 2003; Samuel et al., 2007; Shrestha et al., 2003; Xiao et al., 2001). It causes mononuclear infiltration, perivascular cuffing, and gliosis in the cerebellum, cerebral cortex, diencephalon, mesencephalon, pons, medulla and spinal cord in both species(Agamanolis et al., 2003; Doron et al., 2003; Petropoulou et al., 2005; Sampson et al., 2000; Xiao et al., 2001). WNV neurological disease is also characterized by meningitis, encephalitis, poliomyelitis-like disease, hemi-paralysis and paresis in hamsters (Morrey et al., 2004; Morrey et al., 2008; Xiao et al., 2001) and humans(Chowers et al., 2001; Jeha et al., 2003; Weiss et al., 2001). As in human cases, surviving hamsters exhibit a variable outcome: some exhibit neurological/functional symptoms while others appear healthy.
We verified in this study that some, but not all, hamsters having a spatial memory of the location of the platform’s position in the MWM exhibit poor memory performance as measured on day 32 after viral challenge. This confirms the findings of spatial memory impairment of WNV-infected hamsters in a previous study using the same MWM test(Smeraski et al., 2009). This observation of memory impairment in hamsters may be relevant to human patients with WNV disease, in as much as published results of WNV-patient records and surveys indicate that a subpopulation of patients recovering from the acute WNV infection complain of problems with memory and executive functions (Carson et al., 2006; Cook et al., 2010; Gottfried et al., 2005).
It is well established that the hippocampus plays a prominent role in learning and memory and that this brain region can become infected with WNV in mice (Hunsperger and Roehrig, 2006) and hamsters (Siddharthan et al., 2009; Smeraski et al., 2009) to cause histopathological lesions. The means that the mechanism by which WNV causes neuronal injury and associated neurological sequelae is not yet well established, but it is thought that both direct virus infection and bystander injury induced by neurotoxic factors and inflammatory genes/proteins of non-neuronal brain cells (e.g., glia) may contribute (van Marle et al., 2007). Furthermore, in vitro studies using SK-N-SH cells (transformed human neuroblastoma cell line), have suggested that WNV-infected neurons may also be one of the sources of proinflammatory cytokines that mediate neuronal cell death (Kumar et al., 2010). MGAWN1 therapy administered at 4.5 dpi may have protected against cognitive impairment by either directly arresting viral load, or by indirectly reducing the proinflammatory response, thereby preventing the eventual neuronal injury typically observed by either process.
The presence of anorexic behavior can be utilized as a noninvasive indicator of the onset of sickness behavior induced by cytokine signaling the brain (Asarian and Langhans, 2010).During systemic infections, the innate immune response mounts an acute phase response, a coordinated cascade of physiological and behavioral events that fight the infectious disease agent. Included is a collection of behaviors known as sickness behaviors, and includes hyperthermia, anorexia, reduced social activities, decrease locomotion, weakness, and increased sleep(Maier and Watkins, 1998). Systemic cytokines in response to infectious agents in the periphery signal the brainby neural and/or humoral routes to produce sickness behaviors by inducing expression of cytokines in the brain(Kelley et al., 2003). Uncontrolled increases in circulating or local (brain) cytokines in response to peripheral/centralnervous system infection can further activate resident immune cells in the brain, which may enhance or prime neuronal injury. That MGAWN1-treated animals did not exhibit anorexic behavior or other associated sickness behaviors, suggests that inhibition of the virus halted proinflammatory cytokine signaling to the brain. It is possible that unabated proinflammatory responses in the central nervous system (e.g., hippocampus) may have contributed to neuronal injury during early phases of acute illness in saline-treated WNV-infected hamsters, thus impinging upon neural circuitry underlying spatial memory. Preliminary studies suggest that the hippocampus sampled from saline treated WNV-infected hamsters has elevated levels of interleukin-1β by 8 dpi when compared to hipppocampi sampled from placebo- or MGAWN1-treated WNV-infected hamsters (unpublished observations).
Overall these studies support the possibility that WNV can cause spatial memory impairment and that therapeutic intervention may be warranted.
Acknowledgements
We are grateful for Jeffrey L. Nordstrom, Macrogenics, Inc. for providing MGAWN1, and R.A. Bowen, Colorado State University, in partial support of CAS. This work was supported by U54 A1-065357-04 (to JDM) Rocky Mountain Regional Center for Excellence, NIAID, NIH, and 5R21NS056280-02 (to CAS) NINDS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: C.A.S and V.S. do not have any conflict of interest. J.D.M. is a consultant for Macrogenics Inc.
References Cited
- Agamanolis DP, Leslie MJ, Caveny EA, Guarner J, Shieh WJ, Zaki SR. Neuropathological findings in West Nile virus encephalitis: a case report. Ann. Neurol. 2003;54:547–551. doi: 10.1002/ana.10731. [DOI] [PubMed] [Google Scholar]
- Asarian L, Langhans W. A new look on brain mechanisms of acute illness anorexia. Physiol. Behav. 2010;100:464–471. doi: 10.1016/j.physbeh.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Beigel JH, Nordstrom JL, Pillemer SR, Roncal C, Goldwater DR, Li H, Holland PC, Johnson S, Stein K, Koenig S. Safety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virus. Antimicrob. Agents Chemother. 2010;54:2431–2436. doi: 10.1128/AAC.01178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenz EJ, Rodriguez M, Howe CL. Disrupted spatial memory is a consequence of picornavirus infection. Neurobiol. Dis. 2006;24:266–273. doi: 10.1016/j.nbd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Carson PJ, Konewko P, Wold KS, Mariani P, Goli S, Bergloff P, Crosby RD. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin. Infect. Dis. 2006;43:723–730. doi: 10.1086/506939. [DOI] [PubMed] [Google Scholar]
- Chowers MY, Lang R, Nassar F, Ben-David D, Giladi M, Rubinshtein E, Itzhaki A, Mishal J, Siegman-Igra Y, Kitzes R, Pick N, Landau Z, Wolf D, Bin H, Mendelson E, Pitlik SD, Weinberger M. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg. Infect. Dis. 2001;7:675–678. doi: 10.3201/eid0704.010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Xu X, Yablonsky EJ, Sakata N, Tripp JH, Hess R, Piazza P, Rinaldo CR. Demographic and clinical factors associated with persistent symptoms after West Nile virus infection. Am. J. Trop. Med. Hyg. 2010;83:1133–1136. doi: 10.4269/ajtmh.2010.09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron SI, Dashe JF, Adelman LS, Brown WF, Werner BG, Hadley S. Histopathologically proven poliomyelitis with quadriplegia and loss of brainstem function due to West Nile virus infection. Clin. Infect. Dis. 2003;37:e74–77. doi: 10.1086/377177. [DOI] [PubMed] [Google Scholar]
- Gottfried K, Quinn R, Jones T. Clinical description and follow-up investigation of human West Nile virus cases. South Med. J. 2005;98:603–606. doi: 10.1097/01.SMJ.0000155633.43244.AC. [DOI] [PubMed] [Google Scholar]
- Hunsperger EA, Roehrig JT. Temporal analyses of the neuropathogenesis of a West Nile virus infection in mice. J. Neurovirol. 2006;12:129–139. doi: 10.1080/13550280600758341. [DOI] [PubMed] [Google Scholar]
- Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile virus infection: a new acute paralytic illness. Neurology. 2003;61:55–59. doi: 10.1212/01.wnl.0000073617.08185.0a. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Kupfermann I, Iversen S. Learning and memory. In: Kandel ER, Jessell TM, editors. Principles of Neural Science. 4 ed McGraw-Hill; New York: 2000. pp. 1227–1246. [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003;17(Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kumar M, Verma S, Nerurkar VR. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. J. Neuroinflam. 2010;7:73. doi: 10.1186/1742-2094-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Olsen AL, Sidwell RW, Cheney CD, Blatt LM. Modeling hamsters for evaluating West Nile virus therapies. Antiviral Res. 2004;63:41–50. doi: 10.1016/j.antiviral.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Roper GY, Wang H, Baldwin TJ, Koenig S, Johnson S, Nordstrom JL, Diamond MS. Humanized monoclonal antibody against West Nile virus envelope protein administered after neuronal infection protects against lethal encephalitis in hamsters. J. Infect. Dis. 2006;194:1300–1308. doi: 10.1086/508293. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Wang H, Julander JG, Hall JO, Li H, Nordstrom JL, Koenig S, Johnson S, Diamond MS. Defining limits of treatment with humanized neutralizing monoclonal antibody for West Nile virus neurological infection in a hamster model. Antimicrob. Agents Chemother. 2007;51:2396–2402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Wang H, Hall JO, Motter NE, Skinner RD, Skirpstunas RT. Neurological suppression of diaphragm electromyographs in hamsters infected with West Nile virus. J. Neurovirol. 2010;16:3198–3129. doi: 10.3109/13550284.2010.501847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Wang H, Hall JO, Skirpstunas RT, Olsen AL, Nordstrom JL, Koenig S, Johnson S, Diamond MS. West Nile virus-induced acute flaccid paralysis is prevented by monoclonal antibody treatment when administered after infection of spinal cord neurons. J. Neurovirol. 2008;14:152–163. doi: 10.1080/13550280801958930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omalu BI, Shakir AA, Wang G, Lipkin WI, Wiley CA. Fatal fulminant pan-meningo-polioencephalitis due to West Nile virus. Brain Pathol. 2003;13:465–472. doi: 10.1111/j.1750-3639.2003.tb00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulou KA, Gordon SM, Prayson RA, Ruggierri PM. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am. J. Neuroradiol. 2005;26:1986–1995. [PMC free article] [PubMed] [Google Scholar]
- Rubin SA, Yednock TA, Carbone KM. In vivo treatment with anti-alpha4 integrin suppresses clinical and pathological evidence of Borna disease virus infection. J. Neuroimmunol. 1998;84:158–163. doi: 10.1016/s0165-5728(97)00249-x. [DOI] [PubMed] [Google Scholar]
- Sampson BA, Ambrosi C, Charlot A, Reiber K, Veress JF, Armbrustmacher V. The pathology of human West Nile Virus infection. Hum. Pathol. 2000;31:527–531. doi: 10.1053/hp.2000.8047. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Morrey JD, Diamond MS. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol. 2007;81:2614–2623. doi: 10.1128/JVI.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddharthan V, Wang H, Motter NE, Hall JO, Skinner RD, Skirpstunas RT, Morrey JD. Persistent West Nile virus associated with a neurological sequela in hamsters identified by motor unit number estimation. J. Virol. 2009;83:4251–4261. doi: 10.1128/JVI.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeraski CA, Simpson AM, Bowen RA. Exploring a hamsters model of West Nile virus encephalitis. 39th Annual Meeting of the Society for Neuroscience; Chicago, IL. 2009. [Google Scholar]
- van Marle G, Antony J, Ostermann H, Dunham C, Hunt T, Halliday W, Maingat F, Urbanowski MD, Hobman T, Peeling J, Power C. West Nile virus-induced neuroinflammation: glial infection and capsid protein-mediated neurovirulence. J. Virol. 2007;81:10933–10949. doi: 10.1128/JVI.02422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Carr D, Kellachan J, Tan C, Phillips M, Bresnitz E, Layton M. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg. Infect. Dis. 2001;7:654–658. doi: 10.3201/eid0704.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao SY, Guzman H, Zhang H, da Rosa A.P. Travassos, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg. Infect. Dis. 2001;7:714–721. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]