Abstract
Background
The secreted enzyme EndoS, an endoglycosidase from Streptococcus pyogenes, hydrolyzes the N-linked glycan of the constant region of immunoglobulin G (IgG) heavy chain and renders the antibody unable to interact with Fc receptors and elicit effector functions. In this study we couple targeted allelic replacement mutagenesis and heterologous expression to elucidate the contribution of EndoS to group A Streptococcus (GAS) phagocyte resistance and pathogenicity in vitro and in vivo.
Results
Knocking out the EndoS gene in GAS M1T1 background revealed no significant differences in bacterial survival in immune cell killing assays or in a systemic mouse model of infection. However, exogenous addition and heterologous expression of EndoS was found to increase GAS resistance to killing by neutrophils and monocytes in vitro. Additionally, heterologous expression of EndoS in M49 GAS increased mouse virulence in vivo.
Conclusions
We conclude that in a highly virulent M1T1 background, EndoS has no significant impact on GAS phagocyte resistance and pathogenicity. However, local accumulation or high levels of expression of EndoS in certain GAS strains may contribute to virulence.
Background
Group A Streptococcus (GAS, S. pyogenes) is a human-specific pathogen producing diseases ranging from pharyngitis and impetigo to severe, invasive conditions such as necrotizing fasciitis and streptococcal toxic shock syndrome [1]. Causing an estimated 500,000 deaths annually [2], GAS is one of the world's most important pathogens, reflecting its wide repertoire of virulence factors that interfere with host immune clearance mechanisms [3]. A hypothesized GAS immune evasion factor is the secreted enzyme EndoS, an endoglycosidase possessing a highly specific hydrolyzing activity toward the N-linked glycan of immunoglobulin G (IgG) [4]. The IgG heavy chain is N-glycosylated at asparagine 297 with a complex biantennary oligosaccharide that is crucial for the interaction with Fc gamma receptors (FcγRs) on phagocytic cells [5-7]. Experimentally, enzymatic deglycosylation of murine IgG can decrease complement activation, binding of IgG to FcγRs on macrophages, and antibody-mediated cytotoxicity [5].
EndoS is specific to native IgG, which is in contrast to many related endoglycosidases that requires denaturation of their glycoprotein substrates [8,9]. Furthermore, pretreatment of IgG with recombinant EndoS diminishes its ability to opsonize bacteria and interact with FcγRs on leukocytes [10,11]. The activity of EndoS on IgG heavy chain glycans is well characterized and conserved among GAS serotypes [12]. However, a potential role of endogenous EndoS expression by the GAS bacterium in phagocyte resistance and virulence has not been elucidated. We hypothesize that EndoS contributes to GAS virulence by hydrolyzing the N-linked glycan on IgG and thereby impairing antibody mediated functions in the immune system. Here we couple targeted allelic replacement mutagenesis and heterologous gene expression to study EndoS activity during bacterial-host cell interaction in vitro and in vivo.
Results
Generation of EndoS mutants and heterologous expression
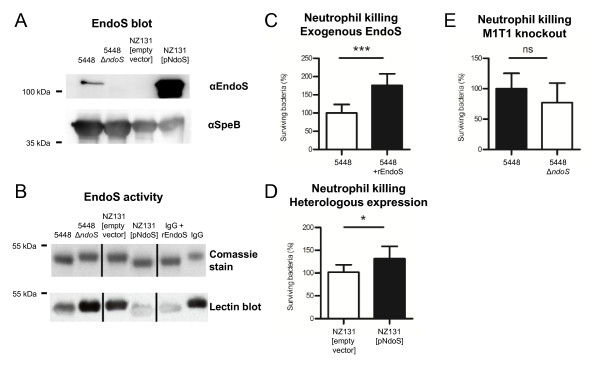
To investigate the contribution of EndoS to GAS and host-cell interactions an allelic replacement knockout in the M1T1 background was constructed and denoted 5448 ΔndoS. Heterologous expression of EndoS in a non-native EndoS producing GAS strain, NZ131 (serotype M49), was established by transformation of the EndoS expressing plasmid pNdoS. Loss- and gain-of-function was confirmed by Western immunoblot (Figure 1A) and IgG glycan hydrolysis assays (Figure 1B) [8]. As suspected no detectable EndoS was identified in the supernatants of the 5448ΔndoS strain, and heterologous expression of EndoS in NZ131 was successful. In addition, higher levels of EndoS were observed in the overexpressing strain NZ131 [pNdoS] compared to the wild-type M1 strain 5448.
Figure 1.
EndoS expression and activity, and neutrophil killing assays. (A) Western immunoblot showing EndoS expression in bacterial supernatants. SpeB is shown as a loading control. (B) Lectin blot analysis of murine IgG incubated with bacterial supernatants or rEndoS as a positive control. Opsonized bacterial survival in the presence of human neutrophils: (C) M1T1 GAS strain 5448 and isogenic ndoS knockout, 5448ΔndoS. (D) Exogenous treatment of plasma with rEndoS prior to opsonization of GAS. (E) Heterologous expression of EndoS in NZ131 (serotype M49). Error bars indicate standard deviation from the mean. Experiments were performed in triplicate. * indicates P < 0.05, *** indicates P < 0.001, ns indicates no significant difference.
Neutrophil killing assay
The phagocytic resistance of GAS with and without EndoS contribution was investigated in a human neutrophil killing assay with GAS strains 5448ΔndoS and wild-type 5448. Loss-of-function did not reveal significant difference in GAS resistance to phagocyte killing in the M1T1 background (Figure 1C). In the same M1T1 background, exogenous recombinant EndoS, rEndoS, or PBS was used to pretreat plasma to investigate phagocytic resistance contribution of the enzyme itself. It was found that rEndoS increases GAS survival in the presence of neutrophils and plasma containing GAS antibodies (Figure 1D). The contribution of EndoS to GAS virulence was also studied in the less virulent strain NZ131 (serotype M49) in gain-of-function analysis. The results reveal that heterologous overexpression of EndoS in M49, NZ131[pNdoS] increased GAS resistance to killing by human neutrophils (Figure 1E).
Monocyte killing assay
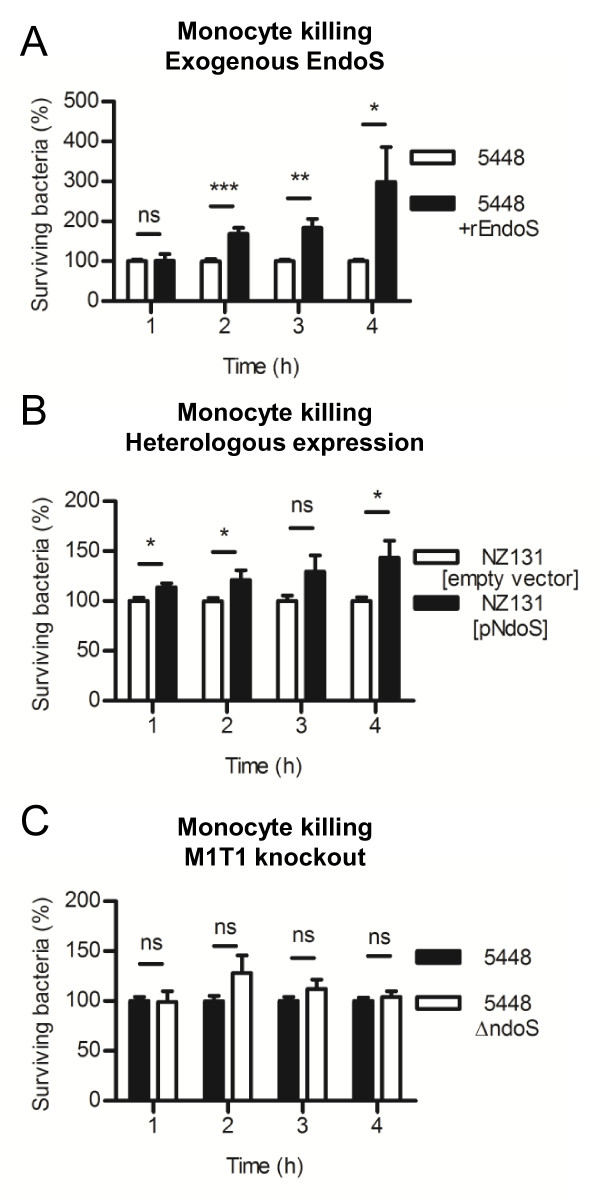
As with neutrophil killing assays, no significant difference in bacterial survival was detected in the monocytic killing assays when comparing M1T1 GAS strain 5448 to the isogenic ndoS knockout strain (Figure 2A). Pretreatment of plasma with exogenous rEndoS resulted in a significant increase in GAS resistance to killing by monocytes (Figure 2B), as did heterologous expression of EndoS in the less virulent strain NZ131 (Figure 2C).
Figure 2.
Opsonized bacterial survival in U937 monocytic cell killing assays. (A) M1T1 GAS strain 5448 and isogenic ndoS knockout, 5448ΔndoS. (B) Exogenous pretreatment of plasma with rEndoS prior opsonization of GAS. (C) Heterologous expression of EndoS in NZ131 (serotype M49). Error bars indicate standard deviation from the mean. * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001, ns indicates no significant difference.
In vivo mouse model
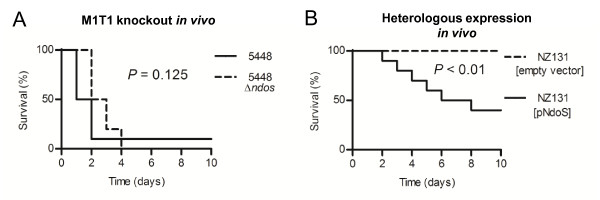
Many major GAS virulence factors have been shown to decrease overall virulence when knocked out and studied in murine infection models [13-16]. It has also been shown that EndoS has activity on all subclasses of murine IgG [17]. Taken together, this led us to believe that the contribution of EndoS to GAS virulence could be studied in vivo. However, in this murine model of infection GAS strain 5448ΔndoS showed no significant difference in virulence compared to wild-type 5448 (Figure 3A).
Figure 3.
Survival curves of female CD-1 mice following intraperitoneal challenge with GAS. (A) M1T1 GAS strain 5448 and isogenic ndoS knockout, 5448ΔndoS, at 2 × 107 cfu with 5% mucin (n = 6). (B) Heterologous expression of EndoS in NZ131 (serotype M49) at 5 × 108 cfu with 5% mucin (n = 10).
However, when we studied the less virulent GAS strain NZ131 (serotype M49) overexpressing EndoS, it was found that strain NZ131[pNdoS] showed increased virulence in vivo (Figure 3B) compared to wild-type NZ131[empty vector]. This may be a function of the relatively high level of expression of EndoS in NZ131[pNdoS] compared to 5448 (Figure 1A).
Discussion
A single clone of the M1T1 serotype has disseminated globally during the last few decades to represent the leading cause of severe, invasive GAS infections [18]. The unique virulence of the M1T1 clone has been associated with many factors including the phage-encoded DNAse Sda1, allowing escape from neutrophil extracellular traps [13,19,20], the streptococcal inhibitor of complement (SIC) protein, promoting serum and antimicrobial peptide resistance [14,21], pro-inflammatory and phagocyte resistance properties of the M1T1 protein [15,22], high level expression of the pore-forming cytotoxin streptolysin O (SLO) [16], and a propensity for genetic mutations in the covR/S regulatory locus promoting hypervirulence [23,24]. There exist many inherent limitations of modeling a secreted bacterial virulence factor in vitro and of the mouse as a surrogate host for GAS infection studies. However, our studies do strongly suggest that the endogenous expression of EndoS may be redundant or dispensable for M1T1 GAS phagocyte resistance and pathogenicity, since targeted mutation of the other factors described above do yield clear attenuation of virulence phenotypes in similar in vitro and in vivo assay systems.
Conversely, pretreatment of plasma containing antibodies against GAS with recombinant EndoS reduced opsonphagocytic killing of GAS, and heterologous overexpression of EndoS in a less virulent M49 GAS strain conferred increased phagocyte resistance and increased lethality in the mouse infection model. These results suggest that high level expression or local accumulation of EndoS in tissues could contribute to virulence in certain GAS strain backgrounds or infection scenarios, a subject that could merit future analysis in larger clinical or molecular epidemiologic surveys.
EndoS is highly conserved among GAS serotypes and can also be found in Streptococcus equi and zooepidemicus [12]. Therefore, it was somewhat surprising that we could not detect a significant contribution to GAS virulence in vivo. This may be due to the limitations of the mouse model used, and the expression levels of EndoS during the murine infection. The expression level of this enzyme during a human infection could have an impact on GAS immune cell killing resistance but this remains to be investigated. The specificity of EndoS activity towards IgG suggests that the enzyme may have an important role in the pathogenesis of GAS, yet to be discovered.
Finally, whether or not GAS can effectively deploy this unique enzymatic activity targeted IgG N-glycosylation to promote its own survival in the host (as is intuitively appealing), the enzyme itself has already proven a promising lead biotherapeutic for treatment of antibody-mediated inflammatory pathologies [17,25-29].
Conclusions
We conclude that in a highly virulent M1T1 background, EndoS has no significant impact on GAS phagocyte resistance and pathogenicity. However, our overexpression experiments could indicate that local accumulation or high levels of expression of EndoS can contribute to virulence in certain GAS strains, or in other infection scenarios than the systemic infection model used in this study.
Methods
Bacterial strains and growth
GAS strain 5448 (serotype M1T1, ndoS-positive) and GAS strain NZ131 (serotype M49, ndoS-negative) are well-characterized and were selected for use in this study [30,31]. Escherichia coli MC1061 was used as cloning tool [32]. The streptococcal and E. coli strains were propagated on Todd-Hewitt agar (THA). For selection, erythromycin (erm) was used at 5 μg/mL (5448), 2 μg/mL (NZ131) and 500 μg/mL (MC1061). GAS and its isogenic mutant were grown in Todd-Hewitt broth (THB (Difco, Detroit, MI)) at 37°C without shaking. For in vitro and in vivo experiments, fresh overnight cultures were diluted 1:10 in THB and grown to mid logarithmic phase (OD600 = 0.4) and resuspended in PBS, or in mid-log supernatants for neutrophil assays with NZ131. For analysis of streptococcal supernatants, strains were grown in C-medium (0.5% (w/v) Proteose Peptone no. 2 (Difco), 1.5% (w/v) yeast extract, 10 mM K2HPO4, 0.4 mM MgSO4, 17 mM NaCl pH 7.5) to maximize EndoS expression.
GAS mutants
EndoS is encoded by the gene ndoS. A precise, in-frame allelic replacement of ndoS with chloramphenicol transferase, cat, was created in M1T1 GAS strain 5448 by a method previously described [13] and was denoted 5448ΔndoS. Briefly, a 798 bp fragment upstream, and 987 bp fragment downstream of ndoS was amplified using polymerase chain reaction, PCR, using primers ndoS-up-F-XbaI (GCATCTAGAGCTTGTCGGTCTTGGGGTAGC), ndoS-up-R (GGTGGTATATCCAGTGATTTTTTTCTCCATTTGGACACTCCTTATTTTTGGTACTAAGT C) and ndoS-dn-F (TACTGCGATGAGTGGCAGGGCGGGGCGTAAACAAAGTAACTTTCTTAGATAGCAACATT CAG), ndoS-dn-R-BamHI (GCGGATCCGTTCTTGCGCCATGACACCTCC) respectively. The primers adjacent to ndoS contained 30 bp overhang of the cat gene corresponding to the 5' and 3' ends of cat, respectively. The upstream and downstream fragments were combined with the 650 bp cat gene in a fusion PCR using primers ndoS-up-F-XbaI and ndoS-dn-R-BamHI. This triple fragment was digested using restriction enzymes XbaI and BamHI and ligated using T4 ligase into the temperature sensitive vector pHY304, bearing erythromycin resistance, to generate the knockout plasmid pHY-ndoS-KO. pHY-ndoS-KO was transformed into GAS 5448 by electroporation and transformants were grown at the permissive temperature of 30°C with erythromycin. Transformants were then grown at the non-permissive temperature of 37°C with erythromycin present to select for homologous recombination and integration of the plasmid into the genome. Single crossovers were confirmed by PCR analysis. Relaxation of the plasmid was carried out at 30°C with no antibiotic selection to allow the plasmid to reform, outside the chromosome. Growing the bacteria at 37°C without antibiotic pressure resulted in loss of the plasmid. Finally, screening for erythromycin sensitive colonies was used to identify double crossover events and allelic replacement mutants were confirmed by PCR. In frame allelic replacement ndoS mutant, 5448ΔndoS, was confirmed by multiple PCR reactions showing the insertion of the cat gene and absence of the ndoS gene in the genome. Heterologous expression of EndoS in M49 GAS strain NZ131 was established by transformation with the EndoS expression plasmid pNdoS. ndoS was amplified from the M1 genome using primers ndoS-F-EcoRI (GCGAATTCATGGATAAACATTTGTTGGTAAAAAGAAC) and ndoS-R-BamHI (GCGGATCCTTATTTTTTTAGCAGCTGCCTTTTCTC), digested with EcoRI and BamHI prior to T4-ligation into the expression vector pDCerm, denoted pNdoS. As a control, GAS strain NZ131 was transformed with the empty vector pDCerm to generate NZ131[empty vector].
Western blot
Supernatants from stationary phase (16 h) GAS strains 5448, 5448ΔndoS, NZ131[empty vector] and NZ131[pNdoS] were precipitated with 5% final concentration of trichloroacetic acid and separated on a 10% SDS-PAGE gel and blotted onto a methanol activated PVDF membrane. The membrane was blocked in 5% skimmed milk (Difco) for 1 h and washed 3 × 10 minutes in phosphate buffered saline, PBS (137 mM NaCl, 2.7 M KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4). The membrane was then incubated with polyclonal rabbit antiserum against rEndoS at 1:2000 dilution in 0.5% skimmed milk and incubated for 1 h at 37°C. The membrane was washed as before and incubated with goat anti-rabbit IgG conjugated with Horse radish peroxidase (Bio-Rad), at 1:5,000 in 0.5% skimmed milk for 1 h at 37°C. After washing, the membrane was developed using Supersignal West Pico Chemiluminescent (Thermo Scientific, Rockford, IL) and analyzed on a Chemidoc XRS (Bio-Rad, Hercules, CA).
Lectin blot
Supernatants from GAS strains 5448, 5448ΔndoS, NZ131[empty vector] and NZ131[pNdoS] at stationary phase (16 h) was incubated with 1 μg murine IgG (mIgG) for 2 h at 37°C at static conditions. As a positive control, IgG was incubated with 1 μg rEndoS. The glycan hydrolyzing activity was analyzed with SDS-PAGE and lectin blot using biotinylated Lens culinaris agglutinin (LCA) (Vector Laboratories, Burlingame, CA). LCA lectin recognizes the α-1,3 mannose residue found on the N-linked glycan on IgG. Briefly, the supernatants and mIgG were separated on 10% SDS-PAGE gels, onestained with Coomassie blue and the other blotted onto Immobilon PVDF membranes (Millipore, Bedford, MA). The membrane was blocked in lectin buffer (10 mM HEPES, 0.15 M NaCl, 0,1% Tween 20, 0.01 mM MnCl2, 0.1 mM CaCl2, pH = 7.5) for 1 h. 10 μg LCA in lectin buffer was incubated with the membrane for 1 h at RT. The membrane was then washed for 3 × 10 min in lectin buffer and incubated with 2 μg streptavidin linked HRP (Vector Laboratories) for 1 h. After washing as above the blot was developed using Supersignal West Pico Chemiluminescent (Thermo Scientific) as described for Western blots.
Neutrophil killing assay
Neutrophils were purified from healthy donors using PolyMorphPrep-kit (Axis-Shield, Oslo, Norway) and RBCs lysed with sterile H20 as previously described [33]. Neutrophils were seeded at 2 × 105 cells/well in 96-well microtiter plates in RPMI.
Plasma was obtained from healthy volunteers as previously described [33]. All neutrophil and plasma donors exhibited high serum titer (>1:20,000) against serotype M1 and M49 GAS (Additional file 1 Table S1). GAS strains were grown as described and opsonized for 1 h at 37°C in 80% plasma, with or without pretreatment using recombinant EndoS (rEndoS) under rotating conditions. For pretreatment, 1 mL of plasma was incubated with 50 μg of rEndoS or PBS (control) at 37°C for 2 h with rotation. The bacteria were then diluted to the desired concentration in RPMI with a final concentration of 2% plasma and added to the neutrophils at a multiplicity of infection (MOI) of 10 bacteria per cell. Control wells contained GAS in RPMI and 2% plasma without neutrophils. The plate was centrifuged at 500 × g for 10 min and incubated for 30 min at 37°C with 5% CO2 before being serially diluted in sterile H2O and triplicate wells were plated on Todd-Hewitt agar (THA) plates for enumeration. Percent survival of the bacteria was calculated relative to control wells. Data from three separate experiments were normalized to 5448 or NZ131[empty vector] and combined.
Monocyte killing assay
The human monocytic cell line U937 was seeded at 5 × 105 cells/well in RPMI supplemented with 10% fetal bovine serum (FBS) in 24-well plates. GAS was grown and pre-opsonized in human plasma with or without rEndoS treatment, as described above. Bacteria were grown as described above and added to the U937 cells at MOI = 10 and incubated at 37°C with 5% CO2. Samples were collected at 1, 2, 3 and 4 h when monocytes were lysed with 0.025% Triton X-100 (MP Biomedicals, Aurora, OH) and triturated vigorously. Surviving bacteria from triplicate wells were plated on THA for enumeration. Percentage of surviving bacteria was calculated relative to the initial innoculum. Data from at least three separate experiments were normalized to 5448 or NZ131[empty vector] and combined.
Determination of donor serum titers
Blood from healthy human donors was collected in glass venous blood collection tubes with no additives (BD Biosciences, San Jose, CA) and clotted at room temperature for 15 min. Blood was centrifuged at 3,200 × g for 10 min at 4°C. The serum fraction was collected and stored at -80°C.
GAS strains NZ131 (serotype M49) and 5448 (serotype M1) were grown to mid-log phase in THB. Bacteria were resuspended in PBS and heat-killed at 95°C for 10 min. Heat-killed bacteria were mixed with a final concentration of 0.1 M NaHCO3 pH 9.6 and 106 bacteria per well were coated to 96-well high-bind ELISA plates (Costar, Cambridge, MA) at 4°C overnight. Plates were washed with PBS + 0.05% Tween (PBS-T) and blocked with 4% BSA + 10% FBS in PBS-T for 1 h at 37°C. Serum samples were diluted in blocking solution and incubated for 2 h at 37°C. Plates were washed with PBS-T and incubated with 1:5000 dilution of HRP-conjugated goat anti-human IgG antibody (Promega, Madison, WI) for 1 h at room temperature. Plates were washed five times with PBS-T and incubated with TMB substrate reagent (BD OptEIA TMB Substrate Reagent Set, BD Biosciences) at room temperature for 30 min. The reaction was stopped with an equal volume of 0.2 N sulfuric acid, and the plate was read at 450 nm. End point titer was determined as the dilution giving signal above a calculation cutoff determined using a mouse serum negative control and the calculation method described in [34].
In vivo mouse model
To evaluate the contribution of EndoS to GAS virulence in vivo, we utilized a murine model of systemic infection. GAS strains were grown as described and resuspended in PBS with 5% mucin for an inoculum of 2 × 107 cfu for WT M1T1 strain 5448 and isogenic mutant 5448ΔndoS, and 5 × 108 cfu for NZ131[empty vector] and NZ131[pNdoS]. 8-10 week old female CD-1 mice (n = 6 for 5448, n = 10 for NZ131) were infected intraperitoneally with GAS strains and mortality was monitored daily for 10 days.
Statistical analysis
Cfu enumeration in neutrophil and monocyte killing assays were statistically analyzed by unpaired Student's t-test. Differences were considered significant if P <0.05. The in vivo results were evaluated with log-rank (Mantel-Cox) test for comparison of survival curves. Differences in survival were considered significant if P < 0.05. All statistical analysis was performed using GraphPad Prism v.5 (GraphPad Software).
Ethical approval
Permission to collect human blood under informed consent was approved by the UCSD Human Research Protections Program. All animal use and procedures were approved by the UCSD Institutional Animal Care and Use Committee.
Conflicts of interests
Patents for the in vitro and in vivo use of EndoS have been applied for by Genovis AB and Hansa Medical AB, respectively. MC is listed as inventor on these applications that are pending. Hansa Medical AB in part funded this study, but had no influence on the design of study, interpretation of data, or the final form of the manuscript. MC is a part time scientific consultant for Hansa Medical AB.
Authors' contributions
JS participated in the design of the study, performed experiments and drafted the manuscript. MC and VN conceived of the study. CO performed experiments. AH designed the study and performed experiments. All authors read and approved the final manuscript.
Supplementary Material
Table S1.
Contributor Information
Jonathan Sjögren, Email: jonathan.sjogren@med.lu.se.
Cheryl YM Okumura, Email: cokumura@ucsd.edu.
Mattias Collin, Email: mattias.collin@med.lu.se.
Victor Nizet, Email: vnizet@ucsd.edu.
Andrew Hollands, Email: anhollands@ucsd.edu.
Acknowledgements and Funding
AH was supported by a Department of Employment Sciences and Technology (Australia) International Science Linkages grant to Prof. Mark Walker (U. Queensland) and VN. Additional support was provided by the Swedish Research Council (projects 2005-4791 and 2010-57X-20240 to MC), the Foundations of Crafoord (MC), Bergvall (MC), Österlund (MC), Wiberg (MC), Söderberg (MC), Kock (MC) the Swedish Society for Medicine (MC), the Royal Physiografic Society (MC), King Gustaf V's Memorial Fund (MC), and Hansa Medical AB (MC). CYMO is a San Diego IRACDA Postdoctoral Fellow supported by NIH Grant GM06852.
References
- Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13(3):470–511. doi: 10.1128/CMR.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- Kwinn LA, Nizet V. How group A Streptococcus circumvents host phagocyte defenses. Future Microbiol. 2007;2:75–84. doi: 10.2217/17460913.2.1.75. [DOI] [PubMed] [Google Scholar]
- Collin M, Olsén A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20(12):3046–3055. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci USA. 1983;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325(5):979–989. doi: 10.1016/S0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- Collin M, Olsén A. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect Immun. 2001;69(11):7187–7189. doi: 10.1128/IAI.69.11.7187-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino AL, Plummer TH Jr. Enzymatic deglycosylation of asparagine-linked glycans: purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol. 1994;230:44–57. doi: 10.1016/0076-6879(94)30006-2. [DOI] [PubMed] [Google Scholar]
- Collin M, Svensson MD, Sjöholm AG, Jensenius JC, Sjöbring U, Olsén A. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect Immun. 2002;70(12):6646–6651. doi: 10.1128/IAI.70.12.6646-6651.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allhorn M, Olin AI, Nimmerjahn F, Collin M. Human IgG/Fc gamma R interactions are modulated by streptococcal IgG glycan hydrolysis. PLoS One. 2008;3(1):e1413. doi: 10.1371/journal.pone.0001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Olsén A. Extracellular enzymes with immunomodulating activities: variations on a theme in Streptococcus pyogenes. Infection and Immunity. 2003;71(6):2983–2992. doi: 10.1128/IAI.71.6.2983-2992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16(4):396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Pence MA, Rooijakkers SH, Cogen AL, Cole JN, Hollands A, Gallo RL, Nizet V. Streptococcal inhibitor of complement promotes innate immune resistance phenotypes of invasive M1T1 group A Streptococcus. J Innate Immun. 2010. [DOI] [PMC free article] [PubMed]
- Herwald H, Cramer H, Mörgelin M, Russell W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Björck L. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell. 2004;116(3):367–379. doi: 10.1016/S0092-8674(04)00057-1. [DOI] [PubMed] [Google Scholar]
- Miyoshi-Akiyama T, Zhao J, Kikuchi K, Kato H, Suzuki R, Endoh M, Uchiyama T. Quantitative and qualitative comparison of virulence traits, including murine lethality, among different M types of group A streptococci. J Infect Dis. 2003;187(12):1876–1887. doi: 10.1086/375348. [DOI] [PubMed] [Google Scholar]
- Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci USA. 2008;105(39):15005–15009. doi: 10.1073/pnas.0808248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz RK, Kotb M. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg Infect Dis. 2008;14(10):1511–1517. doi: 10.3201/eid1410.071660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG. et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci USA. 2005;102(5):1679–1684. doi: 10.1073/pnas.0406641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, McArthur JD, Dinkla K, Aziz RK, Kansal RG. et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13(8):981–985. doi: 10.1038/nm1612. [DOI] [PubMed] [Google Scholar]
- Frick IM, Åkesson P, Rasmussen M, Schmidtchen A, Björck L. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J Biol Chem. 2003;278(19):16561–16566. doi: 10.1074/jbc.M301995200. [DOI] [PubMed] [Google Scholar]
- Påhlman LI, Mörgelin M, Eckert J, Johansson L, Russell W, Riesbeck K, Soehnlein O, Lindbom L, Norrby-Teglund A, Schumann RR. et al. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J Immunol. 2006;177(2):1221–1228. doi: 10.4049/jimmunol.177.2.1221. [DOI] [PubMed] [Google Scholar]
- Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2(1):e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maamary PG, Sanderson-Smith ML, Aziz RK, Hollands A, Cole JN, McKay FC, McArthur JD, Kirk JK, Cork AJ, Keefe RJ, Parameters governing invasive disease propensity of non-M1 serotype group A streptococci. J Innate Immun. 2010. [DOI] [PMC free article] [PubMed]
- Allhorn M, Briceno JG, Baudino L, Lood C, Olsson ML, Izui S, Collin M. The IgG-specific endoglycosidase EndoS inhibits both cellular and complement-mediated autoimmune hemolysis. Blood. 2010;115(24):5080–5088. doi: 10.1182/blood-2009-08-239020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar KS, Collin M, Olsén A, Nimmerjahn F, Blom AM, Ravetch JV, Holmdahl R. Endoglycosidase treatment abrogates IgG arthritogenicity: importance of IgG glycosylation in arthritis. Eur J Immunol. 2007;37(10):2973–2982. doi: 10.1002/eji.200737581. [DOI] [PubMed] [Google Scholar]
- Collin M, Shannon O, Björck L. IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proc Natl Acad Sci USA. 2008;105(11):4265–4270. doi: 10.1073/pnas.0711271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allhorn M, Collin M. Sugar-free antibodies - the bacterial solution to autoimmunity? Ann N Y Acad Sci. 2009;1173:664–669. doi: 10.1111/j.1749-6632.2009.04739.x. [DOI] [PubMed] [Google Scholar]
- van Timmeren MM, van der Veen BS, Stegeman CA, Petersen AH, Hellmark T, Collin M, Heeringa P. IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis. J Am Soc Nephrol. 2010;21(7):1103–1114. doi: 10.1681/ASN.2009090984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussee MS, Ajdic D, Ferretti JJ. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect Immun. 1999;67(4):1715–1722. doi: 10.1128/iai.67.4.1715-1722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansal RG, McGeer A, Low DE, Norrby-Teglund A, Kotb M. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect Immun. 2000;68(11):6362–6369. doi: 10.1128/IAI.68.11.6362-6369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban MJ, Cohen SN. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol. 2005;187(19):6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221(1-2):35–41. doi: 10.1016/S0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.