Abstract
Background
Various studies have shown that the population densities of a number of forest vertebrates, such as orangutans, are higher on Sumatra than Borneo, and that several species exhibit smaller body sizes on Borneo than Sumatra and mainland Southeast Asia. It has been suggested that differences in forest fruit productivity between the islands can explain these patterns. Here we present a large-scale comparison of forest fruit production between the islands to test this hypothesis.
Methodology/Principal Findings
Data on fruit production were collated from Sumatran and Bornean sites. At six sites we assessed fruit production in three forest types: riverine, peat swamp and dryland forests. We compared fruit production using time-series models during different periods of overall fruit production and in different tree size classes. We examined overall island differences and differences specifically for fruiting period and tree size class. The results of these analyses indicate that overall the Sumatran forests are more productive than those on Borneo. This difference remains when each of the three forest types (dryland, riverine, and peat) are examined separately. The difference also holds over most tree sizes and fruiting periods.
Conclusions/Significance
Our results provide strong support for the hypothesis that forest fruit productivity is higher on Sumatra than Borneo. This difference is most likely the result of the overall younger and more volcanic soils on Sumatra than Borneo. These results contribute to our understanding of the determinants of faunal density and the evolution of body size on both islands.
Introduction
Patterns of fruit production vary substantially among tropical forests. Studies of tropical forest phenology have demonstrated differences in fruit production between continents [1], [2], between sites [3]–[6], and between forest types within sites [7]–[13]. This variation is thought to be determined by a range of factors, such as soil nutrients [9], [10], [14]–[18], rainfall [4], [19], [20], latitude [21], altitude [7], [8], [21], levels of carbon dioxide [22], [23], and solar irradiation [1], [24], [25]. Characterizing differences in fruit production at different spatial scales may shed light on a range of questions in forest and vertebrate ecology, vertebrate evolution, biogeography, and conservation biology. For example, fruit productivity is typically thought to set carrying capacity for rain forest vertebrates, which in turn may have effects on plant life history, seed dispersal, and vertebrate population dynamics [16], [26]–[31]. In addition, resource availability differences on islands might affect the evolution of mammal body size [32].
Many studies of tropical forest phenology have been conducted in Malesia (e.g. [5], [33]–[35]), in part due to the unusual patterns of inter-specific gregarious fruiting characteristic of the region (i.e., mast fruiting: [26], [36]–[38]). Despite the existence of a number of long-term data sets, few attempts have been made to compare general patterns of fruit production in different parts of Malesia (but see [5], [33], [39] for comparisons of mast fruiting across sites). It has been suggested that Sumatran rain forests are generally more productive than their Bornean counterparts [39]–[41]. This hypothesis is based on the assumption that ongoing tectonic activity, including uplift, and recent volcanism has created more fertile soils on much of Sumatra than are found on most of Borneo [41], [42]. This region is well-suited for a comparative study because the forests on Sumatra and Borneo have recurrently been connected during the various glacial periods, which has led to mixing of both plant and animal species (e.g. [43]). Hence, systematic differences between Borneo and Sumatra in for instance plant productivity and subsequent differences mammal body size are more likely to be the result of relatively short-term environmental differences such as differences in soil nutrition than long-term differences (allopatric divergence). In addition, the observation that primate biomass is higher on Sumatra than on Borneo suggests that Sumatran forests may be more productive [44]. There are also several differences in the behavioural ecology and life history of orangutans (Pongo spp.) that are thought to have resulted from higher fruit production on Sumatra than Borneo [40], [45]–[47]. In addition, the lower productivity in Borneo has been hypothesised to influence the reduction in body size of several mammal species. Body sizes of the Malayan Sun Bear (Helarctos malayanus: [48]), the greater chevrotain (Tragulus napu: [49]), sambar (Cervus unicolor: [50]) and many others [51] are all smaller on Borneo compared to Sumatra and mainland Asia. Moreover, Meiri et al. [52] found that among an entire ecological guild, the carnivores, Bornean forms were smaller than their mainland conspecifics. Such smaller size evolution could have been the result of lower resource availability [32] and it is thus important to determine whether resource availability indeed is lower on Borneo.
In this paper we compare fruit productivity between forests on the islands of Sumatra and Borneo using a standard measure, the percentage of trees per month that carry fruit. This measure has been used in several studies of forest and vertebrate ecology (e.g. [5], [35], [53]). We hypothesise that on average Sumatran forests have a higher fruit production than forests on Borneo, but that this will only occur in those Sumatran forests that potentially have nutrient influences from fertile soils. Thus Sumatran forests that are fed nutrients from rivers or nutrient run-off from large mountain massifs should show higher fruit production, but those Sumatran forests that do not obtain such nutrient influences are predicted to be similar in productivity to Bornean forests.
Results
Overall Island Differences
The time-series model of fruit productivity accounting for variation in DBH and fruit level category resulted in a significant difference in fruit production between Borneo and Sumatra (overview of sites: Table 1, Figure 1), with Sumatra being characterized as having significantly higher levels of fruit production (Table 2). To further illustrate these differences, we break each analysis down by forest type (Table 3).
Table 1. Habitat characteristics and survey effort at each site.
| Sites | Island | Altitude (m) | Annual rainfall (mm) | Total trees | # trees 15–29.9 cm dbh | # trees 30–44.9 cm dbh | # trees 45–59.9 cm dbh | # trees 60–74.9 cm dbh | # trees 75–89.9 cm dbh | # trees >90 cm dbh | Sample area (ha) | Data collection Period | # months |
| Peat forest | |||||||||||||
| Suaq | S | 5 | 3362 | 424 | 278 | 86 | 35 | 14 | 3 | 8 | Feb 94–Aug 99 | 66 | |
| GP | B | 5–10 | 4300 | 779 | 487 | 181 | 74 | 20 | 11 | 6 | 1.5 | Jan 86–Sep 91 | 79 |
| TP | B | 5–10 | 3026 | 891 | 666 | 181 | 32 | 8 | 4 | 0 | 2.0 | Apr 93–Jun 96 | 40 |
| Riverine forest | |||||||||||||
| Suaq | S | 5 | 3362 | 183 | 100 | 47 | 16 | 9 | 4 | 7 | Feb 94–Aug 99 | 66 | |
| GP | B | 5–10 | 4300 | 890 | 608 | 168 | 69 | 25 | 15 | 5 | 2.0 | Jan 86–Sep 91 | 79 |
| Dryland forest | |||||||||||||
| Ketambe | S | 350–500 | 3288 | 600 | 387 | 110 | 56 | 17 | 14 | 16 | 1.1* | Sep 88–May 01 | 153 |
| Suaq | S | 5–150 | 3362 | 309 | 194 | 55 | 29 | 10 | 6 | 15 | Feb 94–Aug 99 | 67 | |
| Sungai Wain | B | 30–150 | 2968 | 315 | 233 | 46 | 17 | 13 | 0 | 6 | 1.0 | Jan 98–Jul 02 | 55 |
| Barito Ulu | B | 100–300 | 3750 | 134 | 107 | 18 | 7 | 1 | 1 | 0 | - | Nov 90–Jun 00 | 124 |
| GP AB | B | 5–50 | 4300 | 718 | 413 | 168 | 72 | 30 | 16 | 19 | 2.0 | Jan 86–Sep 91 | 79 |
| GP LS | B | 20–200 | 4300 | 1139 | 614 | 280 | 98 | 67 | 30 | 50 | 3.0 | Jan 86–Sep 91 | 79 |
| GP | B | 200–400 | 4300 | 934 | 472 | 273 | 95 | 57 | 20 | 17 | 1.8 | Jan 86–Sep 91 | 79 |
Note: S = Sumatra, B = Borneo, GP = Gunung Palung, AB = alluvial bench, LS = lowland sandstone, LG = lowland granite forest, TP = Tanjung Puting. For some sites there is no sample area as the area in which the trees were located was not measured. Rainfall data from the references in the method section for each site except for Tanjung Puting [75].
*in Ketambe total plot area was extended to 1.6 ha in 1997.
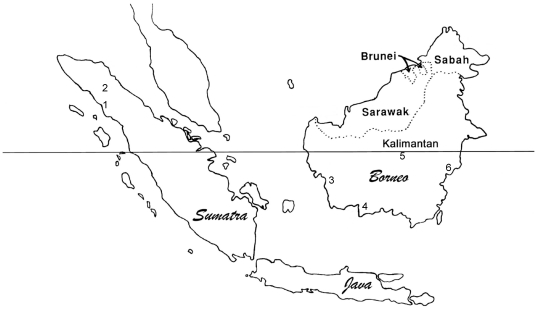
Figure 1. Site locations.
Locations of study sites: 1 = Suaq; 2 = Ketambe; 3 = Gunung Palung; 4 = Tanjung Puting; 5 = Barito Ulu; 6 = Sungai Wain.
Table 2. Differences in time series estimated fruit production between Borneo and Sumatra.
| Mean difference in % fruiting (Sumatra-Borneo) | SE | t-statistic | P-value (two-sided) | |
| All sites combined | 13.06 | 0.22 | 59.54 | <0.0001 |
| Dryland forests | 10.90 | 0.26 | 41.18 | <0.0001 |
| Peat swamp forests | 23.84 | 0.53 | 45.35 | <0.0001 |
| Riverine forests | 6.08 | 0.49 | 12.51 | <0.0001 |
Note: All sites are combined and split by habitat type. Models include estimates for DBH and fruit level category. SE is standard error.
Table 3. Pairwise comparisons of all sites within Borneo and Sumatra by habitat.
| Bornean site | Sumatran Site | Habitat type | Estimated Mean % (Borneo) | Estimated Mean % (Sumatra) | Mean difference in % fruiting (Sumatra-Borneo) | SE | t-value | DF | p-value | Adjusted p-value |
| BU | KET | Dry | 5.41 | 25.11 | 19.7 | 0.5 | 39.65 | 835 | <0.0001 | <0.0001 |
| GP AB | KET | Dry | 6.59 | 25.11 | 18.53 | 0.4 | 46.75 | 1147 | <0.0001 | <0.0001 |
| GP LG | KET | dry | 3.97 | 25.11 | 21.14 | 0.62 | 34.3 | 951 | <0.0001 | <0.0001 |
| GP LS | KET | dry | 6.49 | 25.11 | 18.62 | 0.4 | 46.19 | 1195 | <0.0001 | <0.0001 |
| SW | KET | dry | 3.03 | 25.11 | 22.08 | 0.4 | 54.64 | 1096 | <0.0001 | <0.0001 |
| BU | SB | dry | 5.41 | 6.89 | 1.48 | 0.43 | 3.41 | 497 | 0.0007 | 0.009 |
| GPAB | SB | dry | 6.59 | 6.89 | 0.3 | 0.31 | 0.97 | 495 | 0.3344 | 1 |
| GPLG | SB | dry | 3.97 | 6.89 | 2.92 | 0.57 | 5.16 | 687 | <0.0001 | <0.0001 |
| GPLS | SB | dry | 6.49 | 6.89 | 0.4 | 0.32 | 1.23 | 536 | 0.2177 | 1 |
| SW | SB | dry | 3.03 | 6.89 | 3.86 | 0.32 | 11.97 | 489 | <0.0001 | <0.0001 |
| GP PS | SB | peat | 6.81 | 30.57 | 23.76 | 0.59 | 40.48 | 443 | <0.0001 | <0.0001 |
| TNPUT | SB | peat | 6.65 | 30.57 | 23.92 | 0.52 | 45.96 | 296 | <0.0001 | <0.0001 |
| GPFS | SB | river | 6.08 | 12.17 | 6.08 | 0.49 | 12.51 | 373 | <0.0001 | <0.0001 |
Note: Time series estimated fruit production means (% fruiting). Models include DBH and fruit level category as variables in the model. (For Gunung Palung (GP), AB = alluvial bench, LS = lowland sandstone, LG = lowland granite, PS = peat swamp, FS = freshwater swamp). SE is standard error. DF is degrees of freedom. P-values are two-sided. The adjusted p-value is the maximum of 1 and the p-value times 13.
Island Variation among Habitat Types
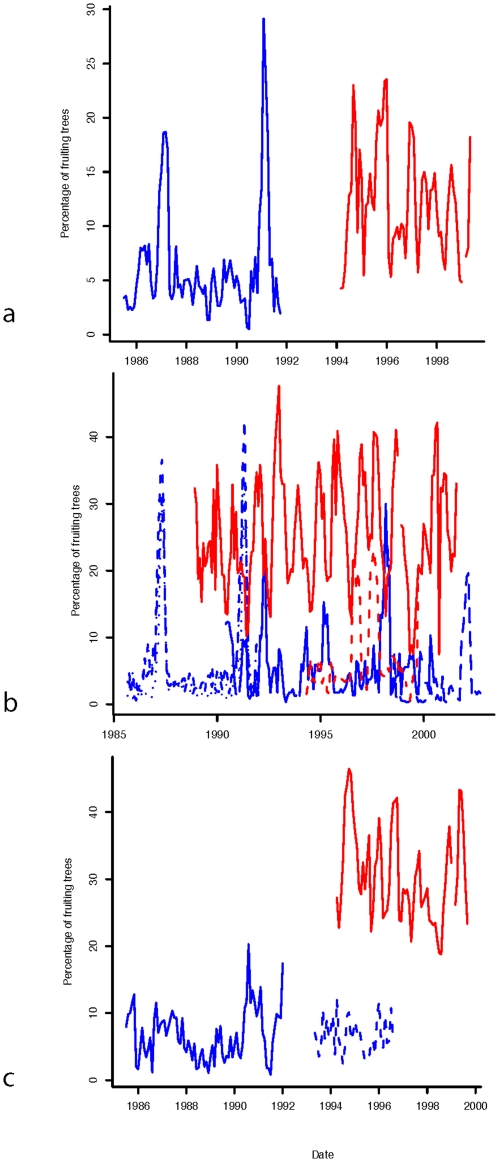
Riverine and dryland forest habitats showed the greatest fluctuations in fruit production, with high peaks characteristic of mast fruiting (Figures 2a, b), while fruit production in peat swamp forests showed less pronounced fluctuation (Figure 2c). Overall, fruit production was consistently higher in Sumatra compared to Borneo across all three habitat types (Figures 2, 3; Riverine: Table 3 and Methods S1, Table S1, S2; Peat Swamp Forest: Table 3 and Tables S3, S4, S5, S6; Dryland Forest: Table 3, Tables S7, S8). This pattern held across most DBH classes and fruiting periods, with the few exceptions occurring primarily for smaller DBH classes during low fruit periods (Tables S2, S4, S5, S6, S8). The only cases where a Bornean forest had higher overall fruit production was for the dryland forest habitats of Gunung Palung AB and Gunung Palung LS compared to Suaq Balimbing in Sumatra (Table 3).
Figure 2. Graph of forest fruit availability.
Time series graphs of each forest type (Fig 2a, b, c) (Riverine (a), Dryland forest (b), Peat (c). Blue = Borneo, Red = Sumatra.
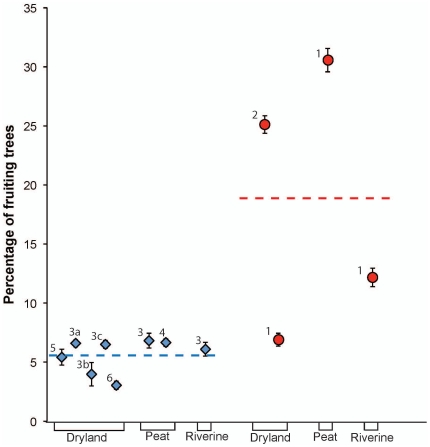
Figure 3. Mean fruit availability per site.
Graph of time-series corrected model mean estimates of the percentage of fruiting trees and 95% confidence intervals for all sites in Borneo and Sumatra separated by habitat type. Field sites in Borneo are represented by blue triangles and those in Sumatra are represented by red circles. The numbers in the figure correspond to the following field areas or sites: 1 = Suaq Balimbing, 2 = Ketambe, 3 = Gunung Palung, 3a = Gunung Palung AB, 3b = Gunung Palung LG, 3c = Gunung Palung LS, 4 = Tanjung Puting, 5 = Barito Ulu, 6 = Sungai Wain.
Discussion
The results in this paper clearly show differences in fruit production among sites. It is, however, important to determine whether these differences vary systematically between the two islands and therefore support our hypothesis or merely reflect site differences that are unrelated to island differences. Overall, fruit production in the three forest types assessed is higher on Sumatra than on Borneo (Table 2). For riverine and peat swamp forests, Suaq (Sumatra) shows a significantly higher fruit production than the Bornean sites. For dryland forests it is clear that Ketambe and Suaq (Sumatra) have a significantly higher fruit production than almost all forests on Borneo. Only for two of the pairwise comparisons did a Sumatran site (Suaq) not show a significantly higher fruit production than a Bornean site (Gunung Palung). The Suaq dryland forest is somewhat unique in that it is a completely isolated low lying hill that does not obtain any nutrient influence from the Leuser mountain massif as the forest at Ketambe does and as do most forests in the area. This nutrient influence for forests such as Ketambe that are located at the base or on slopes of large mountain massifs might explain why some Sumatran sites appear to have such a high fruit production and, at even high altitude, they still show high orangutan densities [31]. Thus although the general trend is that there is an overall higher productivity in Sumatra, it is important to realise that not all inter-site comparisons follow this general trend. More long-term phenological data, especially from Sumatran sites, are needed to examine the general applicability of the trends indicated here. Ideally new studies on both islands should use similar phenological data collection methods that record both fruiting frequency and fruit crop sizes. Such refinements are needed to fully appreciate potential differences between sites and islands.
Because the number of sites is limited it is relevant to assess whether the pattern found here with the two Sumatran sites having a higher fruit production than the four Bornean sites. Under the assumption that all sites are equal, the chance that the two Sumatran sites would be higher than the four Bornean sites is 0.067. This is a very conservative probability because it is purely based on ranking and does not incorporate the real fruit production percentages, which would lead to lower p-values. If one would use the 12 locations instead of the six sites the ranking found has a probability of 0.008. Thus the chance that the pattern found is due to chance is low, which gives confidence into the generality of these results.
It is also important to determine whether there are overall factors that potentially differ between the islands and therefore can explain the variation. Several studies have shown that rainfall is correlated to plant productivity (e.g. [4]). Mean annual rainfall at the Sumatran sites seems similar to that for the Bornean sites (mean Sumatran site rainfall = 3323 mm; Borneo = 3511 mm), which does not support the prediction that higher rainfall should lead to higher plant productivity, although above 2500 mm this effect might be absent [4].
Because the data for these comparisons were compiled post-hoc from a large number of sites where research questions differed, it is important to discuss whether possible differences in research methodology could have influenced the results presented in this paper. Although the data collection between sites differed to the extent that the number of fruits on trees were sometimes scored in different classes, the data always clearly indicate whether fruits were absent or present on a tree and, as such, these data were directly comparable at this level. Another potential bias might be that sampling periods did not always overlap in time and as such might not be directly comparable. Especially since fruit production can be tied to climatic factors such as ENSO [5], [33], systematic differences between Sumatra and Borneo might have confounded comparisons (e.g. due to global climate change). However, for our dataset, the midpoints of the sampling periods did not differ significantly [39]. In addition, a previous analysis between Ketambe and Gunung Palung indicated that for the same time period fruit production is still significantly higher for the Sumatran site [54]. We also attempted to correct for this possible confounding factor by comparing fruit production in three different periods of fruit production. This also reduced biases that may have been introduced through the use of data sets of different durations. Thus, we are confident that our results indeed reflect island differences in fruit production rather than climatically-mediated temporal differences.
It is important to note that our study considered differences in fruiting frequency, and did not incorporate indices of fruit crop size. We suggest that future studies use standardized data collection methods that incorporate crop size measures to permit more detailed future comparisons or conduct analyses at species or community levels (e.g. [55]). Another useful next step in refining comparisons would be to categorise trees as mast- or non mast-type (cf. [56]) and then conduct analyses separately for the two categories. Unfortunately, the duration of data collection at many of the sites included in these analyses did not allow for such an analysis.
These results support a growing body of studies that indicates higher production of forests in Sumatra than on Borneo. These studies show that primate biomass in general, but also specifically for certain species, such as orangutans, is higher on Sumatra than Borneo [39], [40], [43]. Several comparisons also indicate that mammal body size is smaller on Borneo than on Sumatra [48]–[51], which could be the result of an evolution towards smaller body size in an area where resource availability is reduced [32].
Future comparisons of forest production should ideally be conducted by using standardized litterfall protocols, because these yield standard data on actual productivity (kg/ha: e.g. [14], [50], [57]). In combination with collecting litterfall data soil nutrient studies should be conducted that use standard methods to assess soil fertility (e.g. [8]). These studies should ideally also examine productivity measures of similar families, genera or even species on both islands to exclude the influence of island differences in those on the overall analyses. Such future studies will be important in making more fine-grained comparisons between and within the two islands and will help us to better understand the differences in fauna between the islands.
Materials and Methods
Tree fruit phenology data were collected at two study sites on Sumatra and four sites on Borneo (Figure 1, Table 1). All study sites consisted of undisturbed forest. To ensure that our analyses were not biased by comparing different forest types at different sites, all comparisons were done between forests of the same general type. For this analysis we recognized three broad forest types: peat forest (peat), riverine forest (river), and dryland forest (dry). Peat forests are found on relatively acidic (4<pH<6), nutrient-poor, poorly-drained soils that are overlain by variable amounts of organic matter. Tree species diversity in most Asian peat forests is impoverished relative to more well-drained forest types, and the canopy is relatively low and even [40], [58]. Riverine forest, as defined here, encompasses freshwater swamps and frequently inundated alluvial fans. Soils in riverine forest are generally less acidic than peat soils (pH>6) nutrient rich, seasonally flooded, and poorly drained [59]. Dryland forests, as defined here, are found up to 500 m asl on generally well-drained soils. Species diversity in these dryland forests is relatively high and the canopy is tall and well-structured [58]. Although each of these three forest types encompasses a range of variation due to microhabitat heterogeneity, edaphic effects, and differences in rainfall, this broad classification scheme permitted the comparison of sites that are similar in drainage, species diversity, and structure.
Study Sites
Sumatra
Ketambe (KET) (3°41′N, 97°39′E) is located in the upper Alas valley in Gunung Leuser National Park, Leuser Ecosystem. This study area mainly consists of primary dryland rain forest and was described in detail by Rijksen [60], van Schaik and Mirmanto [9] and Wich and van Schaik [5].
Suaq Balimbing (SB) (3°04′N, 97°26′E) is located in the western coastal plain, and consists of a variety of floodplain and hill forest habitats. It forms part of Gunung Leuser National Park, Leuser Ecosystem [5].
Borneo
Barito Ulu (BU) is located in Central Kalimantan, Indonesia, at 114°0′E, 0°06′S. The research area covers 430 ha and contains a mosaic of forest types. These include several types of tropical lowland evergreen rain forest [35], [61].
Gunung Palung (GP) (1°13′S, 110°7′E) is located in Gunung Palung National Park, West Kalimantan, Indonesia. Data were collected in several distinct forest types at the Cabang Panti Research Station: peat swamp (5–10 m a.s.l.), riverine forest (freshwater swamp; 5–10 m a.s.l.), and three types of dryland forest (alluvial bench, lowland sandstone, and lowland granite; 5–400 m a.s.l.). General descriptions and detailed data on the plant composition of each habitat are provided in Webb [62], Cannon and Leighton [63], Marshall [59], and Paoli et al. [64], [65].
Sungai Wain (SW) is located in Sungai Wain Protected Forest, East Kalimantan, Indonesia (1°05′S, 116°49′E) and consists of lowland dipterocarp forest. The topography of the reserve consists of gentle to sometimes steep hills, and is intersected by many small rivers [66].
Tanjung Puting (TP) (2°46′S, 111°52′E) is located in Tanjung Puting National Park, Central Kalimantan, Indonesia. Data were collected at Natai Lengkuas Station in peat swamps that were periodically flooded with freshwater [67].
Field Methods
Trained observers collected monthly presence/absence of fruit (immature and/or mature, cf. [56]) on each tree by using binoculars to examine the canopy of each tree.
Analyses
For each site we calculated the percentage of trees fruiting per month and included all trees with a diameter at breast height (DBH) larger than 10 cm (Table S1, S3, S7a–c, Data S1). At all size most trees in the dataset were identified to the species level or morphospecies level. Since the datasets varied in duration (Table 1) we divided fruit production into three classes for comparison using the following procedure. All monthly percentage scores were standardised by calculating standardised deviates per site/forest type combination (or z-scores: [68], which is the monthly value minus the mean divided by the standard deviation. Months with a z-score<−1 were classified as low fruit periods (LFP), a z-score between −1 and 1 and medium fruit periods (MFP), and z-scores>1 as high fruit periods (HFP).
Because observations over months within a site are correlated over time and not independent, we fit time-series models for each site (Methods S1). These time series patterns influence the variability of estimators such as the mean availability of fruit per month for each site. All models included effects for fruiting level (LFP, MFP, HFP) and DBH (diameter at breast height (1.3 m)) category (defined in Table 1). The time series error structure for each site was selected using the smallest AIC criterion value for the fit to the site (Methods S1). Potential error structures included independence, autoregressive (AR) of order up to 4, moving average (MA) of order up to 4, and ARMA of orders up to (2,2) [69]. Each model provides estimates of fruit production for each fruiting level and DBH category observed at a given site. Time series models were fit to measurements on the original scale.
For forest type comparisons between sites in Borneo and Sumatra, estimates from the time series models within a fruiting level by DBH category were directly averaged within habitat. In order to provide a single summary for Sumatra versus Borneo, an average for each site was produced by averaging its estimates by fruit and DBH categories. Averages were computed according to the distribution of measurements across fruiting level by DBH category within each site. The resulting standard error for the average within a site takes into account the covariance among estimates within a site. The estimates of the sites within each island were then averaged. Satterthwaite approximations were used to determine degrees of freedom, which is a more conservative procedure than directly pooling degrees of freedom. The Satterhwaite approximation for degrees of freedom is appropriate when taking linear combinations of sample variances [70], [71]. Neter et al. [72] and Rice [73] give the formula as df = (MS1+MS2)∧2/(MS1∧2/df1+MS2∧2/df2), where MS1 and MS2 are two estimates of variance and df1 and df2 are the degrees of freedom associated with the variance estimates. This formula does not assume that the two variances are equal as would a pooled variance estimate formula. Using these average estimates, we conducted pairwise comparisons between sites on different islands in similar forest types. For these analyses, we corrected the significance level by multiplying the p-value by 13, the number of comparisons. Results when models were selected using the Bayesian information criterion (BIC) were analogous to the presented results.
Means and standard error are reported in the text for statistical analyses unless otherwise indicated. We ran all analyses in R version 2.12.1 [74]. All probability levels are two-tailed, and the significance for all tests was set at alpha <0.05 unless noted. The R package used was nlme (Linear and Nonlinear Mixed Effects Models) with the commands gls (generalized least squares) and corARMA (ARMA(p,q) Correlation Structure).
Supporting Information
Details on the methods and models used.
(DOC)
Raw data used for analyses.
(XLS)
Counts of observations and time series estimated mean differences between Sumatra and Borneo riverine forest habitats.
(DOC)
Differences in time series estimated fruit production for riverine forests.
(DOC)
Counts of observations in peat swamp forests.
(DOC)
Differences in time series estimated fruit production in peat forests.
(DOC)
Comparison of time series estimated differences in fruit production (% fruiting) at Suaq Balimbing in Sumatra and Gunung Palung in Borneo peat swamp habitats (model includes time series correction, fruit level, and DBH).
(DOC)
Comparison of time series estimated differences in fruit production (% fruiting) at Suaq Balimbing in Sumatra and Tanjung Puting in Borneo for peat swamp habitats (model includes time series correction, fruit level, and DBH).
(DOC)
Counts of observations during low, middle, and high fruit periods in Sumatra and Borneo dryland forest sites.
(DOC)
Differences in time series estimated fruit production in dryland forests.
(DOC)
Acknowledgments
We gratefully acknowledge the co-operation and support of the Indonesian Institute of Science (LIPI, Jakarta), the Indonesian Nature Conservation Service (PHPA), Forest management units and bureaus at each site, and Universitas Nasional (Indonesia). GMF would like to express gratitude to the Forest Research Institute in Samarinda for their assistance. FQB would like to acknowledge the late John Proctor, Rupert Ridgeway and Suriantata for their help. CPY would like to thank Trevor K. Blondal, H. D. Susilo, Pak Ledan, and Jatna Supriatna for their support and assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Financial support was provided by the Netherlands Foundation for the Advancement of Tropical Research (WOTRO), the Netherlands Organisation for Scientific Research (NWO), the Wildlife Conservation Society (WCS), the Tropenbos Foundation, the Borneo Orangutan Foundation, the Conservation Endowment Fund of the American Zoo and Aquarium Association, Conservation International and The Arnold Arboretum of Harvard University, the European Commission and the Government of Indonesia as the funding agencies for the Leuser Development Programme, The National Geographic Society, the Erb Foundation, the Douroucoulli Foundation, the Crystal Channel Foundation, the Margot Marsh Biodiversity Foundation, the Center For Field Research, the International Scientific Support Trust, the American Primatological Association, Page Yeager, and a Clare Booth Luce Professorship for Women in Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.van Schaik CP, Terborgh JW, Wright SJ. The phenology of tropical forests: adaptive significance and consequence for primary consumers. Ann Rev Ecol Syst. 1993;24:353–377. [Google Scholar]
- 2.Whitmore TC. An introduction to tropical rain forests. Oxford: Oxford University Press; 1998. 296 [Google Scholar]
- 3.Terborgh J, van Schaik CP. Convergence vs. nonconvergence in primate communities. In: Gee JHR, Gillar PS, editors. Organization of communities: past and present. Oxford: Blackwell Science; 1987. pp. 205–226. [Google Scholar]
- 4.Kay RF, Madden RH, van Schaik CP, Higdon D. Primate species richness is determined by plant productivity: implications for conservation. Proc Natl Acad Sci. 1997;94:13023–13027. doi: 10.1073/pnas.94.24.13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wich SA, van Schaik CP. The impact of El Niño on mast fruiting in Sumatra and elsewhere in Malesia. J Trop Ecol. 2000;16:563–577. [Google Scholar]
- 6.Clark DA, Brown S, Kicklighter D, Thommlinon JR, Ni J. Net primary production in tropical forests: an evaluation and synthesis of existing field data. Ecol Appl. 2001;11:371–384. [Google Scholar]
- 7.Proctor J, Anderson JM, Chai P, Vallack HW. Ecological studies in four contrasting lowland rain forests in Gunung Mulu National Park, Sarawak. II. Litterfall, litter standing crop and preliminary observations on herbivory. J Ecol. 1983;71:261–283. [Google Scholar]
- 8.Proctor J, Anderson JM, Chai P, Vallack HW. Comparative studies on forests, soils and litterfall at four altitudes on Gunung Mulu, Sarawak. Malay For. 1983;46:60–76. [Google Scholar]
- 9.van Schaik CP, Mirmanto E. Spatial variation in the structure and litter fall of a Sumatran rain forest. Biotropica. 1985;17:196–205. [Google Scholar]
- 10.Gentry AH, Emmons LH. Geographical variation in fertility, phenology, and composition of the understory of Neotropical forests. Biotropica. 1987;19:216–227. [Google Scholar]
- 11.Proctor J, Lee YF, Langley AM, Munro WRC, Nelson T. Ecological Studies on Gunung Silam, a small ultrabasic mountain in Sabah, Malaysia. I. Environment, forest structure and floristics. J Ecol. 1988;76:320–340. [Google Scholar]
- 12.Marshall AJ, Leighton M. How does food availability limit the population density of white-bearded gibbons? In: Hohmann G, Robbins MM, Boesch C, editors. Feeding ecology of the apes and other primates. Cambridge: Cambridge University Press; 2006. pp. 311–333. [Google Scholar]
- 13.Cannon CH, Curran LM, Marshall AJ, Leighton M. Long-term reproductive behavior of woody plants across seven Bornean forest types in the Gunung Palung National Park, Indonesia: suprannual synchrony, temporal productivity, and fruiting diversity. Ecol Lett. 2007;10:956–969. doi: 10.1111/j.1461-0248.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- 14.Vitousek PM. Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology. 1984;65:285–298. [Google Scholar]
- 15.Vitousek PM, Sanford RL. Nutrient cycling in moist tropical forests. Ann Rev Ecol Syst. 1986;17:137–167. [Google Scholar]
- 16.Peres CA. Effects of habitat quality and hunting pressure on arboreal folivore density in Neotropical forests: A case study of howler monkeys (Alouatta spp.). Folia Primatol. 1997;68:199–222. [Google Scholar]
- 17.Reich PB, Grigal DF, Aber JD, Gower ST. Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils. Ecology. 1997;78:335–347. [Google Scholar]
- 18.Mirmanto E, Proctor J, Green J, Nagy L, Suriantata Effects of nitrogen and phosphorus fertilization in a lowland evergreen rainforest. Phil Trans Roy Soc Lond, B. 1999;354:1825–1829. doi: 10.1098/rstb.1999.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker TR, Burslem DFRP, Swaine MD. Associations between tree growth, soil fertility and water production at local and regional scales in Ghanaian tropical rain forest. J Trop Ecol. 2003;19:109–125. [Google Scholar]
- 20.Schurr EAG. Productivity and global climate revisited: the sensitivity of tropical forest growth to precipitation. Ecology. 2003;84:1165–1170. [Google Scholar]
- 21.Lonsdale WM. Predicting the amount of litterfall in forests of the world. Ann Bot. 1988;61:319–324. [Google Scholar]
- 22.LDeau SL, Clark JS. Rising CO2 levels and the fecundity of forest trees. Science. 2001;292:95–98. doi: 10.1126/science.1057547. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd J, Farquhar GD. Effects of rising temperature and [CO2] on the physiology of tropical forest trees. Phil Trans R Soc B. 2008;363:1811–1817. doi: 10.1098/rstb.2007.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JH. Two decades of Hommage to Santa-Rosalia - toward a general theory of diversity. Am Zool. 1981;21:877–88. [Google Scholar]
- 25.Wright SJ, van Schaik CP. Light and the phenology of tropical trees. Am Nat. 1994;143:192–199. [Google Scholar]
- 26.Janzen DH. Tropical blackwater rivers, animals, and mast fruiting by the Dipterocarpaceae. Biotropica. 1974;6:69–103. [Google Scholar]
- 27.Oates JF, Whitesides GH, Davies AG, Waterman PG, Green SM, et al. Determinants of variation in tropical forest primate biomass: New evidence from West Africa. Ecology. 1990;71:328–343. [Google Scholar]
- 28.Janson CH, Chapman CA. Resources and primate community structure. In: Fleagle JJ, Janson C, Reed KE, editors. Primate communities. Cambridge: Cambridge University Press; 1999. pp. 237–267. [Google Scholar]
- 29.Curran LM, Leighton M. Vertebrate responses to spatiotemporal variation in seed production of mast-fruiting Dipterocarpaceae. Ecol Mono. 2000;70:101–128. [Google Scholar]
- 30.Stevenson PR. The relationship between fruit production and primate abundance in Neotropical communities. Biol J Linn Soc. 2001;72:161–178. [Google Scholar]
- 31.Wich SA, Buij R, van Schaik CP. Determinants of orangutan density in the dryland forests of the Leuser Ecosystem. Primates. 2004a;45:177–182. doi: 10.1007/s10329-004-0080-1. [DOI] [PubMed] [Google Scholar]
- 32.Heaney LR. Island area and body size of insular mammals: Evidence from the tri-colored squirrel (Callosciurus prevosti) of Southeast Asia. Evolution. 1978;32:29–44. doi: 10.1111/j.1558-5646.1978.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 33.Ashton PS, Givnish TJ, Appanah S. Staggered flowering in the Dipterocarpaceae: New insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. Am Nat. 1988;132:44–66. [Google Scholar]
- 34.Curran LM, Webb CO. Experimental tests of the spatiotemporal scale of seed predation in mast-fruiting Dipterocarpaceae. Ecol Mono. 2000;70:129–148. [Google Scholar]
- 35.Brearley FQ, Proctor J, Suriantata, Nagy L, Dalrymple G, et al. Reproductive phenology over a 10-year period in a lowland evergreen rain forest of central Borneo. J Ecol. 2007;95:828–839. [Google Scholar]
- 36.Medway L. Phenology of a tropical rainforest in Malaya. Biol J Linn Soc. 1972;4:117–146. [Google Scholar]
- 37.Appanah S. General flowering in the climax rain forests of Southeast Asia. Malay For. 1985;4:37–42. [Google Scholar]
- 38.Numata S, Yasuda M, Okuda T, Kachi N, Noor NSMd. Temporal and spatial patterns of mass flowerings on the Malay Peninsula. Am J Bot. 2003;90:1025–1031. doi: 10.3732/ajb.90.7.1025. [DOI] [PubMed] [Google Scholar]
- 39.Marshall AJ, Ancrenaz M, Brearley FQ, Fredriksson GM, Ghaffar N, et al. The effects of forest phenology and floristics on populations of Bornean and Sumatran orangutans: are Sumatran forests better orangutan habitat than Bornean forests? In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP, editors. Orangutans: Geograhical variation in behavioural ecology and conservation. Oxford: Oxford University Press; 2009. pp. 97–118. [Google Scholar]
- 40.Delgado R, van Schaik CP. The behavioural ecology and conservation of the orangutan (Pongo pygmaeus): A tale of two islands. Evol Anthro. 2000;9:201–218. [Google Scholar]
- 41.van Schaik CP. Among orangutans: red apes and the rise of human culture. Cambridge, MA: Harvard University Press (Belknap); 2004. 256 [Google Scholar]
- 42.MacKinnon K, Hatta G, Halim H, Mangalik A. The Ecology of Kalimantan. Singapore: Periplus Editions; 1996. 870 [Google Scholar]
- 43.Cannon CH, Morley RJ, Bush ABG. The current refugial rainforests of Sundaland are unrepresentative of their biogeographic past and highly vulnerable to disturbance. Proc Natl Acad Sci. 2009;106:11188–11193. doi: 10.1073/pnas.0809865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meijaard E, Sheil D, Nasi R, Augeri D, Rosenbaum B, et al. Life after logging. Bogor: Indonesia, CIFOR; 2005. 354 [Google Scholar]
- 45.Wich SA, Utami Atmoko SS, Mitra Setia T, Rijksen HR, Schürmann C, et al. Life history of wild Sumatran orangutans (Pongo abelii). J Hum Evol. 2004b;47:385–398. doi: 10.1016/j.jhevol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Wich SA, Utami Atmoko SS, Mitra Setia T, Djoyosudharmo S, Geurts ML. Dietary and energetic responses of Pongo abelii to fruit availability fluctuations. Int J Primatol. 2006a;27:1535–1550. [Google Scholar]
- 47.Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP. Orangutans: Geograhical Variation in behavioural ecology and conservation. Oxford University Press, Oxford; 2009. 440 [Google Scholar]
- 48.Meijaard E. Craniometric differences among Malayan sun bears (Ursus malayanus) : Evolutionary and taxonomic implications. Raff Bull Zool. 2004;52:665–672. [Google Scholar]
- 49.Meijaard E, Groves CP. Morphometrical relationships between South-East Asian Cervinae; evolutionary and biogeographic implications. J Zool. 2004a;263:179–196. [Google Scholar]
- 50.Meijaard E, Groves CP. A taxonomic revision of the Tragulus mouse-deer (Artiodactyla). Zool J Linn Soc. 2004b;140:63–102. [Google Scholar]
- 51.Meiri S, Meijaard E, Wich S, Groves CP, Helgen KM. Mammals of Borneo - small size on a large island. J Biogeo. 2008;35:1087–1094. [Google Scholar]
- 52.Meiri S, Dayan T, Simberloff D. Body size of insular carnivores: little support for the island rule. Am Nat. 2004;163:469–479. doi: 10.1086/382229. [DOI] [PubMed] [Google Scholar]
- 53.van Schaik CP. Phenological changes in a Sumatran rainforest. J Trop Ecol. 1986;2:327–347. [Google Scholar]
- 54.Wich SA, Geurts ML, Mitra Setia T, Utami Atmoko SS. Influence of fruit availability on Sumatran orangutan sociality and reproduction. In: Hohmann G, Robbins MM, Boesch C, editors. Feeding ecology of the apes and other primates. Cambridge: Cambridge University Press; 2006b. pp. 335–356. [Google Scholar]
- 55.Zimmerman JK, Wright SJ, Calderón O, Pagan MA, Paton S. Flowering and fruiting phonologies of seasonal and aseasonal neotropical forests: the role of annual change in irradiance. J Trop Ecol. 2007;23:231–251. [Google Scholar]
- 56.Sakai S, Momose K, Yumoto T, Nagamitsu T, Nagamasu H, Hamid AA, Nakashizuka T. Plant reproductive phenology over four years including an episode of general flowering in a lowland Dipterocarp forest, Sarawak, Malaysia. Am J Bot. 1999;86:1414–1436. [PubMed] [Google Scholar]
- 57.Chave J, Navarette D, Almeida S, Álvarez E, Aragão LEOC, et al. Regional and seasonal patterns of litterfall in tropical South America. Biogeosciences. 2010;7:43–55. [Google Scholar]
- 58.Whitmore TC. Tropical rain forests of the Far East. Oxford: Oxford University Press; 1984. 376 [Google Scholar]
- 59.Marshall AJ. Population ecology of gibbons and leaf monkeys across a gradient of Bornean forest types. 2004. PhD-Dissertation, Harvard University, USA.
- 60.Rijksen HR. A fieldstudy on Sumatran orang utans (Pongo pygmaeus abelii Lesson 1827) Wageningen: H. Veenman & Zonen, B. V; 1978. 420 [Google Scholar]
- 61.Brearley FQ, Prajadinata S, Kidd PS, Proctor J, Suriantata Structure and floristics of an old secondary rain forest in Central Kalimantan, Indonesia, and a comparison with adjacent primary forest. For Ecol Manag. 2004;195:385–397. [Google Scholar]
- 62.Webb CO. Seedling ecology and tree diversity in a Bornean rain forest. 1998. PhD-Dissertation, Dartmouth College, USA.
- 63.Cannon CH, Leighton M. Tree species distributions across five habitats in a Bornean rain forest. J Veg Sci. 2004;15:257–66. [Google Scholar]
- 64.Paoli GD, Curran LM, Zak DR. Soil nutrients and beta diversity in the Bornean Dipterocarpaceae: evidence for niche partitioning by tropical rain forest trees. J Ecol. 2006;94:157–170. [Google Scholar]
- 65.Paoli GD, Curran LM. Soil nutrients limit aboveground productivity in mature lowland tropical forests of Southwestern Borneo. Ecosystems. 2007;10:503–518. [Google Scholar]
- 66.Fredriksson GM, Wich SA, Trisno Frugivory in sun bears (Helarctos malayanus) is linked to El Niño-related fluctuations in fruiting phenology, East Kalimantan, Indonesia. Biol J Linn Soc. 2006;89:489–508. [Google Scholar]
- 67.Yeager CP. Feeding ecology of the proboscis monkey (Nasalis larvatus). Int J Primatol. 1989;10:497–530. [Google Scholar]
- 68.Sokal RR, Rohlf FJ. Biometry. New York: W. H. Freeman and Company; 1994. 880 [Google Scholar]
- 69.Cowpertwait PSP, Metcalfe AV. Introductory time series with R. Dordrecht: Springer Science+Business Media, LLC; 2009. 272 [Google Scholar]
- 70.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometr Bull. 1946;2:110–114. [PubMed] [Google Scholar]
- 71.Welch BL. The generalization of “student's" problem when several different population variances are involved. Biometrika. 1947;34:28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- 72.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4th edition. Chicago: Irwin; 1996. 720 [Google Scholar]
- 73.Rice JA. Mathematical statistics and data analysis. Wadsworth, Inc. 2nd edition. Belmont, CA: Duxbury Press; 1995. 602 [Google Scholar]
- 74.R Development Core Team. R: A language and environment for statistical computing. 2009. R Foundation for Statistical Computing. Austria, Vienna.
- 75.Galdikas BMF. Orangutan adaptation at Tanjung Puting Reserve, Central Borneo. 1978. PhD-Dissertation, University of California, Los Angeles, USA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details on the methods and models used.
(DOC)
Raw data used for analyses.
(XLS)
Counts of observations and time series estimated mean differences between Sumatra and Borneo riverine forest habitats.
(DOC)
Differences in time series estimated fruit production for riverine forests.
(DOC)
Counts of observations in peat swamp forests.
(DOC)
Differences in time series estimated fruit production in peat forests.
(DOC)
Comparison of time series estimated differences in fruit production (% fruiting) at Suaq Balimbing in Sumatra and Gunung Palung in Borneo peat swamp habitats (model includes time series correction, fruit level, and DBH).
(DOC)
Comparison of time series estimated differences in fruit production (% fruiting) at Suaq Balimbing in Sumatra and Tanjung Puting in Borneo for peat swamp habitats (model includes time series correction, fruit level, and DBH).
(DOC)
Counts of observations during low, middle, and high fruit periods in Sumatra and Borneo dryland forest sites.
(DOC)
Differences in time series estimated fruit production in dryland forests.
(DOC)