Abstract
The active vitamin D metabolite, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), has been shown to be an important regulator of innate and adaptive immune function. In addition, synthesis of 1,25(OH)2D3 from 25-hydroxyvitamin D3 (25(OH)D3) by the enzyme 1α-hydroxylase in monocytes upon activation by TLR signaling has been found to regulate innate immune responses of monocytes in an intracrine fashion. In this study we wanted to determine what cells expressed 1α-hydroxylase in stimulated peripheral blood mononuclear cell (PBMC) cultures and if conversion of 25(OH)D3 to 1,25(OH)2D3 in PBMC cultures regulated antigen-specific immune responses. Initially, we found that stimulation of PBMCs from animals vaccinated with Mycobacterium bovis (M. bovis) BCG with purified protein derivative of M. bovis (M. bovis PPD) induced 1α-hydroxylase gene expression and that treatment with a physiological concentration of 25(OH)D3 down-regulated IFN-γ and IL-17F gene expression. Next, we stimulated PBMCs from M. bovis BCG-vaccinated and non-vaccinated cattle with M. bovis PPD and sorted them by FACS according to surface markers for monocytes/macrophages (CD14), B cells (IgM), and T cells (CD3). Sorting the PBMCs revealed that 1α-hydroxylase expression was induced in the monocytes and B cells, but not in the T cells. Furthermore, treatment of stimulated PBMCs with 25(OH)D3 down-regulated antigen-specific IFN-γ and IL-17F responses in the T cells, even though 1α-hydroxylase expression was not induced in the T cells. Based on evidence of no T cell 1α-hydroxylase we hypothesize that activated monocytes and B cells synthesize 1,25(OH)2D3 and that 1,25(OH)2D3 down-regulates antigen-specific expression of IFN-γ and IL-17F in T cells in a paracrine fashion.
Introduction
Substantial evidence supports the notion that vitamin D insufficiency (serum 25(OH)D3 concentrations <32 ng/mL or 80 nM) results in inadequate immune function and thus increased risk for infectious and autoimmune diseases [1]. For instance, an inverse correlation exists between serum 25(OH)D3 and the risk for upper respiratory tract infections [2], tuberculosis [3], [4], and multiple sclerosis [5], [6]. Vitamin D supplementation also decreases the risk influenza A infection [7], decreases the relapse rate in multiple sclerosis patients [8], and enhances ex vivo immunity to Mycobacteria tuberculosis [9]. The actions of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3; the active hormone) on innate and adaptive immunity and the ability of immune cells to synthesize 1,25(OH)2D3 [10] provides further evidence for a link between vitamin D status and immune function. Understanding the mechanisms of vitamin D signaling in the immune system, consequently, provides critical insight for the vitamin D requirements of the immune system.
The vitamin D hormone has been known for some time to regulate key responses of innate and adaptive immunity. The actions of 1,25(OH)2D3 on the immune system occur through the vitamin D receptor (VDR). The VDR is present in most populations of immune cells [11], [12], [13] and controls the expression of genes that have promoters with accessible vitamin D response elements [14], [15]. In human monocytes and macrophages, 1,25(OH)2D3 induces cathelicidin, CD14, defensin beta 4, and NOD2 gene expression [16], [17], [18], [19]. In contrast to human monocytes, 1,25(OH)2D3 enhances inducible nitric oxide synthase (iNOS) and RANTES/CCL5 gene expression in bovine monocytes [20]. In regards to adaptive immunity, 1,25(OH)2D3 is a potent suppressor of lymphocyte proliferation and this has been observed for humans, cattle, and mice [21], [22], [23], [24]. In addition, 1,25(OH)2D3 suppresses IFN-γ responses of T cells from humans, cattle and mice in vitro [25], [26], [27], [28], [29]. Recently, 1,25(OH)2D3 also was found to suppress IL-17A responses of human and mouse T cells [25], [30], [31]. In mouse models of autoimmune disease, 1,25(OH)2D3 suppresses Th1 and Th17-mediated inflammation [32], [33], [34], and T cell VDR expression is required for 1,25(OH)2D3-mediated inhibition of experimental autoimmune encephalomyelitis (EAE) [35]. Altogether, in vitro and in vivo evidence show that 1,25(OH)2D3 acts on immune cells to regulate both innate and adaptive immunity, and that the actions of 1,25(OH)2D3 on adaptive immunity are similar among humans, cattle and mice.
The metabolism of 1,25(OH)2D3 is critical for immune function because of the potent effects of 1,25(OH)2D3 on innate and adaptive immunity. The enzyme that synthesizes 1,25(OH)2D3 from 25-hydroxyvitamin D3 (25(OH)D3) is 1α-hydroxylase (1α-OHase) [36]. In the vitamin D endocrine system, 1α-OHase is expressed in the kidney and is tightly regulated in response to calcium homeostasis via the parathyroid hormone in order to control the circulating concentration of 1,25(OH)2D3 [37]. However, the circulating concentration of 1,25(OH)2D3 does not affect vitamin D-mediated immune responses [38], [39] and circulating 1,25(OH)2D3 does not increase when the immune system is activated [40]. Rather, monocytes and macrophages express 1α-OHase in response to toll-like receptor (TLR) signaling, and this has been shown for humans, cattle, and mice [20], [41], [42]. In addition, dendritic cells, B cells and T cells also have been found to express 1α-OHase to some degree upon activation [43], [44]. However, 1α-OHase is predominantly upregulated in the CD14+ cells (monocytes/macrophages) from the inflamed mammary gland during mastitis in cattle [45]. Consequently, induction of 1α-OHase in immune cells enables regulation of 1,25(OH)2D3 concentration at sites of inflammation and this localized regulation is evident from animal models of inflammation. In cattle, the gene for 24-hydroxylase, the vitamin D catabolic enzyme that is highly upregulated by 1,25(OH)2D3, is expressed much higher in inflamed mammary tissue than in healthy tissue or circulating immune cells during mastitis [45]. Also in cattle, 1,25(OH)2D3 accumulated in granulomas during tuberculosis [46]. Finally, the concentration of 1,25(OH)2D3 increased in the spinal cords of mice during EAE, but did not change in serum [47]. Therefore, the immune system has a mechanism to control 1,25(OH)2D3 concentration locally independent of the endocrine system.
Subsequently, local control of 1,25(OH)2D3 metabolism by the immune system has been shown to have a significant impact on innate immunity [48]. For example, synthesis of 1,25(OH)2D3 by 1α-OHase in human monocytes induces their expression of cathelicidin [41]. Similarly, synthesis of 1,25(OH)2D3 by 1α-OHase in bovine monocytes enhances their expression of iNOS and RANTES [20]. So, 1,25(OH)2D3 produced in monocytes acts in an intracrine fashion to regulate vitamin-responsive genes.
As for adaptive immunity, monocyte production of 1,25(OH)2D3 has been suggested to also regulate T cell responses in a paracrine fashion [48]. However, lymphocytes also may be a source of 1,25(OH)2D3 and regulation of antigen-specific immune responses of T cells by conversion of 25(OH)D3 to 1,25(OH)2D3 in either monocytes or lymphocytes has yet to be shown. Therefore, the objectives of this study were to evaluate 1α-OHase gene expression in PBMC cultures in response to antigen stimulation and determine the effects of 25(OH)D3 on innate and adaptive immune responses in PBMC cultures.
To accomplish the objectives of this study we use PBMCs from calves vaccinated with Mycobacterium bovis bacilli Calmette-Guerin (M. bovis BCG), which elicits strong IFN-γ and IL-17 responses to purified protein derivative (PPD) of M. bovis [49]. The calf immune system has been found to serve as a good model of the human immune system for the study of tuberculosis and M. bovis BCG vaccination [50], [51]. In addition, the concentration of 25(OH)D3 circulating in blood is similar between cattle and humans with typical concentrations ranging from 20 to 100 ng/mL in both species [52], [53], [54]. In cattle and humans symptoms of vitamin D toxicity is rarely observed with circulating 25(OH)D3 concentrations below 200 ng/mL [8], [55], [56]. Finally, as mentioned already, local control of 1,25(OH)2D3 synthesis by the immune system and 1,25(OH)2D3 –regulation of T cell responses is similar between cattle and humans. Therefore, the outcome of this study will provide insight on the mechanisms of vitamin D signaling in the human and bovine immune systems.
Materials and Methods
Animals
Twelve male Holstein calves that were approximately 5 months to 12 months of age were used for this study. At 14 d of age, 8 calves were vaccinated subcutaneously in the midcervical region with 107 cfu of M. bovis BCG (Pasteur strain). M. bovis BCG was prepared for vaccination as previously described [57]. The remaining 4 calves were not vaccinated. The NADC animal care and use committee approved the care and treatment of animals used in this study (Animal Protocol #ARS-3982).
Peripheral blood mononuclear cell cultures
Blood from the jugular vein was collected in 2× acid citrate dextrose. Blood was centrifuged and buffy coats were collected. Contaminating RBCs were removed by hypotonic lysis. PBMCs were isolated by density gradient centrifugation. PBMC were resuspended in RPMI 1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 50 µg/ml gentamicin (Invitrogen, Carlsbad, CA). For gene expression assays, PBMCs were cultured at a concentration of 1.5×107 cells/ml in 96-well (200 µl/well) or 6-well (2 ml/well) tissue culture plates for 24 h at 37°C in 5% CO2. For determination of nitric oxide and IFN-γ production, PBMCs were cultured at 1×106 cells/mL in a 96-well plate (200 µl/well) for 24, 48, and 72 h.
LPS from Serratia marcescens (Sigma-Aldrich), pokeweed mitogen (PWM; Sigma-Aldrich) and purified protein derivative from M. bovis (M. bovis PPD) (Prionics, Zurich, Switzerland) were added at 100 ng/ml, 5 µg/ml, and 10 µg/ml, respectively, to PBMC cultures. The vitamin D metabolites, 25(OH)D3 and 1,25(OH)2D3, (Sigma-Aldrich) were diluted in 100% ethanol and added to fetal bovine serum (FBS; Hyclone, Waltham, MA) at 10× the final desired concentration. The concentrations of 25(OH)D3 and 1,25(OH)2D3 in ethanol were confirmed by UV spectroscopy. FBS with ethanol (vehicle) or the vitamin D metabolites was added to PBMC cultures to a final concentration of 10% FBS. The final concentration of ethanol did not exceed 0.04%.
Cell sorting
PBMCs from 7 BCG-vaccinated and 4 non-vaccinated calves were cultured with 10 µg/ml M. bovis PPD and 0 or 100 ng/ml 25(OH)D3 for 24 h in 6 well plates. Cells were removed from the wells with cold PBS and scraping. Cells were labeled with anti-CD14 (CAM36A; mouse IgG1), anti-IgM (PIG45A; mouse IgG2b), or a cocktail of anti-CD3 (MM1A; mouse IgG1), anti-CD4 (CACT83B; mouse IgM), anti-CD8 (MAQ111A; mouse IgM) and anti-γδTCR (CACT61A; mouse IgM). Cells labeled with the cocktail of CD3, CD4,CD8, and γδTCR antibodies are simply referred to as CD3+ cells. All primary antibodies were purchased from VMRD, Pullman, WA. Cells were then labeled with anti-mouse IgG1-PE (Becton Dickinson, San Jose, CA), anti-mouse IgG2b-Cy5 (Southern Biotech, Birmingham, AL), or a combination of anti-mouse IgG1-PE and anti-mouse IgM-PE (Becton Dickinson) secondary antibodies. Labeled cells were separated based on fluorescence intensity using the BD FACSAria Cell Sorting System (BD Biosciences, San Jose, CA). Approximately 106 cells of each sub-population with >95% purity were collected from each PBMC culture.
Relative gene expression
RNA was isolated from PBMC using the RNeasy Mini Kit (Qiagen, Valencia, CA). RNA samples were reverse transcribed to cDNA in 20 µl reactions using the High Capacity Reverse Transcription Kit with RNase inhibitor and random primers (Applied Biosystems, Foster City, CA). The reverse transcription reactions were incubated for 2 h at 37°C followed by 5 s at 85°C and finally cooled to 4°C. The cDNA samples were diluted 1∶10 in water and stored at −20°C.
The amount of specific cDNA transcripts in each sample was determined using the 7300 Real-Time PCR System (Applied Biosystems). Each reaction contained 12.5 µl SYBR Green Master Mix (Applied Biosystems), 7.5 µl of cDNA sample, and 5 µl of 10 µM forward and 10 µM reverse primers. Reactions were incubated as follows: 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Primer sets were designed with Primer3 (http://frodo.wi.mit.edu/primer3) [58] to span intron-exon boundaries and are listed in Table 1. Primers were purchased from Integrated DNA Technologies (Coralville, IA). The efficiency of each primer set was determined as previously described [20] and fit the criteria required for quantification by real-time PCR [59]. The specificity of each primer set was verified by melting curve analysis and gel electrophoresis. The amounts of cDNA transcripts were normalized to ribosomal protein S9 (RPS9) cDNA. Expression of RPS9 also was compared to β-actin and GAPDH expression to verify its stability over treatment conditions. The relative expression of each gene was determined using the 2−ΔΔCt method [59]. The expression of each gene is relative to the normalized amount of each cDNA transcript in the non-stimulated controls for each experiment.
Table 1. Primer sequences for real-time PCR.
| Gene (alternate name) | Accesion no.1 | Strand | Sequence (5′ - 3′) |
| 1α-OHase (CYP27B1)2 | NM_001192284 | Forward Reverse | TGGGACCAGATGTTTGCATTCGC TTCTCAGACTGGTTCCTCATGGCT |
| 24-OHase (CYP24A1)2 | NM_001191417 | Forward Reverse | GAAGACTGGCAGAGGGTCAG CAGCCAAGACCTCGTTGATT |
| IFN-γ | NM_174086 | Forward Reverse | GATTCAAATTCCGGTGGATG GCAGGAGGACCATTACGTTG |
| IL-17A | NM_001008412 | Forward Reverse | TCCATCTCACAGCGAGCACAAG AGCCACCAGACTCAGAAGCAGTA |
| IL-17F | NM_001192082 | Forward Reverse | CTCCCCTTGGGATTACAACA TTCAGGGTCCTGTCTTCCTG |
| iNOS2 | NM_001076799 | Forward Reverse | GATCCAGTGGTCGAACCTGC CAGTGATGGCCGACCTGATG |
| RANTES (CCL5)2 | NM_175827 | Forward Reverse | CACCCACGTCCAGGAGTATT CTCGCACCCACTTCTTCTCT |
| RPS92 | NM_001101152 | Forward Reverse | GTGAGGTCTGGAGGGTCAAA GGGCATTACCTTCGAACAGA |
| VDR | NM_001167932 | Forward Reverse | AGCCACCGGCTTCCATTTCA AACAGCGCCTTCCGCTTCAT |
Accesion numbers for mRNA sequences from NCBI database.
Primer sequences have been published previously [20].
Measurement of nitric oxide production
Production of nitric oxide by PBMCs was determined by measurement of nitrite in the culture supernatant by using the Griess assay as previously described [20]. Supernatants (100 µL) from PBMC cultures were added to an equal volume of Griess reagent [0.5% sulfanilamide, 2.5% phosphoric acid, and 0.05% N-(1-naphthyl) ethylenediamine dihydrochloride; Sigma-Aldrich] in a 96-well clear bottom plate. Absorbance at 550 nm in each well was measured using a FlexStation 3 plate reader (Molecular Devices, Sunnyvale, CA). Absorbance values were converted to micromoles per liter using a standard curve that was generated by addition of 0 to 100 µM sodium nitrite to fresh culture media.
Measurement of IFN-γ production
The concentration of IFN-γ in PBMC culture supernatants was determined by an ELISA using the Endogen Bovine IFNγ Screening Set (Pierce Biotechnology, Rockford IL) according to the manufacturers instructions. The absorbance at 450 nm minus the absorbance at 550 nm was measured with the FlexStation 3 plate reader and the values were converted to picograms per milliliter by using a standard curve.
Statistical Analysis
Analysis of variance was performed using PROC GLM of SAS (SAS Institute INC., Cary, NC). The model accounted for effects of treatment, cell type, and calf or vaccination status. ΔΔCt values were used in the analyses of gene expression. The average ΔΔCt values ± SE were transformed using the equation 2−ΔΔCt. The expression of each gene is presented as the mean fold increase ± SE relative to non-stimulated controls. Multiple comparison tests of the means were made using the Tukey adjustment.
Results
M. bovis PPD-activation of vitamin D signaling in PBMCs
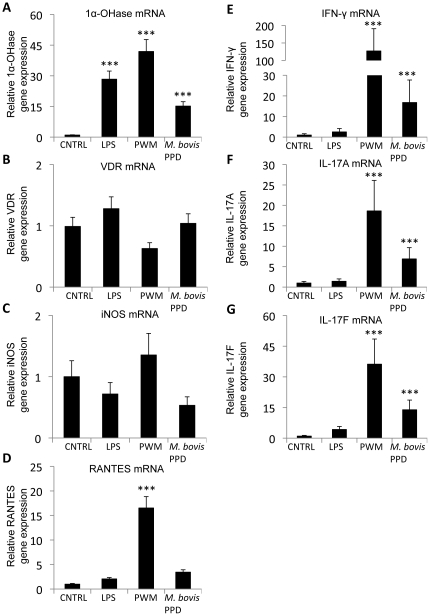
By stimulating PBMCs from M. bovis-BCG-vaccinated calves with LPS, PWM, or M. bovis PPD, we found that 1α-OHase gene expression in the PBMCs was upregulated by LPS, PWM, and M. bovis PPD stimulation relative to non-stimulated PBMCs (P<0.001; Fig. 1A). In contrast, VDR gene expression in the PBMC cultures was not upregulated by any of the stimulants (Fig. 1B). We also measured iNOS, RANTES, IFN-γ, IL-17A, and IL-17F gene expression. Neither iNOS nor RANTES was affected by M. bovis PPD or LPS stimulation, but RANTES was upregulated by PWM stimulation (Fig. 1C and D). IFN-γ, IL-17A, and IL-17F were upregulated in PBMCs stimulated with PWM or M. bovis PPD, however, they were not affected by LPS stimulation (Fig. 1E–G).
Figure 1. 1α-hydroxylase (1α-OHase) gene expression in PBMC cultures.
PBMC cultures from eight M. bovis-BCG-vaccinated calves were treated with 100 ng/ml LPS, 5 µg/ml PWM, or 10 µg/ml of M. bovis PPD or received no treatment (CTRL) as indicated for 24 h. The amount of 1α-OHase (A), VDR (B), iNOS (C), RANTES (D), IFN-γ (E), IL-17A (F), and IL-17F (G) mRNA was determined by quantitative real-time RT-PCR and was normalized to the amount of RPS9 mRNA in each sample. Expression of each gene is relative to non-stimulated cultures. Error bars represent SE. *** Mean is different from non-stimulated PBMC; P<0.001.
Previously, treatment of M. bovis PPD-stimulated PBMCs with exogenous 1,25(OH)2D3 was found to suppress IFN-γ production [29]. And recently, we showed that conversion of 25(OH)D3 to 1,25(OH)2D3 by 1α-OHase in activated bovine monocytes up-regulated iNOS and RANTES expression [20]. Because 1α-OHase gene expression was upregulated in M. bovis PPD-stimulated PBMC, we wanted to determine the effect of 25(OH)D3 on gene expression in M. bovis PPD-stimulated PBMCs.
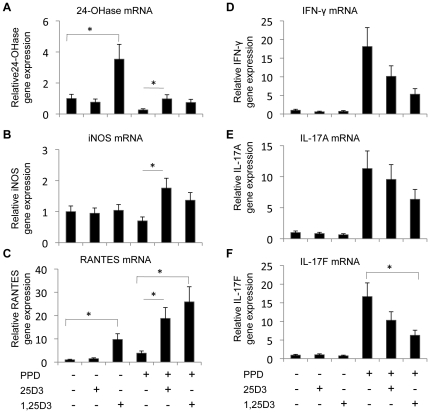
Addition of 100 ng/mL 25(OH)D3, a physiological concentration [54], to resting PBMCs did not affect expression of any of the genes tested (Fig. 2). Addition of 4 ng/mL 1,25(OH)2D3, a concentration 2 to 3 orders of magnitude greater than normal serum 1,25(OH)2D3, did upregulate 24-OHase and RANTES gene expression in resting PBMCs (P<0.05; Fig. 2A and C). Stimulation of PBMCs with M. bovis PPD suppressed 24-OHase gene expression (P<0.05; Fig. 2A), which is consistent with LPS stimulation of bovine monocytes [20]. However, addition of either 25(OH)D3 or 1,25(OH)2D3 to stimulated PBMCs increased 24-OHase gene expression relative to PBMCs that were stimulated with M. bovis PPD alone. Similarly, iNOS and RANTES were upregulated in M. bovis PPD-stimulated PBMCs treated with 25(OH)D3 or 1,25(OH)2D3 compared to PBMCs that were stimulated with M. bovis PPD alone (P<0.05; Fig. 2B and C). IL-17F gene expression was decreased in stimulated PBMCs that were treated with 1,25(OH)2D3 (P<0.05; Fig. 2F). IFN-γ, IL-17A, and IL-17F gene expression was decreased by 25(OH)D3 treatment, but the decrease was not statistically significant (P>0.05; Fig. 2D–F).
Figure 2. Effects of 1,25(OH)2D3 and 25(OH)D3 on gene expression in PBMC cultures.
PBMC cultures from eight M. bovis-BCG-vaccinated calves were treated with 0 or 10 µg/ml of M. bovis purified protein derivative (PPD), 100 ng/ml of 25(OH)D3, and 4 ng/ml 1,25(OH)2D3 as indicated for 24 h. The amount 24-OHase (A), iNOS (B), RANTES (C), IFN-γ (D), IL17A (E), and IL-17F (F) mRNA was determined by quantitative real-time RT-PCR. Each gene was normalized to the amount of RPS9 mRNA in each sample. Expression of each gene is relative the expression of that gene in non-stimulated cultures. Error bars represent SE. * Means are different; P<0.05.
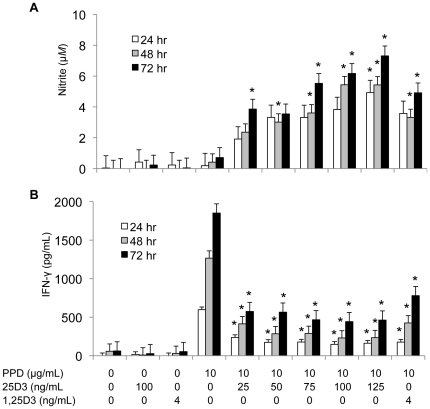
In addition to gene expression, we measured nitric oxide and IFN-γ production by M. bovis PPD-stimulated PBMCs treated with graded doses of 25(OH)D3 form 0 to 125 ng/mL (Fig. 3). Like iNOS gene expression, 100 ng/mL 25(OH)D3 increased nitric oxide production, as measured by nitrite in the culture supernatant, after 48 and 72 h in culture (Fig. 3A). Furthermore, treatment with 25 to 125 ng/mL 25(OH)D3 increased nitric oxide production by the stimulated PBMCs in a dose dependent manner. Addition of 25(OH)D3 suppressed IFN-γ production in M. bovis PPD-stimulated cultures after 24, 48, and 72 h in culture (P<0.05; Fig. 3B), but the effect of 25(OH)D3 did not occur in linear fashion.
Figure 3. Effects of 1,25(OH)2D3 and 25(OH)D3 on nitrite oxide and IFN-γ production.
PBMCs from seven M. bovis-BCG-vaccinated calves were cultured at a concentration of 1×106 cells/mL with 0 or 10 µg/ml of M. bovis purified protein derivative (PPD), 25 to 125 ng/ml of 25(OH)D3, or 4 ng/ml 1,25(OH)2D3 as indicated for 24, 48, and 72 h. A) The nitrite concentration in the culture supernatants was measured by using the Griess assay and used as an indicator of nitric oxide production by the PBMCs. B) The concentration of IFN-γ in the culture supernatants was measured by an ELISA specific for bovine IFN-γ. Data represents the average concentration of nitrite or IFN-γ in culture supernatants harvested at 24, 48, and 72 h. Error bars represent SE. * Mean is different (P<0.05) from PBMC cultures treated with M. bovis PPD alone for the corresponding time point.
Cell type-specific expression of 1α-OHase and VDR
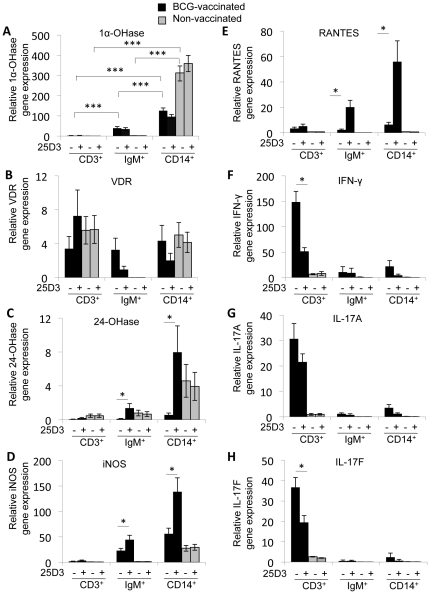
Several cell types have been reported to express 1α-OHase, including activated monocytes, T cells, and B cells [41], [43], [44]. We sorted PBMCs that had been stimulated with M. bovis PPD from BCG-vaccinated animals according to surface expression of CD3, IgM, and CD14 by using FACS (Fig. 4) to determine what populations of cells in PBMCs were expressing 1α-OHase upon activation. By sorting the stimulated PBMCs, we found that 1α-OHase was predominantly expressed in the CD14+ population of cells (P<0.001; Fig. 4A). 1α-OHase expression was also induced in IgM+ cells from vaccinated calves (P<0.001). Relative to 1α-OHase expression in non-stimulated, non-sorted PBMCs, the expression of 1α-OHase did not increase in the CD3+ cells isolated from the stimulated PBMC cultures (Fig. 4A). Unlike 1α-OHase, VDR gene expression did not differ significantly between cell types in PBMC cultures from vaccinated calves, but IgM+ cells from 25(OH)D3 treated cultures did have somewhat lower VDR expression (Fig. 4B).
Figure 4. Cell-type specific regulation of vitamin D signaling.
PBMCs from seven M. bovis-BCG-vaccinated calves (black bars) and four non-vaccinated calves (grey bars) were treated with 10 µg/ml of M. bovis PPD and 0 or 100 ng/ml 25(OH)D3 as indicated for 24 h. After treatment, PBMCs were sorted by FACS according to CD3/4/8/γδTCR (CD3; T cell), IgM (B cell), and CD14 (monocyte) expression on the cell surface. The amount of 1α-OHase (A), VDR (B), 24-OHase (C), iNOS (D), RANTES (E), IFN-γ (F), IL-17A (G), and IL-17F (H) mRNA in the sorted cells was determined by quantitative real-time RT-PCR. The amount of mRNA for each gene was normalized to the amount of RPS9 mRNA in each sample. Expression of each gene in each cell type is relative to non-stimulated, non-sorted PBMCs. Error bars represent SE. * P<0.05, *** P<0.001, Means are different.
Cell type-specific effects of 25(OH)D3 on gene expression
We also compared gene expression in cells from M. bovis PPD-stimulated PBMCs that were treated with 100 ng/ml 25(OH)D3 with cells from M. bovis PPD-stimulated PBMCs that were not treated with 25(OH)D3. Treatment with 25(OH)D3 increased 24-OHase, iNOS, and RANTES gene expression in both CD14+ cells and IgM+ cells from the BCG-vaccinated calves (P<0.05; Fig. 4C–E). In contrast, 25(OH)D3 treatment decreased expression of IFN-γ by over 60% and IL-17F by nearly 50% in the CD3+ cells from the BCG-vaccinated calves (P<0.05; Fig. 4F and H). IL-17A expression in the CD3+ cells was also down-regulated by 25(OH)D3 treatment, but to a lesser extent (P>0.05; Fig. 4G).
Comparison of responses between BCG-vaccinated and non-vaccinated animals
Finally, we compared changes in gene expression caused by M. bovis PPD stimulation and 25(OH)D3 in cells from non-vaccinated animals to the changes observed in cells from BCG-vaccinated animals. In PBMCs from non-vaccinated animals, 1α-OHase was induced in CD14+ cells by M. bovis PPD stimulation like in CD14+ cells from BCG-vaccinated animals (Fig. 4A). Unlike the PBMCs from BCG-vaccinated calves, VDR was not detected in IgM+ cells from the non-vaccinated animals (Fig. 4B). VDR expression in CD3+ and CD14+ cells was similar between vaccinated and non-vaccinated calves. Neither 24-OHase, iNOS, or RANTES expression was affected by 25(OH)D3 in CD14+ cells and IgM+ cells from the non-vaccinated animals like it was in the BCG-vaccinated animals (Fig. 4C–E). Finally, IFN-γ, IL-17A, and IL-17F were not induced by M. bovis PPD stimulation in the CD3+ cells from non-vaccinated animals as they were in CD3+ cells from BCG-vaccinated animals (Fig. 4F–H).
Discussion
For over two decades now, 1,25(OH)2D3 has been known as an important regulator of adaptive immunity, suppressing lymphocyte proliferation and IFN-γ production [28], [60]. The implications of 1,25(OH)2D3 on adaptive immunity are further realized in animal models of T cell-mediated autoimmunity as 1,25(OH)2D3 inhibits disease progression [32], [34], [61], [62]. Recently, 1,25(OH)2D3 also was found to be an important regulator of innate immunity by enhancing antimicrobial properties of macrophages [16], [18]. Vitamin D-mediated immune responses, however, do not correlate with the circulating concentration of 1,25(OH)2D3 [38], [39]. Therefore, local synthesis of 1,25(OH)2D3 is a critical factor in regulating both innate and adaptive immunity. Previously, induction of 1α-OHase expression in macrophages was shown to occur upon activation by TLR 2/1 or TLR 4 signaling and enable them to convert 25(OH)D3 to 1,25(OH)2D3 [20], [41], [63]. Synthesis of 1,25(OH)2D3 in macrophages, in turn, enhanced their innate antimicrobial properties in an intracrine fashion [48]. In this study, we give evidence that endogenous synthesis of 1,25(OH)2D3 also occurs in antigen-stimulated PBMC cultures and regulates key aspects of adaptive immunity.
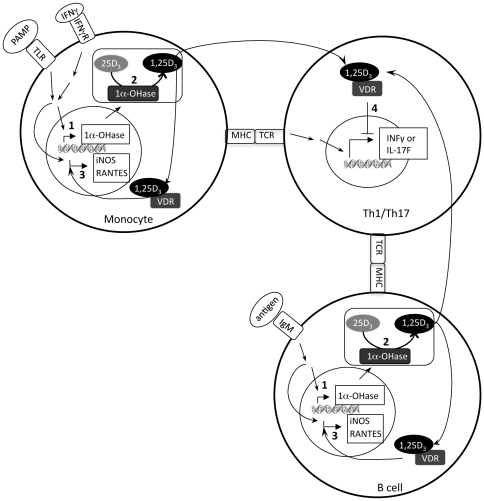
In this study we found that 1α-OHase gene expression was induced in CD14+ cells (monocytes/macrophages) and IgM+ (B cells), but not in CD3+ (T cells) cells in M. bovis PPD-stimulated PBMC cultures. Furthermore, treatment of M. bovis PPD-stimulated PBMC cultures with 25(OH)D3 enhanced iNOS and RANTES expression in monocytes and B cells and suppressed antigen-specific IFN-γ and IL-17F responses in T cells. Based on this evidence, we hypothesize that 1,25(OH)2D3 was produced in monocytes and B cells acted on monocytes and B cells in an intracrine fashion to upregulate iNOS and RANTES expression and on T cells in a paracrine fashion to suppress M. bovis PPD-specific IFN-γ and IL-17F responses (Fig. 5).
Figure 5. Regulation of T cells through a paracrine mechanism of vitamin D signaling.
1) 1α-hydroxylase (1α-OHase) gene expression is induced in monocytes by recognition of pathogen associated molecular patterns (PAMP) by toll-like receptors (TLR) and interferon-γ (IFN-γ) from Th1 cells, and in B cells by antigen recognition by the B cell receptor (IgM) along with co-stimulation by antigen-specific T cells. 2) 25-hydroxyvitamin D3 (25D3) is converted to 1,25-dihydroxyvitamin D3 (1,25D3) by 1α-OHase. 3) 1,25(OH)2D3 produced in monocytes and B cells activates the vitamin D receptor (VDR) and enhances iNOS and RANTES expression in monocytes and B cells. 4) 1,25(OH)2D3 secreted from monocytes and B cells suppresses IFN-γ and interleukin-17F (IL-17F) expression in T cells. Direct suppression of IFN-γ and IL-17F expression by the 1,25(OH)2D3, however, is not certain. Instead, 1,25(OH)2D3 may influence T cell development, suppress proliferation, or sensitize them to apoptosis.
M. bovis PPD is a crude extract and as such likely contains antigens that activate both the innate and adaptive immune systems. Therefore, in PBMC cultures innate antigen presenting cells (e.g., monocytes) recognize TLR ligands, such as lipoproteins, become activated and then express 1α-OHase. The APCs also internalize protein from the M. bovis PPD, process it and then present it on their surface as peptide associated with MHC. Activation of T cells specific for M. bovis PPD is then caused by the interaction of the specific T cell receptor (TCR) with its cognate MHC/antigen. Likewise, B cells recognize antigen through IgM on their surfaces, and along with co-stimulation from T cells, become activated and express 1α-OHase. We suggest that production of 1,25(OH)2D3 by 1α-OHase in activated monocytes and B cells can alter the IFN-γ and IL-17F responses that are the result of the TCR/MHC/antigen interaction between T cells and APCs.
There are multiple possibilities as to how 1,25(OH)2D3 suppressed IFN-γ and IL-17F gene expression in T cells. VDR expression in the T cells was similar to that in monocytes in this study and purified T cells do respond to 1,25(OH)2D3 [25], . Also, T cell VDR expression is required for 1,25(OH)2D3-mediated inhibition of experimental autoimmune encephalomyelitis in mice [35]. Therefore, the T cells in the PBMC cultures likely had the ability to respond to 1,25(OH)2D3 secreted from the monocytes and B cells. Consequently, activation of the VDR in T cells could have directly suppressed IFN-γ and IL-17F expression. However, 1,25(OH)2D3 failed to suppress IFN-γ production in fully differentiated Th1 cells [61]; so, 1,25(OH)2D3 may have regulated genes in T cells that influenced T cell differentiation or sensitized them to apoptosis. Alternatively, up-regulation of nitric oxide production by 1,25(OH)2D3 in monocytes and B cells could have induced apoptosis in the surrounding T cells and resulted in suppressed IFN-γ and IL-17F expression. A combination of several mechanisms also is possible and we have not ruled out the possibility that T cells are able to synthesize their own 1,25(OH)2D3. Therefore, further experiments are needed to determine if regulation of T cell responses by 1,25(OH)2D3 strictly depends on synthesis of 1,25(OH)2D3 in monocytes or B cells and how 1,25(OH)2D3 is regulating T cell responses.
In any case, treatment of antigen-stimulated PBMCs with 25(OH)D3 suppressed antigen-specific IFN-γ and IL-17F expression in T cells, which indicates that synthesis of 1,25(OH)2D3 by immune cells has significant implications in regulating adaptive immunity. IFN-γ is a potent activator of macrophages and is mainly produced by Th1 cells [65]. Th1-mediated responses are critical in the defense against intracellular infections, such as tuberculosis [66], [67]. IL-17A and IL-17F are produced by Th17 cells and play major roles in neutrophil recruitment and protection against intracellular and extracellular bacterial infections [68], [69], [70]. Self reactive Th1 and Th17 cells, however, are involved in the development of autoimmune disorders [71] and inhibition of animal models of autoimmunity by 1,25(OH)2D3 is thought to occur, in part, by suppression of self-reactive Th1 and Th17 cells [72]. Although suppression of Th1 and Th17 responses to bacterial antigens by 1,25(OH)2D3 would seem to attenuate the immune response against bacterial infections, keep in mind that 1,25(OH)2D3 also enhances the antimicrobial activity of macrophages [41]. So overall, production of 1,25(OH)2D3 by immune cells serves to limit inflammation caused by Th1 and Th17 effector cells, but ultimately improves defense against bacterial infections by boosting the innate antimicrobial response.
In addition to suppression of T cell responses by 1,25(OH)2D3 synthesis in PBMC cultures, treatment of PBMCs with 25(OH)D3 upregulated antigen-specific B cell iNOS and RANTES expression. We had previously shown that monocyte iNOS and RANTES expression depends on availability of 25(OH)D3 [20], but not B cell iNOS and RANTES. Nitric oxide produced by iNOS in macrophages is considered to be an antimicrobial molecule. However, nitric oxide produced by the monocytes and B cells may suppress proliferation of T cells [73]. So, as mentioned above, 1,25(OH)2D3 may suppress T cell responses in part by enhancing B cell and monocyte iNOS expression. RANTES is a chemokine originally found to be expressed by T cells [74], but also has been found to be expressed by alveolar macrophages in cattle [75]. We speculate that upregulation of RANTES in monocytes and B cells by 1,25(OH)2D3 would enhance recruitment of immune cells to the site of inflammation, but the implications of 1,25(OH)2D3-upregulation of RANTES in monocytes and B cells will need to be investigated.
Finally, the ability of 1α-OHase in monocytes and B cells to synthesize 1,25(OH)2D3, and subsequently regulate 1,25(OH)2D3-mediated immune responses, depends on the availability of 25(OH)D3. The circulating concentration of 25(OH)D is primarily regulated by dietary intake of vitamin D3 and sun exposure [76]. Current recommendations for vitamin D in humans and cattle target a circulating concentration of 25(OH)D of 20 to 50 ng/ml [55], [77]. However, 25(OH)D concentrations above 30 ng/ml may be necessary for optimal immune function [1]. In addition, vitamin D insufficiency (serum 25(OH)D below 30 ng/ml) and even deficiency (serum 25(OH)D below 20 ng/ml) is widespread, indicating that current recommendations for vitamin D3 intake may be inadequate [78], [79]. Previously, and here, we have shown that 1,25(OH)2D3-regulated innate immune responses increase linearly from 0 to 125 ng/ml of 25(OH)D3 [20]. This observation leads to the question of what concentration is necessary for optimal immune functionality if below 30 ng/ml is insufficient? Based on the requirement of 25(OH)D3 by the immune system for signaling mechanisms and evidence from epidemiological studies, vitamin D requirements need to be re-evaluated to ensure proper immune function.
Acknowledgments
We thank Bruce Pesch, Duane Zimmerman, and Randy Atchison (National Animal Disease Center, USDA, Ames, IA) for their technical assistance. We also thank Philip Liu (Department of Orthopedic Surgery, University of California at Los Angeles) and Justin Spanier (Department of Biochemistry, University of Wisconsin Madison) for their critical review of this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by United States Department of Agriculture, Agriculture Research Service Internal Funding, Project Number: 3625-32000-094-00. The study design, data collection and analysis, decision to publish, or preparation of the manuscript is determined by the USDA. No current external funding sources for this study.
References
- 1.Adams JS, Hewison M. Update in vitamin d. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 4.Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr Infect Dis J. 2008;27:941–942. doi: 10.1097/INF.0b013e31817525df. [DOI] [PubMed] [Google Scholar]
- 5.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 6.Munger KL, Zhang SM, O'Reilly E, Hernan MA, Olek MJ, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 7.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010 doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 8.Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74:1852–1859. doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 10.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 12.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 13.Reinhardt TA, Horst RL, Littledike ET, Beitz DC. 1,25-Dihydroxyvitamin D3 receptor in bovine thymus gland. Biochem Biophys Res Commun. 1982;106:1012–1018. doi: 10.1016/0006-291x(82)91812-5. [DOI] [PubMed] [Google Scholar]
- 14.Koszewski NJ, Reinhardt TA, Horst RL. Vitamin D receptor interactions with the murine osteopontin response element. J Steroid Biochem Mol Biol. 1996;59:377–388. doi: 10.1016/s0960-0760(96)00127-6. [DOI] [PubMed] [Google Scholar]
- 15.Pike JW, Meyer MB, Watanuki M, Kim S, Zella LA, et al. Perspectives on mechanisms of gene regulation by 1,25-dihydroxyvitamin D3 and its receptor. J Steroid Biochem Mol Biol. 2007;103:389–395. doi: 10.1016/j.jsbmb.2006.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB Journal. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 17.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang TT, Nestel FP, Bourdeau V, Naga iY, Wang Q, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of Immunology. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 19.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson CD, Reinhardt TA, Thacker TC, Beitz DC, Lippolis JD. Modulation of the bovine innate immune response by production of 1alpha,25-dihydroxyvitamin D(3) in bovine monocytes. J Dairy Sci. 2010;93:1041–1049. doi: 10.3168/jds.2009-2663. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi T, Nakao Y, Matsui T, Nakagawa T, Katakami Y, et al. Effect of 1,25-dihydroxyvitamin D3 on cytokine-induced thymocyte proliferation. Cell Immunol. 1985;96:455–461. doi: 10.1016/0008-8749(85)90377-6. [DOI] [PubMed] [Google Scholar]
- 22.Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller K, Bendtzen K. Inhibition of human T lymphocyte proliferation and cytokine production by 1,25-dihydroxyvitamin D3. Differential effects on CD45RA+ and CD45R0+ cells. Autoimmunity. 1992;14:37–43. doi: 10.3109/08916939309077355. [DOI] [PubMed] [Google Scholar]
- 24.Nonnecke BJ, Franklin ST, Reinhardt TA, Horst RL. In vitro modulation of proliferation and phenotype of resting and mitogen-stimulated bovine mononuclear leukocytes by 1,25-dihydroxyvitamin D3. Vet Immunol Immunopathol. 1993;38:75–89. doi: 10.1016/0165-2427(93)90114-j. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemire JM. Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 1995;53:599–602. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 27.Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168:1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 28.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84:3385–3389. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waters WR, Nonnecke BJ, Rahner TE, Palmer MV, Whipple DL, et al. Modulation of Mycobacterium bovis-specific responses of bovine peripheral blood mononuclear cells by 1,25-dihydroxyvitamin D(3). Clin Diagn Lab Immunol. 2001;8:1204–1212. doi: 10.1128/CDLI.8.6.1204-1212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer MT, Lee YK, Maynard CL, Oliver JR, Bikle DD, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem. 2011;286:997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang J, Zhou R, Luger D, Zhu W, Silver PB, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182:4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. European Journal of Immunology. 2011 doi: 10.1002/eji.201040632. In press. [DOI] [PubMed] [Google Scholar]
- 36.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–134. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 37.Horst RL, Goff JP, Reinhardt TA. Role of vitamin D in calcium homeostasis and its use in prevention of bovine periparturient paresis. Acta Vet Scand Suppl. 2003;97:35–50. [PubMed] [Google Scholar]
- 38.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smolders J, Menheere P, Thewissen M, Peelen E, Cohen Tervaert JW, et al. Regulatory T cell function correlates with serum 25-hydroxyvitamin D, but not with 1,25-dihydroxyvitamin D, parathyroid hormone and calcium levels in patients with relapsing remitting multiple sclerosis. J Steroid Biochem Mol Biol. 2010;121:243–246. doi: 10.1016/j.jsbmb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Waldron MR, Nonnecke BJ, Nishida T, Horst RL, Overton TR. Effect of lipopolysaccharide infusion on serum macromineral and vitamin D concentrations in dairy cows. J Dairy Sci. 2003;86:3440–3446. doi: 10.3168/jds.S0022-0302(03)73948-4. [DOI] [PubMed] [Google Scholar]
- 41.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 42.Stoffels K, Overbergh L, Bouillon R, Mathieu C. Immune regulation of 1alpha-hydroxylase in murine peritoneal macrophages: unravelling the IFNgamma pathway. J Steroid Biochem Mol Biol. 2007;103:567–571. doi: 10.1016/j.jsbmb.2006.12.091. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 44.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 45.Nelson CD, Reinhardt TA, Beitz DC, Lippolis JD. In vivo activation of the intracrine vitamin d pathway in innate immune cells and mammary tissue during a bacterial infection. PLoS One. 2010;5:e15469. doi: 10.1371/journal.pone.0015469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhodes SG, Terry LA, Hope J, Hewinson RG, Vordermeier HM. 1,25-dihydroxyvitamin D3 and development of tuberculosis in cattle. Clin Diagn Lab Immunol. 2003;10:1129–1135. doi: 10.1128/CDLI.10.6.1129-1135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175:4119–4126. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 48.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.02.013. DOI: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vordermeier HM, Villarreal-Ramos B, Cockle PJ, McAulay M, Rhodes SG, et al. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect Immun. 2009;77:3364–3373. doi: 10.1128/IAI.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Endsley JJ, Waters WR, Palmer MV, Nonnecke BJ, Thacker TC, et al. The calf model of immunity for development of a vaccine against tuberculosis. Vet Immunol Immunopathol. 2009;128:199–204. doi: 10.1016/j.vetimm.2008.10.312. [DOI] [PubMed] [Google Scholar]
- 51.Van Rhijn I, Godfroid J, Michel A, Rutten V. Bovine tuberculosis as a model for human tuberculosis: advantages over small animal models. Microbes Infect. 2008;10:711–715. doi: 10.1016/j.micinf.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 53.Horst RL, Goff JP, Reinhardt TA. Adapting to the transition between gestation and lactation: differences between rat, human and dairy cow. J Mammary Gland Biol Neoplasia. 2005;10:141–156. doi: 10.1007/s10911-005-5397-x. [DOI] [PubMed] [Google Scholar]
- 54.Nonnecke BJ, Foote MR, Miller BL, Beitz DC, Horst RL. Short communication: Fat-soluble vitamin and mineral status of milk replacer-fed dairy calves: effect of growth rate during the preruminant period. J Dairy Sci. 2010;93:2684–2690. doi: 10.3168/jds.2009-2892. [DOI] [PubMed] [Google Scholar]
- 55.Heaney RP. Vitamin D: criteria for safety and efficacy. Nutr Rev. 2008;66:S178–181. doi: 10.1111/j.1753-4887.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- 56.Horst RL, Goff JP, Reinhardt TA. Calcium and vitamin D metabolism in the dairy cow. J Dairy Sci. 1994;77:1936–1951. doi: 10.3168/jds.S0022-0302(94)77140-X. [DOI] [PubMed] [Google Scholar]
- 57.Foote MR, Nonnecke BJ, Beitz DC, Waters WR. Antigen-specific B-cell responses by neonatal calves after early vaccination. J Dairy Sci. 2007;90:5208–5217. doi: 10.3168/jds.2007-0285. [DOI] [PubMed] [Google Scholar]
- 58.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 60.Lemire JM, Adams JS, Kermani-Arab V, Bakke AC, Sakai R, et al. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032–3035. [PubMed] [Google Scholar]
- 61.Nashold FE, Hoag KA, Goverman J, Hayes CE. Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D(3) prevention of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;119:16–29. doi: 10.1016/s0165-5728(01)00360-5. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85:2480–2490. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 63.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, et al. Immune regulation of 25-hydroxyvitamin-D3-1-hydroxylase in human monocytes. Journal of Bone and Mineral Research. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 64.Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D(3) in the immune system. J Steroid Biochem Mol Biol. 2010 doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 65.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cooper AM. T cells in mycobacterial infection and disease. Curr Opin Immunol. 2009;21:378–384. doi: 10.1016/j.coi.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Almeida DE, Colvin CJ, Coussens PM. Antigen-specific regulatory T cells in bovine paratuberculosis. Vet Immunol Immunopathol. 2008;125:234–245. doi: 10.1016/j.vetimm.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 68.Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev. 2008;226:80–86. doi: 10.1111/j.1600-065X.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immun. 2010;78:32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dittel BN. CD4 T cells: Balancing the coming and going of autoimmune-mediated inflammation in the CNS. Brain Behav Immun. 2008;22:421–430. doi: 10.1016/j.bbi.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc. 2010;69:286–289. doi: 10.1017/S0029665110001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bogdan C. Regulation of lymphocytes by nitric oxide. Methods Mol Biol. 2011;677:375–393. doi: 10.1007/978-1-60761-869-0_24. [DOI] [PubMed] [Google Scholar]
- 74.Levy JA. The unexpected pleiotropic activities of RANTES. J Immunol. 2009;182:3945–3946. doi: 10.4049/jimmunol.0990015. [DOI] [PubMed] [Google Scholar]
- 75.Widdison S, Watson M, Piercy J, Howard C, Coffey TJ. Granulocyte chemotactic properties of M. tuberculosis versus M. bovis-infected bovine alveolar macrophages. Mol Immunol. 2008;45:740–749. doi: 10.1016/j.molimm.2007.06.357. [DOI] [PubMed] [Google Scholar]
- 76.Holick MF. Sunlight, UV-radiation, vitamin D and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2008;624:1–15. doi: 10.1007/978-0-387-77574-6_1. [DOI] [PubMed] [Google Scholar]
- 77.NRC. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition. Washington, DC: Natl Acad Sci; 2001. [Google Scholar]
- 78.Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2007;22(Suppl 2):V64–68. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 79.Hollis BW. Nutrition: US recommendations fail to correct vitamin D deficiency. Nat Rev Endocrinol. 2009;5:534–536. doi: 10.1038/nrendo.2009.178. [DOI] [PubMed] [Google Scholar]