Abstract
G protein-coupled receptors (GPCRs) activate mitogen-activated protein kinases through a number of distinct pathways in cells. Increasing evidence has suggested that endosomal signaling has an important role in receptor signal transduction. Here we investigated the involvement of endocytosis in α1A-adrenergic receptor (α1A-AR)-induced activation of extracellular signal-regulated kinase 1/2 (ERK1/2). Agonist-mediated endocytic traffic of α1A-AR was assessed by real-time imaging of living, stably transfected human embryonic kidney 293A cells (HEK-293A). α1A-AR was internalized dynamically in cells with agonist stimulation, and actin filaments regulated the initial trafficking of α1A-AR. α1A-AR-induced activation of ERK1/2 but not p38 MAPK was sensitive to disruption of endocytosis, as demonstrated by 4°C chilling, dynamin mutation and treatment with cytochalasin D (actin depolymerizing agent). Activation of protein kinase C (PKC) and C-Raf by α1A-AR was not affected by 4°C chilling or cytochalasin D treatment. U73122 (a phospholipase C [PLC] inhibitor) and Ro 31–8220 (a PKC inhibitor) inhibited α1B-AR- but not α1A-AR-induced ERK1/2 activation. These data suggest that the endocytic pathway is involved in α1A-AR-induced ERK1/2 activation, which is independent of Gq/PLC/PKC signaling.
Introduction
α1A-Adrenergic receptor (α1A-AR) is one of 3 members of the α1-AR subfamily (α1A, α1B, and α1D) of G protein-coupled receptors (GPCRs) [1]–[3]. α1A-AR plays a key role in physiological effects such as contraction of vascular and cardiac muscle, contraction of the spleen, liver glycogenesis, or melatonin secretion in the pineal gland [2]–[5]. Mice with cardiac-restricted overexpression of the wild-type α1B-AR that were treated with α1-AR agonist (phenylephrine [PE]) exhibited poor survival, markedly exaggerated cardiac hypertrophy, myocardial fibrosis, and suppressed left ventricular function [6]. In contrast, animals with α1A-AR overexpression showed improved survival and even abrogated cardiac remodeling in response to thoracic aorta constriction-induced pressure overload or myocardial infarction [7], [8]. The activation of extracellular signal-regulated kinase (ERK), a regulator of myocyte survival, is critical in mediating α1-AR survival signaling in cardiac myocytes [9]–[11]. Recent studies of selective inactivation of α1-ARs indicate that the activation of ERK1/2 induced by α1A-AR is critical for cardiomyocyte survival. Reconstitution of α1A-AR but not α1B-AR induced ERK1/2 activation and rescued α1ABKO myocytes from cell death induced by norepinephrine, doxorubicin, and H2O2 [12]. The observation that α1A-AR specifically restored ERK1/2 activation in α1ABKO myocytes suggests that α1A-AR and α1B-AR activate ERK1/2 through differential mechanisms. However, studies to date have not consistently identified major differences in immediate signaling responses initiated by α1A-AR and α1B-AR.
α1A-AR is regulated by many mechanisms, including phosphorylation, protein-protein interaction, protein traffic, and transcription [13]. After stimulation by their ligands, α1-ARs activate intracellular effectors, including phospholipase C β (PLCβ), inositol trisphosphate, protein kinase C (PKC), mitogen-activated protein kinase (MAPK) and calcium signals, often through a heterotrimeric G protein-dependent manner [14], [15]. An α1A-AR variant, which was unable to couple to Gq, could also induce calcium influx when coactivated by β2-AR [16]. Thus, α1A-AR, even though uncoupled from Gq, may remain competent for induction of signaling events through yet unknown pathways.
Increasing evidence has shown the existence of receptor signaling from the endocytic process. For instance, activation of ERK1/2 via epidermal growth factor receptor (EGFR) and β2-AR were suppressed in cells transfected with dynamin-mutant K44A (Dyn-K44A), which is defective in GTPase activity [17], [18]. Signaling from GPCR inside the cell is persistent and appears to trigger specific downstream effects [19]. Visualizing and tracking receptors stimulated by agonists in living cells contributes to understanding the molecular mechanisms of receptor signaling [20]. However, the association of α1A-AR endocytic trafficking and activation of MAPKs is still unknown.
We aimed to investigate whether an endocytic process is involved in ERK1/2 activation induced by α1A-AR. By real-time tracking, we found a time-dependent dynamic pattern of α1A-AR endocytosis with stimulation and the involvement of the cytoskeleton, especially actin-filaments, in this process. This relationship was further examined by colocalization of α1A-AR with reorganized cytoskeletons. We provide evidence for an involvement of endocytosis in α1A-AR-induced activation of ERK1/2, which differs from that of α1B-AR via a Gq/PLC/PKC pathway.
Materials and Methods
Materials
Cytochalasin D, nocodazole, PE and U73122 were from Sigma (St. Louis, MO). Ro 31–8220, prazosin and phorbol 12-myristate, 13-acetate (PMA) were from Calbiochem (La Jolla, CA). Phospho-p38 MAPK (Thr180/Tyr182), -p42/44 MAPK (Thr202/Tyr204), -PKC (pan) (Ser660), and -C-Raf (Ser338) antibodies were from Cell Signaling Technology (Beverly, Mass). Antibodies against ERK1/2, PKC (pan), C-Raf, p38, FLAG-tag and HA-tag were from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse and anti-rabbit antibodies were from Beijing Zhongshan Golden Bridge Biotechnology. Alexa 488-conjugated WGA, Alexa 555 and 633 IgG and Alexa 488-conjugated phalloidin were from Invitrogen. All other chemicals were of analytical grade.
Cell culture, plasmids and transfection
HEK-293A cell lines were obtained from Invitrogen. Receptor constructs and HEK-293A cells stably transfected with α1A-AR or α1B-AR were described previously [21]. Dyn-K44A was a gift from Ming Zhao (La Jolla Institute for Molecular Medicine, San Diego, CA). Amphiphysin I construct was a gift from Pietro De Camilli (Yale University School of Medicine, New Haven, CT). Transfection involved use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Membrane receptor labeling and tracking
FLAG-tagged receptors were labeled with anti-FLAG monoclonal antibody (12.5 µg/ml) for 10 min and then Alexa FLour® 555 goat anti-mouse IgG (Invitrogen) (3.75 µg/ml) for 10 min as described previously [21], [22]. Before fluorescence experiments, cells were washed 3 times in phosphate buffered saline (PBS) buffer (pH 7.4; 37°C). Live imaging involved use of a wide-field fluorescence microscope equipped with a 100×/1.40NA Plan Apochromat objective (Olympus, Japan) and a 14-bit, back-illuminated, electron-multiplying charge-coupled device camera (Andor iXon DU-897 BV). The microscope was also equipped with a cell incubation system (INU-ZIL-F1, TOKAI HIT), which ensured live-cell imaging at 37°C in 5% CO2. Fluorescence was excited at 532-nm by an argon laser (Melles Griot, Carlsbad, CA). Movies were acquired at a frame rate of 20 Hz by use of MetaMorph software (Molecular Devices). Trajectories from cells observed under the given labeling procedure were plotted and resolved as described previously [21].
Fluorescence microscopy
After drug treatment, cells were fixed for 15 min in 4% paraformaldehyde in PBS and permeabilized with 0.2% Triton X-100. After washes with PBS, cells were incubated for 25 min with TRITC-labeled phalloidin (Sigma). The samples were viewed under a laser scanning confocal microscope (TCS SP2, Leica Microsystems) with a Plan-Apo 63×/1.32 oil immersion objective (Leica Microsystems); images were collected by use of Leica TCS SP2 v2.611537. The 488- and 532-nm laser beam was focused by a Leica Apochromat with <200 lW power irradiation. The pinhole size was 1 airy unit.
Western blot analysis
Protein expression was examined by western blot analysis as previously described [23]. Briefly, samples were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. After being blocked, blots were probed with the appropriate primary antibodies overnight at 4°C or for 2 h at room temperature, then washed and incubated with HRP-conjugated secondary antibody. Bands were visualized by use of a super-western sensitivity chemiluminescence detection system (Pierce). Autoradiographs were quantitated by densitometry (Science Imaging System, Bio-Rad).
Results
Tracking α1A-AR stimulated by agonist in single living cells in real time
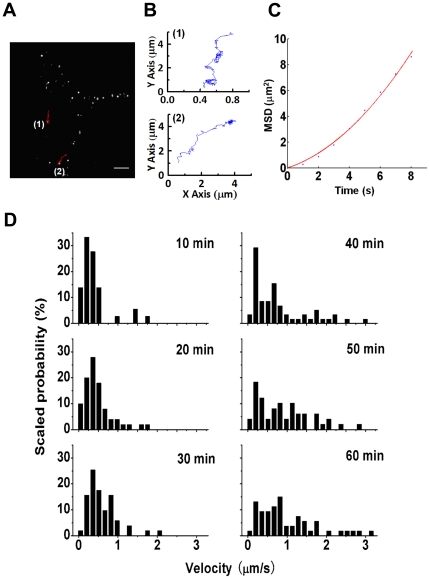
We studied the dynamic properties and mechanisms of receptor transport in HEK-293A cells stably transfected with a FLAG-tagged α1A-AR construct. α1A-AR was detected on the surface of living HEK-293A–α1A-AR cells by use of a monoclonal primary antibody and Alexa-555 IgG (Fig. 1A). After incubation with PE, an α1-AR agonist, some of the α1A-AR particles trafficked inward in the cells. From recorded movies, we tracked the trajectories of trafficking α1A-AR particles. Figure 1B shows 2 sample trajectories of α1A-AR particles (as marked in Fig. 1A) with directed movement within 8 sec on PE stimulation. To quantify the velocities of α1A-AR movements, we plotted the mean square displacement (MSD) versus time (Fig. 1C), which also showed the directional movement of these particles.
Figure 1. Tracking α1A-AR in response to agonist stimulation.
(A) α1A-ARs were detected with anti-FLAG antibody and Alexa-555 IgG in live HEK-293A–α1A-AR cells at 37°C. Images were captured after 30-min stimulation with 10 µM phenylephrine (PE). Two sample trajectories of α1A-AR particles are shown with red lines (1 and 2). Bar: 10 µm. (B) The trajectories in (A) were plotted (1 and 2, respectively). (C) The plot of the mean square displacement (<r2>) against time (t) to the trajectory in Fig. B(2). The red line is a fit by <r2> = 4Dt+(vt)2. Directed movement was confirmed by the superlinear MSD-Δt plots. (D) Velocities of directional movements of α1A-AR resolved from tracked trajectories at various times after 10 µM PE stimulation plotted in probability histograms. (n = 36, 61, 51, 58, 49 and 53 trajectories in separated cells, respectively).
We then resolved the velocities at different time after PE stimulation [21]. Figure 1D shows the time-dependent velocity distribution of endocytic α1A-AR with PE stimulation during 1 hour (10-min intervals). At the early stage of the activation (first 30 min), the receptor mainly moved at a low velocity at a peak of about 0.3 µm/s. After stimulation for 40 to 60 min, movements became much faster, with high velocity trajectories increased gradually. The main peak of the highest velocity was about 0.8 µm/s. Thus, in general, the active movement of α1A-AR vesicles was slower at the early phase of endocytosis and faster at the later phase.
Actin filament-mediated endocytosis of α1A-AR
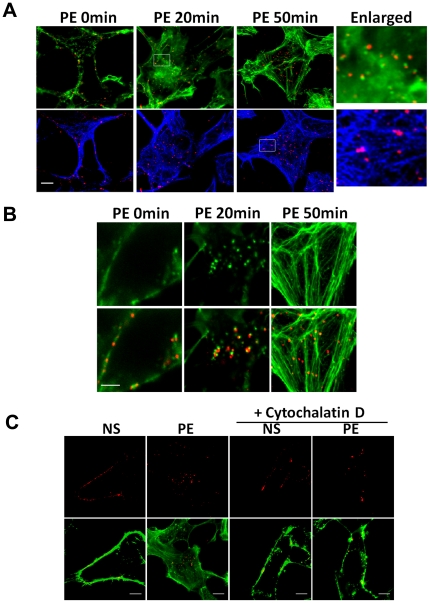
We used confocal microscopy to determine the association of endocytic receptors with cytoskeleton, actin and microtubules, respectively. α1A-AR vesicles largely colocalized with F-actin after 20-min PE stimulation (Fig. 2A). With 50-min PE stimulation, some of the α1A-AR vesicles colocalized with microtubules. With higher resolution imaging, we observed a more relevant relation between reorganized actin and α1A-AR; at 20 min after PE stimulation, small actin patches and tails appeared in the cells (Fig. 2B). Most of the actin patches showed colocalization of a α1A-AR vesicle. Actin may use α1A-AR-associated actin patches as polymerization sites, as was reported for virus internalization [24]. The changes were transient, and after 50-min stimulation, most of actin patches and tails disappeared. And α1A-AR vesicles became located on the filamentous actin. Thus, PE-induced α1A-AR endocytic trafficking in the early phase depends on F-actin.
Figure 2. α1A-AR endocytosis is regulated by cytoskeleton.
(A) Colocalization of α1A-AR with F-actin and microtubules after agonist stimulation. Cells were stimulated with 10 µM PE for 20 or 50 min. Untreated cells were used as control. α1A-AR was labeled with anti-FLAG antibodies and Alexa 555 IgG (red). F-actin was labeled with Alexa 488-conjugated phalloidin (green). Microtubules were labeled with antibodies and Alexa 633 IgG (blue). Last column: 5× magnification of selected boxed regions. Bar: 10 µm. (B) High-resolution imaging of colocalization of α1A-AR with reorganized actin after stimulation. Cells were treated with agonist for 20 or 50 min, and then labeled with antibodies against α1A-AR and Alexa 488-conjugated phalloidin against F-actin (red) (bottom row). Bar: 5 µm. (C) Inhibition of α1A-AR endocytosis by Cytochalasin D. HEK-293A–α1A-AR cells were pre-incubated with cytochalasin-D (Cyto-D; 5 µM, 5 min), then stimulated with 10 µM PE for 20 min. F-actin was stained by Alexa 488-conjugated Phalloidin (green), α1A-ARs were detected with anti-FLAG antibody and Alexa-555 IgG (red). NS: no stimulation. Bar: 10 µm.
To justify the role of F-actin in regulation of α1A-AR endocytosis, cytochalasin D was used before PE stimulation to inhibit the actin polymerization. Incubated for 5 min with 5 µM cytochalasin D, α1A-AR congregated on membrane even after PE stimulation (Fig. 2C). It provides further evidence that α1A-AR endocytosis is regulated by actin filaments.
Receptor endocytosis is required for ERK1/2 activation induced by α1A-AR
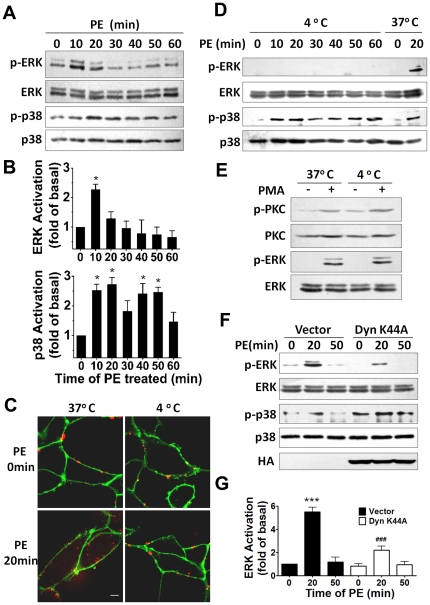
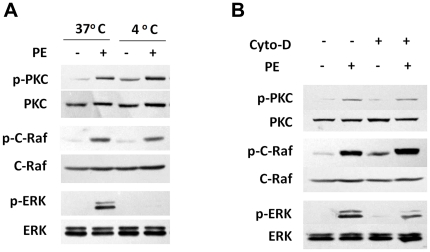
To test whether endocytosis is involved in the α1A-AR induced signaling, we first examined the activation of ERK1/2 and p38 MAPK with PE stimulation. ERK1/2 and p38 MAPK phosphorylation significantly increased at 10 and 20 min after PE treatment and then decreased to the basal level (Fig. 3A,B). PE also caused a secondary increase of p38 MAPK phosphorylation after 50-min treatment. We then used 4°C incubation to inhibit α1A-AR endocytosis [25], [26]. α1A-AR remained on the membrane after PE stimulation at 4°C (Fig. 3C). α1A-AR endocytosis was markedly inhibited at 4°C as compared with at 37°C. 4°C chilling almost completely abrogated the α1A-AR-induced ERK1/2 activation, whereas activation of p38 was not modified (Fig. 3D). To ensure that the ERK1/2 was not defective in phosphorylation at 4°C incubation, we measured PMA-induced activation of ERK1/2 in 4°C. PMA activated both PKC and ERK1/2 at 4°C and at 37°C (Fig. 3E), which suggests that the failure to activate ERK1/2 by α1A-AR at 4°C was not due to a defect in ERK1/2 signaling but rather to a defect in receptor endocytosis.
Figure 3. Receptor endocytosis is involved in ERK1/2 activation by α1A-AR stimulation.
(A) Representative western blot showing activation of ERK1/2 and p38 after 10-µM PE treatment for the indicated times. (B) Relative ERK1/2 and p38 activeity after PE stimulation are shown. Data are means±SEM of results obtained in three independent experiments. Statistical significance of the difference was assessed using one-way ANOVA analysis. *, p<0.05 versus 0 min. (C) Effect of 4°C chilling on agonist-induced α1A-AR endocytosis. Cells were incubated in 4°C or 37°C for 30 min then stimulated with 10 µM PE for 20 min. α1A-ARs were detected with anti-FLAG antibody and Alexa-555 IgG (red), and plasma membrane was labeled with Alexa 488-conjugated WGA (green). (D) Western blot analysis of ERK1/2 and p38 phosphorylation with PE stimulation for the indicated times at 4°C. Stimulation at 37°C is a control. (E) Phorbol 12-myristate, 13-acetate (PMA)-induced activation of protein kinase C (PKC) and ERK1/2 at 4°C. Cells were incubated in 4°C or 37°C for 30 min then stimulated with 0.1 µM PMA for 20 min. (F) Dynamin mutation inhibits the activation of ERK1/2 by α1A-AR. HEK-293A–α1A-AR cells were cultured and infected with Dyn-K44A or vectors. After 40 h, cells were treated for 20 min with 10 µM PE. Protein expression of phospho-ERK1/2 and total ERK1/2 and p38 were measured. Expression of Dyn-K44A (HA-tagged) was identified with blotting of HA-tag. (G) Quantification of relative ERK1/2 activation corresponding to (F) was performed by densitometric analysis. Data are means±SEM of results obtained in three independent experiments. Statistical significance of the difference was assessed using one-way ANOVA analysis. ***, p<0.001 versus Vector 0 min. ###, p<0.001 versus Vector 20 min.
To further define the role of endocytosis in α1A-AR induced signaling, we tested the effect of the mutant Dyn-K44A, used to induce trafficking defects of receptors in many cells [27], [28], on ERK1/2 and p38 activation. Overexpression of HA-tagged Dyn-K44A in HEK-293A–α1A-AR cells suppressed the activation of ERK1/2 but not p38 (Fig. 3F, 3G). Therefore, endocytosis is required for α1A-AR-induced ERK1/2 but not p38 MAPK activation.
Actin organization is involved in ERK1/2 activation induced by α1A-AR
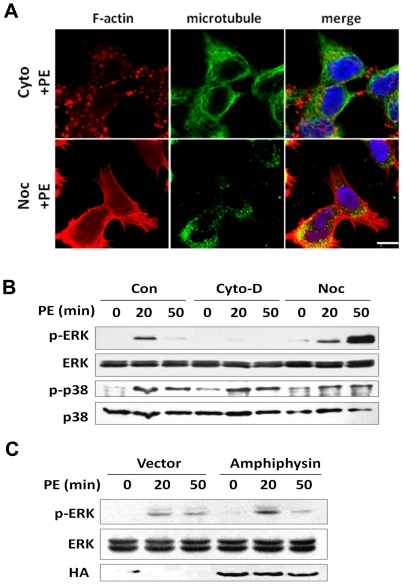
Actin filaments play a pivotal role in α1A-AR trafficking, especially in the early phase of endocytosis (Fig. 1, 2). Therefore, we tested the contribution of actin polymerization to α1A-AR-induced activation of ERK1/2. We pretreated cells with cytochalasin D or nocodazole before PE stimulation to disrupt to disrupt organizing actin or microtubules, respectively. In cells treated for 5 min with 5 µM cytochalasin D, filamentous form of actin was depolymerized and was replaced by aggregated actin, with microtubules appearing normal (Fig. 4A). Treatment with 20 µM nocodazole for 30 min resulted in a loss of organizing microtubule but left F-actin intact. These data confirm the specific effects of cytochalasin D and nocodazole on depolymerizing F-actin and microtubules, respectively. Treatment with cytochalasin D impaired the ability of α1A-AR to activate ERK1/2 but that with nocodazole did not affect the ERK1/2 activation at 20 min of PE stimulation and even increased the phosphorylation levels of ERK1/2 at a later stage of stimulation (Fig. 4B). However, activation of p38 seemed insensitive to the disruption of F-actin or microbules.
Figure 4. Actin organization is involved in ERK1/2 activation induced by α1A-AR.
(A) Effect of actin and microtubule-disrupting drugs on cytoskeleton organization after agonist stimulation. HEK-293A–α1A-AR cells were pre-incubated with cytochalasin-D (Cyto-D; 5 µM, 5 min) or nocodazole (noc; 20 µM, 20 min) at 37°C, then stimulated with 10 µM PE for 20 min. F-actin was stained by TRITC-conjugated Phalloidin (red), and microtubules were stained with anti-α-tubulin antibody and Alexa-488 IgG (green). Nuclei were stained with Hochest-33342 (blue). Bar: 10 µm. (B) Effect of cytoskeleton disrupton on the activation of ERK1/2 and p38 after α1A-AR stimulation. HEK-293A–α1A-AR cells were pre-incubated with Cyto-D (5 µM, 5 min) or nocodazole (20 µM, 20 min) in 37°C, then treated with 10 µM PE for 20 and 50 min. Cell lysates were immunoblotted for the phosphrylation of ERK1/2 and p38. (C) Overexpression of amphiphysin enhanced the activation of ERK1/2 after agonist stimulation. HEK-293A–α1A-AR cells were transfected with amphiphysin plasmid, then treated with 10 µM PE for 20 and 50 min. Cell lysates were immunoblotted for analysis of phospho and total ERK1/2.
To further justify the role of endocytic trafficking in the ERK1/2 activation, we overexpressed amphiphysin, found to be critical for actin polymerization [29], in HEK-293A–α1A-AR cells. Overexpressed amphiphysin increased the phosphorylation level of ERK1/2 at 20- but not 50-min stimulation (Fig. 4C), which agrees with results showing that F-actin preferentially mediates the early-stage but later-stage trafficking of α1A-AR (Fig. 1). These data identified the association of F-actin in the ERK1/2 activation with α1A-AR agonist stimulation.
ERK1/2 activation by α1A-AR is independent of the Gq/PLC/PKC pathway
One of the earliest events in the signaling cascade initiated by α1A-AR is Gq-mediated activation of PLC, with a resulting increase in PKC phosphorylation [14]. To test whether the Gq/PLC/PKC pathway is involved in the α1A-AR-induced activation of ERK1/2, we examined the phosphorylation level of PKC and its downstream C-Raf molecule by α1A-AR with 4°C incubation or cytochalasin-D treatment. As compared with 37°C, 4°C incubation had no effect on the α1A-AR-activation of PKC or C-Raf, but it inhibited the activation of ERK1/2 (Fig. 5A). Similarly, pretreatment with cytochalasin-D before PE did not impair α1A-AR-induced PKC and C-Raf activation (Fig. 5B). Thus, α1A-AR endocytosis was not involved in the Gq/PLC/PKC pathway.
Figure 5. PKC and C-Raf activation with α1A-AR stimulation is not impaired by 4°C chilling or F-actin disruption.
(A) HEK-293A–α1A-AR cells were pre-incubated at 4°C or 37°C for 30 min, then stimulated with 10 µM PE for 20 min. PKC, C-Raf and ERK1/2 activity was measured by western blot analysis. (B) HEK-293A–α1A-AR cells were pre-incubated with or without 5 µM cyto-D for 5 min, then stimulated with 10 µM PE for 20 min. PKC, C-Raf and ERK1/2 activity was measured by western blot analysis.
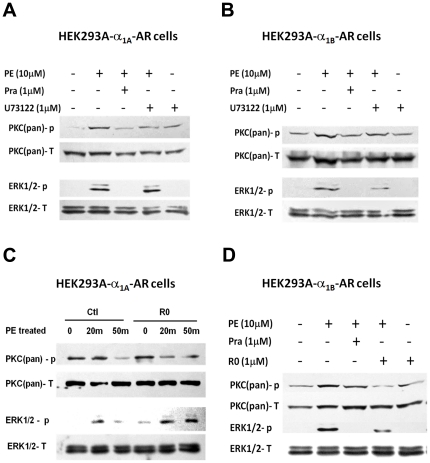
To detemine the role of Gq/PLC/PKC signaling pathway in α1A-AR-induced ERK1/2 activation, HEK-293A–α1A-AR cells were treated with U73122 before PE exposure (Fig. 6A). U73122 is a pharmacological agent commonly used to demonstrate a role of Gq activation of PLC, which inhibits the PLC-dependent process [30]. U73122 suppressed PKC phosphorylation but did not alter the activation of ERK1/2 with PE stimulation. To compare the role of Gq/PLC/PKC signaling in the activation of ERK1/2 by α1A-AR and α1B-AR, we also examined the effect of U73122 in HEK-293A cells stably transfected with α1B-AR. U73122 inhibited both PKC phosphorylation and ERK1/2 activation in HEK-293A–α1B-AR cells with PE stimulation (Fig. 6B). The PKC inhibitor Ro 31–8220 used before PE stimulation showed inhibition of PKC but not ERK1/2 activation with α1A-AR induction (Fig. 6C), and inhibition of both PKC and ERK1/2 with α1B-AR induction (Fig. 6D). The effects of U73122 and Ro 31–8220 on HEK-293A–α1A-AR or –α1B-AR cells suggest that the Gq/PLC/PKC pathway is required for ERK1/2 activation induced by α1B-AR but not α1A-AR.
Figure 6. ERK1/2 activation by α1A-AR is independent of Gq/PLC/PKC pathway.
(A, B) Inhibition of phospholipase C (PLC) by U73122 affected ERK1/2 activation by α1B-AR but not α1A-AR. HEK-293A–α1A-AR or –α1B-AR cells were pre-incubated with 1 µM U73122 or prazosin or not for 30 min. Then cells were treated with 10 µM PE for 20 min. PKC and ERK1/2 activity was measured by western blot analysis. (C, D) Inhibition of PKC by Ro 31–8220 affected ERK1/2 activation by α1B-AR but not α1A-AR. HEK-293A–α1A-AR or –α1B-AR cells were pre-incubated with 1 µM Ro 31–8220 or prazosin or not for 30 min, then were treated with 10 µM PE for 20 min. PKC and ERK1/2 activity were measured by western blot analysis.
Discussion
GPCRs activate MAPKS through distinct pathways in cells, and endosomal signaling has an important role in receptor signal transduction. We investigated the involvement of endocytosis in α1A-AR-induced activation of ERK1/2 in HEK-293A cells. Agonist-mediated endocytic trafficking of α1A-AR was assessed by real-time imaging of living, stably transfected cells. α1A-AR was internalized dynamically in cells with agonist stimulation, and actin filaments regulated the initial trafficking of α1A-AR. α1A-AR-induced activation of ERK1/2 but not p38 MAPK was sensitive to disruption of endocytosis. α1A-AR-induced activation of PKC and C-Raf by was not affected by endocytosis disruption. Thus, the endocytic pathway is involved in α1A-AR-induced ERK1/2 activation and is independent of Gq/PLC/PKC signaling.
Real-time microscopy with high-tempo resolution has novel implications in the investigation of the behavior of receptors. For single particle tracking in this study, α1A-AR was detected on the surface of living HEK-293A–α1A-AR cells by use of a monoclonal primary antibody and Alexa-555 IgG. It should be aware of the potential effects of this labeling method on receptor trafficking and signaling. We found that the labeling with antibodies did not impair the calcium response of α1A-AR among the labeled cells, as compared with non-labeled ones [31]. And it did not affect the ERK1/2 signaling (See Supporting Information Figure S1). Previously, we showed α1A-AR transport with an average step size of 33 nm [21]. In the present work, α1A-AR showed a spatial and time-dependent trafficking pattern with PE stimulation. Near the plasma membrane, the α1A-AR particles were transported a relatively short distance (as shown in Fig. 1A and 1B, trajectory 1) within 8 sec. Inside the cells, the α1A-AR particles moved farther (as shown in Fig.1A and 1B, trajectory 2) in the same time interval. Long-distance transportation allows α1A-AR-containing vesicles to reach late endosomes or lysosomes, which were mostly located near the nucleus. At the early stage of the activation, the receptor mainly moved at a slow velocity, at a peak of about 0.3 µm/sec, which is consistent with the velocity of a single myosin motor walking along actin in vitro [32], [33]. After 40- to 60-min stimulation, the main peak of the higher velocity was about 0.8 µm/sec, which is similar to the value reported for movement along microtubules under in vitro conditions [34], [35]. Thus, the movement of α1A-AR vesicles mainly depends on actin at the early phase of endocytosis and on microtubules at the later phase. Confocal microscopy further verified that endocytic traffic of α1A-AR was mediated by reorganized actin (Fig. 2A, 2B).
Disruption of endocytosis by 4°C chilling, dynamin mutation and F-actin depolymerizing all indicated that the endocytic process was associated in α1A-AR-induced activation of ERK1/2. Many studies have implied a critical role for the ERK1/2 signaling pathway in cardiac myocytes [9]–[11]. Recently, α1A-AR was shown to signal through ERK1/2 to promote cardiac hypertrophy [36] or survival signals [12]. α1A-AR but not α1B-AR rescued α1ABKO cardiac myocytes from cell death, and only α1A-AR could mediate ERK1/2 activation in the myocytes [12]. Activation of phosphoinositide-3-kinase (PI3K) and Ras protein induces activation of a series of growth or proliferation-related protein kinase cascades [37]. Investigation of the role of PI3K and Ras in the activation of ERK1/2 by 2 α1-ARs in NIH3T3 cells revealed that overexpression of a dominant-negative Ras mutant attenuated the α1B-AR– but not α1A-AR-mediated activation of ERK1/2. And overexpression of a dominant-negative PI3K mutant (p85 subunit) attenuated α1A-AR- but not α1B-AR-induced ERK1/2 activation [38]. Therefore, α1A-AR and α1B-AR differentially activate downstream effectors, which further underscores the complexity of α1-AR signaling pathways. We found endocytic trafficking involved in α1A-AR induced ERK1/2 activation and that it was independent of Gq/PLC/PKC signaling. By contrast, α1B-AR-induced ERK1/2 activation required Gq/PLC/PKC signaling, as was previously reported [39]. Thus, α1A-AR uses pathways different from those of α1B-AR to activate ERK1/2.
A number of studies have found a non-Gq-protein signaling pathway in α1A-AR function. An α1A-AR mutant defective in Gq coupling could also activate calcium influx when coactivated with β2-AR [16]. Thus, the functional interaction of these 2 receptors involves heterodimer formation and/or the presence of unidentified non-Gq signaling events in response to α1A-AR stimulation. Activation of calcium influx and PKC may be key events coupling Gq- and Gi-coupled receptor activation to MAPK activation [40]. Berts et al. showed that the Ca2+ chelator BAPTA dose-dependently abolished norepinephrine (NE) -stimulated Ca2+ responses but not ERK1/2 activation. The potent PKC inhibitor bisindolylmaleimide I dose dependently inhibited ERK1/2 activation by phorbol ester tumor-promoting agent but not NE. Thus, Ca2+ release and PKC activation are neither necessary nor sufficient for α1A-AR-mediated activation of ERK1/2 in PC12 cells stably transfected with α1A-AR [41]. Therefore, ERK1/2 activation induced by α1A-AR may be independent of traditional Gq-coupled second-messenger pathways. We found that α1A-AR-induced ERK1/2 activation was unaffected by the PLC inhibitor U73122 and PKC inhibitor R0 31–822, which further identifies a non-Gq signaling pathway involved in α1A-AR-mediated ERK1/2 activation.
Arrestins are critically important for desensitization, endocytosis, and G protein-independent signaling of GPCRs [42]. One of the early evidences that β-arrestins are active participants in signaling was the observation that dominant-negative mutants of β-arrestins inhibited β2-AR-induced activation of ERK1/2 [18]. β-arrestins similarly participate in ERK1/2 signaling by other GPCRs, including neurokinin-1 receptor, protease activated receptor 2, angiotensin II type 1A receptor, and vasopressin V2 receptor [43]–[46]. The stability of receptor–β-arrestin complex controlled the mechanism and extent of ERK1/2 activation [44]. However, results from both coimmunoprecipitation experiments and β-arrestin translocation assays indicated that the agonist-induced interaction of α1A-AR with β-arrestins was much weaker than that of α1B-AR. In addition, α1A-AR did not bind AP50, a subunit of the clathrin adaptor complex AP2. Moreover epinephrine-induced increase of the association of the α1A-AR and β-arrestin 1 or 2 was not statistically significant [47]. Thus, defining the role of β-arrestins in the endocytosis of α1A-AR and α1A-AR-induced ERK1/2 activation is important. PI3K has a potential role in the α1A-AR induced ERK1/2 activation [38] and has been found to regulate intracellular vesicular transport at multiple steps [48], [49]. PI3K may be involved in α1A-AR mediated ERK1/2 activation through an endocytosis pathway but remains to be elucidated.
In summary, we identified a putative role of endocytosis in the regulation of the MAPK pathway under α1A-AR stimulation in cells. Through analysis of receptor tracking in live cells and confocal microscopy, we conclude that the α1A-AR endocytic process is actin-related. Endocytosis and actin reorganization are involved in α1A-AR induced activation of ERK1/2 but not p38, and Gq/PLC/PKC signaling is not required in this process. Thus, we reveal a novel pathway of ERK1/2 activation in α1-AR subtypes. We provide the first biological evidence for a role of endocytosis in the signaling of the α1-AR family, which challenges the classical view that α1A-AR act through Gq/PLC/PKC signaling. Moreover, the mechanism by which α1A-AR induces ERK1/2 activation differs from that of α1B-AR. We provide a possible molecular explanation for the difference between α1A-AR and α1B-AR in activation of ERK1/2 in cardiac myocytes. Because receptor vesicles could link to various intracellular membrane compartments in the endocytic process, the distinct spatiotemporal profile of ERK1/2 activation induced by α1A-AR has profound implications in α1A-AR-mediated survival signaling in cardiac myocytes. In-depth studies of this signaling pathway should add great potential for developing more efficacious and/or safer treatment of heart failure and other clinical conditions.
Supporting Information
Labeling with antibodies did not impair the ERK1/2 signaling induced by α1A-AR stimulation. HEK-293A–α1A-AR cells were pre-incubated with or without anti-FLAG antibody and Alexa-555 IgG. After phenylephrine (PE) treatment, the phosphorylation level of ERK1/2 did not show significant difference among labeled and unlabeled cells. Prazosin (Pra, antagonist of α1A-AR) inhibited the PE-induced ERK1/2 activation in both.
(TIF)
Acknowledgments
We thank Dr. M. Zhao, Dr. P. De Camilli, and Dr. K.P, Minneman for kindly providing us with the pDT–α1A-AR, pDT–α1B-AR, Dyn-K44A, and Amphiphysin I plasmid, respectively.
We also thank Laura Smales for language editing.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Key Basic Research Program of People's Republic of China (2007CB935601, 2006CB910300) and the Natural Science Foundation of China (81030001, 30821001, 20733001) (http://www.most.gov.cn/eng/programmes1/200610/t20061009_36223.htm and http://www.nsfc.gov.cn/Portal0/default106.htm). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Terzic A, Puceat M, Vassort G, Vogel SM. Cardiac alpha 1-adrenoceptors: an overview. Pharmacol Rev. 1993;45:147–75. [PubMed] [Google Scholar]
- 2.Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci. 2010;67:405–17. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang YY, Xu KM, Han C. Alpha(1)-adrenoceptor subtypes mediating inotropic responses in rat heart. J Pharmacol Exp Ther. 1999;291:829–36. [PubMed] [Google Scholar]
- 4.Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, et al. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–7. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Flechtner-Mors M, Jenkinson CP, Alt A, Biesalski HK, Adler G, et al. Sympathetic regulation of glucose uptake by the alpha1-adrenoceptor in human obesity. Obes Res. 2004;12:612–20. doi: 10.1038/oby.2004.70. [DOI] [PubMed] [Google Scholar]
- 6.Iaccarino G, Keys JR, Rapacciuolo A, Shotwell KF, Lefkowitz RJ, et al. Regulation of myocardial betaARK1 expression in catecholamine-induced cardiac hypertrophy in transgenic mice overexpressing alpha1B-adrenergic receptors. J Am Coll Cardiol. 2001;38:534–40. doi: 10.1016/s0735-1097(01)01396-1. [DOI] [PubMed] [Google Scholar]
- 7.Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, et al. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res. 2001;89:343–50. doi: 10.1161/hh1601.095912. [DOI] [PubMed] [Google Scholar]
- 8.Du XJ, Gao XM, Kiriazis H, Moore XL, Ming Z, et al. Transgenic alpha1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res. 2006;71:735–43. doi: 10.1016/j.cardiores.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Yue TL, Wang C, Gu JL, Ma XL, Kumar S, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86:692–9. doi: 10.1161/01.res.86.6.692. [DOI] [PubMed] [Google Scholar]
- 10.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flesch M, Margulies KB, Mochmann HC, Engel D, Sivasubramanian N, et al. Differential regulation of mitogen-activated protein kinases in the failing human heart in response to mechanical unloading. Circulation. 2001;104:2273–6. doi: 10.1161/hc4401.099449. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, et al. An alpha1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–72. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 13.Michelotti GA, Price DT, Schwinn DA. Alpha 1-adrenergic receptor regulation: basic science and clinical implications. Pharmacol Ther. 2000;88:281–309. doi: 10.1016/s0163-7258(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Katz A, Lee CH, Simon MI. Activation of phospholipase C by alpha 1-adrenergic receptors is mediated by the alpha subunits of Gq family. J Biol Chem. 1992;267:25798–802. [PubMed] [Google Scholar]
- 15.Koshimizu TA, Tanoue A, Hirasawa A, Yamauchi J, Tsujimoto G. Recent advances in alpha1-adrenoceptor pharmacology. Pharmacol Ther. 2003;98:235–44. doi: 10.1016/s0163-7258(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 16.Copik AJ, Ma C, Kosaka A, Sahdeo S, Trane A, et al. Facilitatory interplay in alpha 1a and beta 2 adrenoceptor function reveals a non-Gq signaling mode: implications for diversification of intracellular signal transduction. Mol Pharmacol. 2009;75:713–28. doi: 10.1124/mol.108.050765. [DOI] [PubMed] [Google Scholar]
- 17.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–9. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 18.Daaka Y, Luttrell LM, Ahn S, Della RGJ, Ferguson SS, et al. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–8. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 19.Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci. 2010;31:221–8. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Bliss SP, Navratil AM, Breed M, Skinner DC, Clay CM, et al. Signaling complexes associated with the type I gonadotropin-releasing hormone (GnRH) receptor: colocalization of extracellularly regulated kinase 2 and GnRH receptor within membrane rafts. Mol Endocrinol. 2007;21:538–49. doi: 10.1210/me.2006-0289. [DOI] [PubMed] [Google Scholar]
- 21.Liang ZY, Xu N, Guan YH, Xu M, He QH, et al. The transport of alpha(1A)-adrenergic receptor with 33-nm step size in live cells. Biochem Biophys Res Commun. 2007;353:231–7. doi: 10.1016/j.bbrc.2006.11.116. [DOI] [PubMed] [Google Scholar]
- 22.Puthenveedu MA, von ZM. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–24. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Gong K, Li Z, Xu M, Du J, Lv Z, et al. A novel protein kinase A-independent, beta-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by beta2-adrenergic receptors. J Biol Chem. 2008;283:29028–36. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–9. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 25.Kohlhaas SL, Craxton A, Sun XM, Pinkoski MJ, Cohen GM. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem. 2007;282:12831–41. doi: 10.1074/jbc.M700438200. [DOI] [PubMed] [Google Scholar]
- 26.Muratoglu SC, Mikhailenko I, Newton C, Migliorini M, Strickland DK. Low density lipoprotein receptor-related protein 1 (LRP1) forms a signaling complex with platelet-derived growth factor receptor-beta in endosomes and regulates activation of the MAPK pathway. J Biol Chem. 2010;285:14308–17. doi: 10.1074/jbc.M109.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–34. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayer N, Schober D, Huttinger M, Blaas D, Fuchs R. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J Biol Chem. 2001;276:3952–62. doi: 10.1074/jbc.M004722200. [DOI] [PubMed] [Google Scholar]
- 29.Yamada H, Padilla-Parra S, Park SJ, Itoh T, Chaineau M, et al. Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J Biol Chem. 2009;284:34244–56. doi: 10.1074/jbc.M109.064204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O-Uchi J, Sasaki H, Morimoto S, Kusakari Y, Shinji H, et al. Interaction of alpha1-adrenoceptor subtypes with different G proteins induces opposite effects on cardiac L-type Ca2+ channel. Circ Res. 2008;102:1378–88. doi: 10.1161/CIRCRESAHA.107.167734. [DOI] [PubMed] [Google Scholar]
- 31.Xu N, Liang ZY, Xu M, Guan YH, He QH, et al. Real-time detection of alpha1A-AR movement stimulated by phenylephrine in single living cells. Acta Pharmacol Sin. 2007;28:796–802. doi: 10.1111/j.1745-7254.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- 32.Mehta AD, Rock RS, Rief M, Spudich JA, Mooseker MS, et al. Myosin-V is a processive actin-based motor. Nature. 1999;400:590–3. doi: 10.1038/23072. [DOI] [PubMed] [Google Scholar]
- 33.Rief M, Rock RS, Mehta AD, Mooseker MS, Cheney RE, et al. Myosin-V stepping kinetics: a molecular model for processivity. Proc Natl Acad Sci U S A. 2000;97:9482–6. doi: 10.1073/pnas.97.17.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, et al. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science. 2003;300:2061–5. doi: 10.1126/science.1084398. [DOI] [PubMed] [Google Scholar]
- 35.Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–52. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- 36.Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB, et al. MEK1/2-ERK1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol. 2001;33:779–87. doi: 10.1006/jmcc.2001.1348. [DOI] [PubMed] [Google Scholar]
- 37.Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61:269–80. doi: 10.1016/j.phrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Hu ZW, Shi XY, Lin RZ, Hoffman BB. Contrasting signaling pathways of alpha1A- and alpha1B-adrenergic receptor subtype activation of phosphatidylinositol 3-kinase and Ras in transfected NIH3T3 cells. Mol Endocrinol. 1999;13:3–14. doi: 10.1210/mend.13.1.0215. [DOI] [PubMed] [Google Scholar]
- 39.Toews ML, Prinster SC, Schulte NA. Regulation of alpha-1B adrenergic receptor localization, trafficking, function, and stability. Life Sci. 2003;74:379–89. doi: 10.1016/j.lfs.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Hawes BE, van BT, Koch WJ, Luttrell LM, Lefkowitz RJ. Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J Biol Chem. 1995;270:17148–53. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- 41.Berts A, Zhong H, Minneman KP. No role for Ca++ or protein kinase C in alpha-1A adrenergic receptor activation of mitogen-activated protein kinase pathways in transfected PC12 cells. Mol Pharmacol. 1999;55:296–303. doi: 10.1124/mol.55.2.296. [DOI] [PubMed] [Google Scholar]
- 42.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 43.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, et al. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–81. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000;97:11086–91. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–54. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, et al. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–67. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 47.Stanasila L, Abuin L, Dey J, Cotecchia S. Different internalization properties of the alpha1a- and alpha1b-adrenergic receptor subtypes: the potential role of receptor interaction with beta-arrestins and AP50. Mol Pharmacol. 2008;74:562–73. doi: 10.1124/mol.107.043422. [DOI] [PubMed] [Google Scholar]
- 48.Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 49.Abe M, Setoguchi Y, Tanaka T, Awano W, Takahashi K, et al. Membrane protein location-dependent regulation by PI3K (III) and rabenosyn-5 in Drosophila wing cells. PLoS One. 2009;4:e7306. doi: 10.1371/journal.pone.0007306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Labeling with antibodies did not impair the ERK1/2 signaling induced by α1A-AR stimulation. HEK-293A–α1A-AR cells were pre-incubated with or without anti-FLAG antibody and Alexa-555 IgG. After phenylephrine (PE) treatment, the phosphorylation level of ERK1/2 did not show significant difference among labeled and unlabeled cells. Prazosin (Pra, antagonist of α1A-AR) inhibited the PE-induced ERK1/2 activation in both.
(TIF)