Abstract
The endoplasmic reticulum (ER) chaperone protein glucose-regulated protein 78 (GRP78/BiP) is a master regulator of ER homeostasis and stress response which are implicated in pathogenesis of metabolic disorders. By applying the LoxP-Cre strategy, we generated mice with liver specific GRP78 loss. Our studies using this novel mouse model revealed that liver GRP78 was required for neonatal survival and more than 50% loss of GRP78 in the adult liver caused ER stress response and dilation of ER compartment, accompanied by onset of apoptosis, suggesting critical involvement of GRP78 in maintaining hepatocyte ER homeostasis and viability. Further, these mice exhibited elevation of serum alanine aminotransferase and fat accumulation in the liver, and were sensitized to a variety of acute and chronic hepatic disorders by alcohol, high fat diet, drugs and toxins, which were alleviated by simultaneous administration of the molecular chaperone, 4-phenylbutyrate. Analysis by microarray followed by 2D protein profile revealed major perturbation of unfolded protein response (UPR) targets and common enzymes/factors in lipogenesis as well as new factors including liver major urinary proteins, fatty acid binding proteins, adipose differentiation-related protein, cysteine-rich with EGF-like domains 2 (Creld2), nuclear protein 1, and growth differentiation factor 15 (gdf15) possibly contributing to liver steatosis or fibrosis under ER stress. Our findings underscore the importance of GRP78 in managing the physiological client protein load and suppressing apoptosis in hepatocytes, supporting the pathologic role of ER stress in the evolution of fatty liver and disease under adverse conditions of drugs, diet, toxins and alcohol.
INTRODUCTION
The endoplasmic reticulum (ER) is an essential organelle for protein synthesis, folding and post translational modifications, biosynthesis of lipids and sterols, metabolism of drugs, and for maintenance of Ca2+ homeostasis. Molecular chaperones in the ER insure proper folding and targeting of nascent proteins. Physiological or pathological conditions stress the ER triggering adaptive unfolded protein response (UPR)1–4. The UPR signaling pathways are associated with a variety of disorders in both animal models and patients1–5.
The liver plays a central role in the homeostasis of glucose and lipids. Hepatocytes are rich in ER which is the site of synthesis of a large amount of secretory and complex lipoproteins. This high level of secretory function renders the liver particularly susceptible to ER stress. UPR plays a pivotal role in the liver in maintaining ER homeostasis under basal conditions and in adapting to fluctuations in nutrient availability. Mounting evidence indicates that ER stress plays an integral role in the pathogenesis of the most commonly encountered liver diseases1,3–5. Studies using animal models lacking or overexpressing factors involved in ER stress signaling revealed that one common feature of these diseases mediated by ER stress is impaired lipid metabolism1–5. Aberrant lipid accumulation is often an early stage leading to advanced and more irreversible liver injuries such as fibrosis and cirrhosis or even liver cancer. However, the cause versus effect linkage of ER stress to all stages of liver injury including early and advanced liver disorders remains poorly understood.
The chaperone protein GRP78/BiP is a master regulator of ER homeostasis as in addition to facilitating protein folding and assembly, it interacts with all three major UPR sensors PERK, IRE1a and ATF66. The interaction allows GRP78 to act as a repressor of activation of the UPR transducers influencing outcomes of ER stress response. Induction of GRP78 upon ER stress is a hallmark of the UPR. Over expression of GRP78 in the liver protected against insulin and ER stress-induced SREBP1c activation and hepatic steatosis in mice7. Thus, GRP78 represents an ideal target to test directly the role of ER stress in various diseases. However, global deletion of Grp78 gene is lethal as it is required for embryonic development8. In this study, we have generated mice with liver specific deletion of Grp78 using the LoxP-Cre system and demonstrate that the liver specific deletion results in ER stress response and a spectrum of spontaneous or stress-aggravated pathological consequences including fatty liver, insulin resistance, and increased susceptibility to drug, toxin, diet, alcohol induced injury and fibrosis.
METHODS
Animal breeding and experiments
Mice with liver-specific Grp78 deletion were created by the LoxP-Cre strategy (see supplemental methods). PCR genotyping with tail or liver genomic DNA was performed to distinguish Grp78 alleles of wild-type, floxed and deleted9. The presence and quantity of the Alb-Cre transgene were determined by duplex quantitative PCR using Cre-specific primers (Table S1). Male mice at the age of 4–12 weeks were selected for acute or chronic alcohol or drug administrations. Liquid diet containing 4.3% (v/v) of alcohol (#710301, Dyets, Inc., Bethlehem, PA) or isocaloric control diet were pair-fed for 6–8 weeks. For the high fat diet experiments, animals were fed modified AIN-93G with purified high fat diet (Dyets #180529) for 6 weeks. HIV protease inhibitors: ritonavir and lopinavir (10–20 mg/kg body weight (bw)) mixed with the liquid diet from Dyets containing alcohol (4.8 g/kg bw) were gavaged into the animals after fasting for 10 hr. In some experiments, 4-phenylbutyrate (PBA, 1g/kg bw) was mixed with control liquid diet and gavaged into the animals 1 hr before the treatment of alcohol and drug. Carbon tetrachloride (CCl4; 0.8 μl of CCl4/g bw) dissolved in corn oil (1:5) with or without PBA was injected (i.p.) into the animals twice per week for 6 weeks. For examination of insulin signaling, mice were fasted for 6 h after the start of the light cycle and i.p. injected with glucose (2.5 g/kg) or insulin (1.0 unit/kg). Blood glucose was determined with a Diabetes Monitoring Kit (Roche Diagnostics, IN). Insulin resistance was assessed with hometostasis model assessment of insulin resistance using the formula HOMA-IR=[fasting glucose (mg/dL) × fasting insulin (mU/L)]/40510. Littermate controls were used for all experiments and age was matched for each experiment where possible. All animals were treated in accordance with the Guide for Care and Use of Laboratory Animals approved by local committee.
Pathological parameters, molecular analysis and liver histology
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), plasma homocysteine, liver lipid extraction and analysis, primary hepatocyte isolation, extractions and analysis of RNA and whole cell or nuclear proteins, liver histology for Hematoxylin and Eosin (H&E), and Sirius Red and TUNEL staining were described previously11,12. Primers are listed in supplemental Table S1. Histological changes were confirmed by a pathologist blinded to the genotypes. Quantitation of Sirius Red staining was done with the NIH ImageJ software.
Statistical Analysis
Values are expressed as means ± s.e.m. unless otherwise indicated. Statistical analyses were performed using the Student's t test for paired if littermates in each n and for unpaired data if combined littermates in each n or ANOVA for comparison of multiple groups. P < 0.05 or less was defined as statistically significant. See supplemental methods for breeding detail, insulin detection, antibodies, immunoblotting, Phos-tag gel, proteasome activities, electron microscope, DNA microarrays, 2-D DIGE and mass spectrometry.
RESULTS
Pathological consequences of liver-specific deletion of Grp78
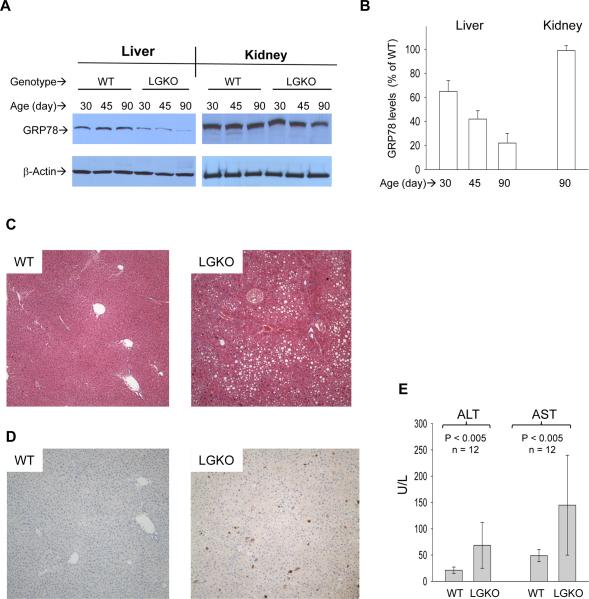
Mice with liver specific deletion of Grp78 were generated (Figure S1A&B; Grp78f/f Alb-CreTg/0 or LGKO). The liver specific deletion was detected in the genomic DNA from liver but not from kidney of LGKO (Figure S1C). In the LGKO liver, GRP78 protein was reduced by 35% to 70% at ages of 30 to 90 days compared to wild type (WT) (Figure 1A&B). GRP78 heterozygous (WK) protein was reduced by 15% to 25% compared to WT at ages of 30 to 90 days (Figure S1D). Immunohistochemistry of liver tissue using anti-GRP78 antibodies confirmed the liver GRP78 decrease (Figure S1E). Some of the remaining brown spots identified possible stroma cells in which the alb-Cre was not active. The viability of primary hepatocytes from LGKO was 68%, whereas the viability from WT or WK was greater than 90%. Interestingly, complete loss of GRP78 in the primary hepatocytes of LGKO was not detected and the GRP78 protein level in the LGKO hepatocytes was 27% of WT whereas the GRP78 protein levels from WK were 74% of WT (Figure S1F).
Figure 1. Liver specific loss of GRP78/BiP and liver injury.
(A) Western blots showing liver specific loss of GRP78 protein. WT, wild type littermates; LGKO, carrying the homozygous Grp78 floxed alleles and half copies of the cre transgene. (B) Graph depicts % of GRP78 of LGKO compared to that of WT. (C) H&E staining (×100). Showing fat accumulation in LGKO. (D) Hepatic apoptosis (brown spots) revealed by immunohistochemistry (100×) with anti-active caspase 3 antibodies. (E) Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Values are mean ± sd.
The LGKO mice are viable and smaller than WT or WK but otherwise appear grossly normal (Figures S1B and S2A&B). However, under unchallenged conditions, mild to moderate fatty liver, apoptosis and necroinflammation were observed in 12 of 21 LGKO mice killed at 90 days (Figures 1CD & S2C). Serum ALT and AST in LGKO were significantly elevated (Figure 1E). In electromicrographs, ER in the LGKO hepatocytes was dilated, vesiculated and accompanied by lipid inclusions (Figure S2D), indicating ER damage as a consequence of Grp78 deletion.
New borne pups containing homozygous Grp78 floxed alleles and full copies of the Cre transgene (tLGKO) usually died within one week after birth. Liver GRP78 protein levels in tLGKO (32% of WT) were lower than those in LGKO (83% of WT) whereas Cre levels were higher in tLGKO (38% of Cre transgenic adult) than in LGKO (18% of Cre transgenic adult) (Figure S3). Severe hepatic inflammation and massive cell death (as high as 30% of hepatocytes) were observed in tLGKO (Figure S3).
Molecular validation of hepatic ER stress response in LGKO
Analysis with cDNA microarrays revealed that expression of 450 out of 18,833 transcripts was altered in the LGKO liver. Molecular chaperones including GRP94, ORP15, PDI, p58IPK, ERdj5 and calreticulin; ubiquitin and protein degradation factors including Usp 4 and 18, Herp1, Ube3b, EDEM2 and derlin3 (derl3); transcription factors regulating apoptosis including Nupr1, CHOP, Trib3, Gadd45 and FoxO; some NFκB targeted genes including IL-6 receptor α, C1q (TNF related protein 1) and TNFR1 were among the genes with increased expression whereas Biklk and the CREBH targeted gene hepcidin 2 were decreased in LGKO (Table S2).
Proteomic 2D-DIGE analysis identified alterations of 35 out of 2350 protein spots in the liver of LGKO (Figure S3F). The proteins with altered expression include GRP94, ORP150, all isoforms of PDI, catalase, GSTμ1 and GSTπ1, UCCR, cytochrome b-5, glyoxylase 1, MUPs; ADRP, FPP and L-FABP (Table S3), confirming the constitutive UPR and impaired energy metabolism at protein levels in LGKO.
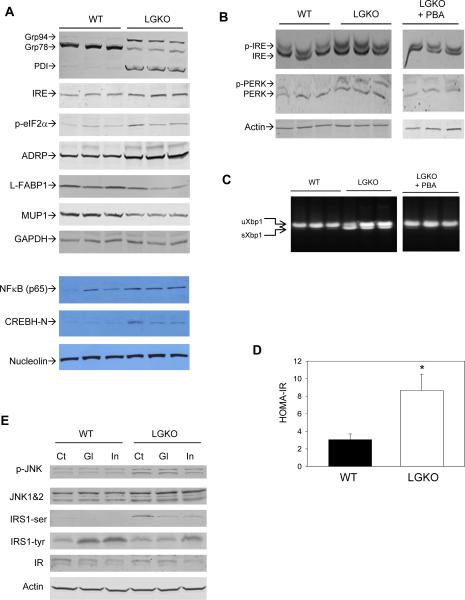
Using immunoblotting, we detected the altered expression of GRP94, PDI, IRE, p-eIF2α, ADRP, L-FABP1 and MUP1, and slight activations of NFκB and CREBH in the LGKO liver (Figure 2A). Phosphorylation of IRE and PERK as well as unconventional splicing of Xbp1 (sXbp1) were also detected, which were reduced by administration of 4-phenylbutyrate (PBA) (Figure 2BC).
Figure 2. Unfolded protein response and insulin resistance in LGKO.
(A) Western blots of whole cell or nuclear proteins. (B) Western blots of Phos-tag gel detecting phosphorylation. PBA, LGKO mice received i. p. injection of 4-phenylbutyrate (PBA) after weaning. Each lane represents an individual mouse. (C) Unconventional splicing of Xbp1 mRNA by RT-PCR assay. uXbp1, unspliced Xbp1; sXbp1, spliced Xbp1. (D) Comparison of HOMA-IR index in WT versus LGKO. *P< 0.05, n= 6. (E) Immunoblots of mouse liver proteins. Ct, injected with PBS; Gl, injected with glucose; In, injected with insulin; IR, insulin receptor; IRS1-tyr, phospho-tyrosine of IRS1; IRS1-ser, phosphoserine of IRS1.
Insulin resistance in LGKO
The HOMA-IR index in LGKO was increased by more than two fold compared to WT (Figure 2D). Immunoblotting of selective proteins involved in insulin signaling indicated that protein levels of phospho-JNK 1 & 2 and phospho-Ser 307 of IRS1 were increased in LGKO compared to WT in the presence or absence of glucose or insulin injection (Figure 2E). In contrast, levels of phospho-Tyr 989 of IRS1 were reduced in the liver of LGKO compared to WT after injection of glucose or insulin. Insulin receptor (IR) protein was not changed in either genotype. These results indicate that the liver specific loss of GRP78 impaired insulin signaling.
Loss of Grp78 exacerbates alcohol- or high fat diet-induced fatty liver injury
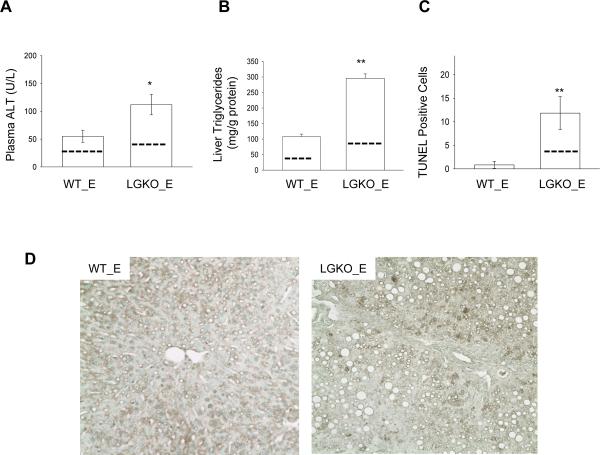
Chronic intragastric alcohol infusion is associated with hyperhomocysteinemia, ER stress response and fatty liver injury4, 5,11. To test directly whether ER stress response contributed to alcohol-induced liver injury, we fed orally a liquid alcohol diet to LGKO and WT. No significant changes of plasma homocysteine between pair-fed and alcohol-fed LGKO and WT were detected at a reduced alcohol dose for 45 days (data not shown). The ALT and liver triglycerides for pair-fed WT were 19.3±2.1 (U/L) and 43±4.6 (mg/g protein) respectively and for pair-fed LGKO were 37.9±3.4 (U/L) and 97.5±8.2 (mg/g protein) respectively. In response to alcohol feeding, the ALT level in LGKO was increased by 100% compared to WT (Figure 3A–D); Triglyceride in the liver of LGKO was increased by 3 fold compared to WT; Cell death revealed by TUNEL positive hepatocytes was significantly increased in LGKO fed alcohol whereas cell death was not increased in WT fed alcohol. These data suggest that loss of GRP78 increases the susceptibility to alcohol-induced fatty liver and injury.
Figure 3. Liver specific deletion of GRP78 exacerbates alcohol-induced ER stress and fatty liver injury.
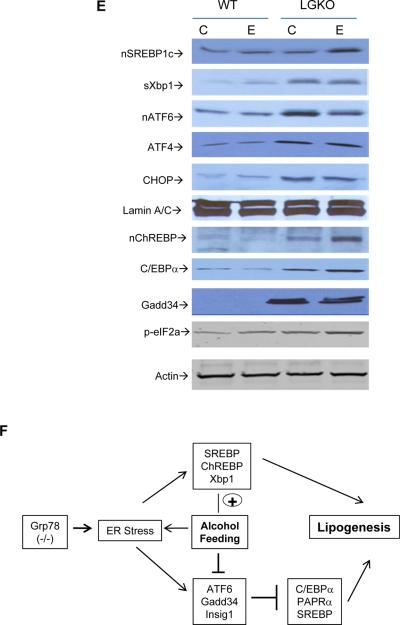
Comparisons of serum ALT (A), liver triglycerides (B) and cell death (C) between WT and LGKO fed ethanol (WT_E and LGKO_E). Dash lines inside the bars represent levels of pair-fed control. Animals in this group of experiments were 4–6 week old. *p<0.05 and **p<0.01 compared to WT, n=6. (D) TUNEL-stained liver section (×200). (E) Western blots of nuclear protein extracts for activated nSREBP1c and nChREBP, sXbp-1, activated ATF6; C/EBPα using Lamin A/C as loading control, and of whole protein extracts for phosphorylated eIF2α (p-eIF2a) using β-actin as loading control; (F) Diagram depicts impact of Grp78 deletion and ER stress factors on alcohol-induced lipogenesis.
Multiple transcription and lipogenic factors potentially involved in ER stress-induced lipogenesis were examined, including SREBP1c, ChREBP, Xbp-1, ATF6, Gadd34, ATF4, CEBPα, CHOP, Insig1 & 2, CYP2E1, PPARα and γ, Glucokinase, SCD1 and FAS (Figures 3E & S4A). Only slight changes of the transcription factors and phosphorylation of eIF2α were detected in the WT fed the low dose alcohol, which was consistent with the mild fat accumulation observed above. However, in the LGKO liver, most of these factors were altered. Alcohol feeding had either enhancing or decreasing effects on the ER stress factors tested (Figures 3E & S4). In LGKO, alcohol further increased expression of nuclear SREBP1c, ChREBP and C/EBPα and increased mRNA of Insig 2. Alcohol decreased ATF6, Gadd34 and Insig1 and ATF6 transcriptional target ERp57 and Derl3. Alcohol had no further effects on sXbp1, CHOP or ATF4 which were already increased after GRP78 deletion. Alcohol had no effects on PPARα. CYP2E1 was increased in response to alcohol, but GRP78 deletion had no apparent effect on CYP2E1 expression. SCD1, FAS and PPARγ were increased by alcohol or GRP78 deletion alone and increased further in LGKO fed alcohol, indicating that interplay between ER stress and alcohol feeding aggravates fat accumulation in the liver (Figure 3F).
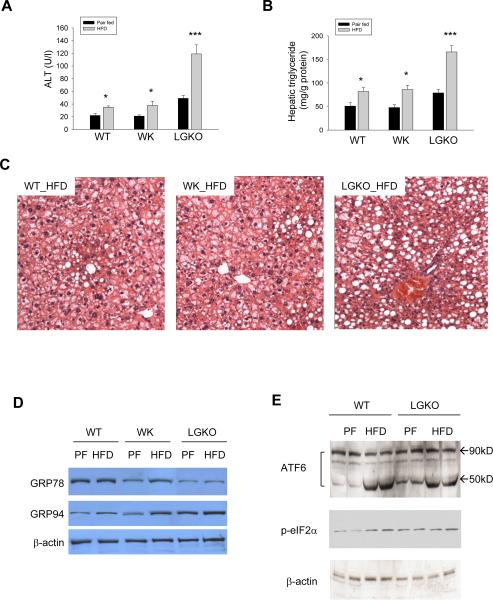
To know whether the liver Grp78 deletion worsens non-alcoholic steatosis, we fed the mice high fat diet (HFD). HFD induced moderate (1.5 fold increase in LGKO compared to pair-fed control) fatty liver injury within 6 weeks and there was no significant difference in the HFD-induced liver injury between WT and WK (Figure 4A–C). GRP78 and GRP94 were increased in WK in response to HFD which may compensate for the heterozygous loss of Grp78 in the liver (Figure 4D). Slight activation of ATF6 was detected in LGKO without HFD and apparent activation was observed in both WT and LGKO fed HFD (Figure 4E). In contrast, HFD doubled both hepatic triglycerides and ALT in LGKO, which was accompanied by greater GRP94 induction and eIF2α phosphorylation compared to WT fed HFD, indicating a severe ER stress response in LGKO fed HFD.
Figure 4. Effects of high fat diet (HFD) feeding on wild type (WT) versus mice with heterozygous (WK) or homozygous (LGKO) liver Grp78 deletion.
(A) Serum ALT; (B) Liver triglycerides; (C) Liver tissue with H&E staining showing fatty liver (×200); (D) Western blots showing liver protein levels of GRP78 and 94. (E) Western blots of liver proteins showing activation of ATF6α and phosphorylation of eIF2α; PF, pair-fed control; Black bar, pair-fed control (PF); Grey bar, HFD; *p<0.05, **p<0.01 and ***p<0.005 compared to pair-fed control, n=3.
Loss of Grp78 sensitizes to HIV PI-induced ER stress and CCl4 induced fibrosis
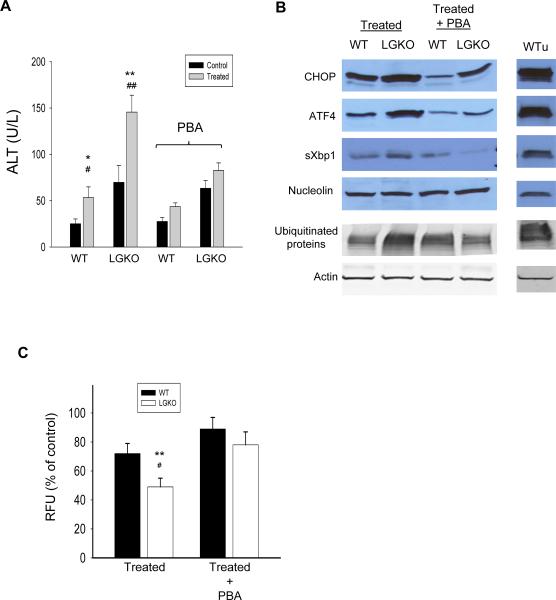
HIV-infected patients under anti-HIV therapeutics with protease inhibitors (HIV PIs) often concomitantly consume or abuse alcohol13. To address if HIV PIs alone or in combination with alcohol trigger ER stress in mouse liver, we treated mice with alcohol and/or ritonavir and lopinavir by gavage. Neither liver injury as indicated by serum ALT and AST levels or the ER stress response was detected when the animals were treated with acute alcohol alone or ritonavir/lopinavir combined for 16 hours (data not shown). However, combined treatment with alcohol and the HIV PIs caused significant increase in plasma ALT (Figure 5A) as well as activation of CHOP, ATF4 and sXbp1 in both WT and LGKO (Figure 5B), which was comparable to the response of WT injected with tunicamycin for 24 hours. In response to the combined treatment, the ALT values and ER stress response were greater in LGKO than in WT. Pre-treatment with PBA partially reduced the alcohol- and HIV PI-induced ER stress response and decreased the elevated ALT by more than 50% in both WT and LGKO. In addition, accumulation of ubiquitinated proteins was detected in LGKO but not WT treated with alcohol plus the HIV PIs. Alcohol and HIV PI reduced proteasome activity by 15% in WT and by more than 50% in LGKO. The PBA treatment restored the proteasome activities in both WT and LGKO (Figure 5C).
Figure 5. Effects of Grp78 deletion on acute alcohol and HIV protease inhibitor-induced ER stress and liver injury.
Control, animals were gavaged with isocaloric liquid diet only; Treated, animals were gavaged with liquid diet containing alcohol and HIV protease inhibitors (HIV PIs), ritonavir and lopinavir with or without PBA. (A) Comparisons of ALT in WT versus LGKO. (B) Immunoblots of ER stress related transcription factors in nuclear protein extracts and ubiquitinated proteins in whole cell protein extracts. WTu, WT treated with Tunicamycin for 24 hours as positive control of ER stress response in vivo; (C) Comparison of 20S proteasome activities between WT and LGKO. Data are presented as RFU (relative fluorescent unit) recorded with a fluorometer. *p<0.05 and **p<0.01 compared to WT; #p<0.05 or ##p<0.01 compared to WT or LGKO with PBA, n= 5 mice.
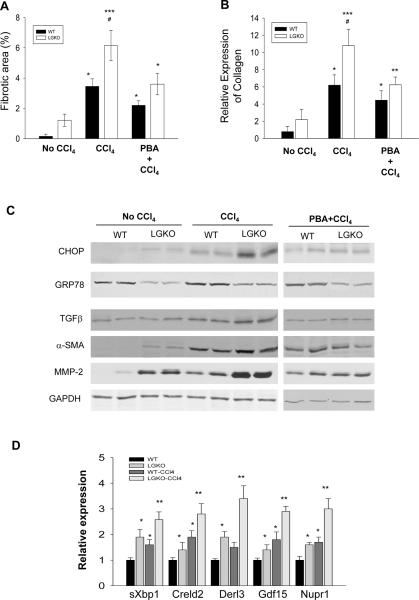
To know the effects of liver specific Grp78 deletion on progressive stages of liver injury, we examined fibrotic changes in LGKO versus WT. Spontaneous mild fibrotic changes were observed in liver tissues of 2 out of 10 LGKO stained with Sirius Red which was not detected in WT (Figures 6A & S5A). Chronic treatment with CCl4 induced fibrotic changes in both WT and LGKO. However, the fibrosis was greater in LGKO than in WT. Quantitatively, the red-stained collagen was increased 15 fold in LGKO compared to WT without CCl4 (Figure 6A). The collagen deposition was increased by 24 fold in WT mice and by 41 fold in LGKO after the chronic CCl4 compared to WT without CCl4. Type I Collagen mRNA in WT and LGKO was increased by 5 and 12.5 fold respectively in response to CCl4 (Figure 6B). There were apparent differences in expression of selective markers of fibrosis between WT and LGKO. Without CCl4, TGFβ, αSMA and MMP-2 were increased by 1.5 to 2.5 fold in LGKO compared to WT (Figure 6C). With CCl4, these markers were increased by 2 to 3.5 fold in WT with enhanced GRP78 and by 3 to 5 fold in LGKO, indicating that GRP78 deletion worsened CCl4 induced fibrosis. PBA treatment reduced CCl4-induced fibrosis by more than 50% in LGKO which was accompanied by decreased expression of Type I collagen mRNA and protein levels of CHOP, TGFβ, α-SMA and MMP-2 (Figure 6). PBA treatment in WT appeared not as effective as in LGKO in reducing CCl4-induced fibrosis, indicative of an ER stress contribution. In addition, mRNA of sXbp1 (Figures 6D & S5B), Creld2, Derl3, Gdf15 and Nup1 was increased in WT treated with CCl4 and increased more in LGKO treated with CCl4 (Figure 6D). Twenty four hours after a single injection of CCl4, both ALT and number of cell death were increased more in LGKO than in WT (Figure S5C&D), suggesting that repeated gradual hepatocellular injury lead to greater fibrosis in LGKO over the course of CCl4 administration.
Figure 6. Liver specific deletion of GRP78 exacerbates carbon tetrachloride (CCl4)-induced ER stress and liver fibrosis.
(A) Fibrotic/cirrhotic changes after treatment with CCl4. WT_C, WT injected with corn oil; WT_CCl4, WT injected with CCl4 mixed with corn oil (1:5); LGKO_C, knockout injected with corn oil; LGKO_CCl4, knockout injected with CCl4 mixed with corn oil (1:5); PBA, 4-phenylbutyrate. (B) Expression of collagen α1 analyzed with quantitative PCR. (C) Immunoblots of protein samples from mice with chronic CCl4 injection. (D) RT-PCR of mRNA of sXbp1, Derl3, Creld2, Gdf15 and Nupr1. *P<0.05 or **P<0.01 compared to WT without CCl4; #P<0.05 compared to PBA treatment; n=4.
DISCUSSION
By deleting GRP78 specifically in the mouse liver, we observed liver injury as indicated by elevated serum ALT. The LGKO mice with liver specific deletion of Grp78 developed ER dilatation, hepatic apoptosis, necroinflammation, fatty liver, insulin resistance and mild fibrosis. At molecular level, consistent with the literature on the predominant role of GRP78 in UPR, the loss of GRP78 activated the three branches of UPR as indicated by increased phosphorylation of IRE1α, PERK, eIF2, JNK and serine of IRS, altered expression of GRP94, ORP15, PDI, CHOP, ATF4, Trib3, Gadd34, FoxO, IL-6Rα, C1q, TNFR1, and hepcidin 2 involved in UPR or ER stress response. The loss of GRP78 also affects the ubiquitin pathway and protein degradation as alterations of Usp 4 and 18, Ube3b, EDEM2 and derl3 were detected. Therefore, the pathogenic mechanisms upon the GRP78 loss can be hepatic cell death mediated by CHOP and JNK; oxidative stress resulting from altered expression of catalase and GSTμ1 and GSTπ1; inflammation resulting from NFκB and CREBH activations, impaired insulin signaling by abnormal phosphorylation of IRS1; impaired energy metabolism mediated by UCCR, cytochrome b-5, and glyoxylase 1. The exact contribution of each of these pathways is not certain at this moment. The cell death as a consequence of GRP78 deletion may or may not be dependent on the ER stress-induced lipogenesis as the early sequence of the two events has been difficult to determine in vivo. However, it is likely that there is interplay between lipogenesis and cell death as the stress continues. In addition, the broad impact of GRP78 deletion on UPR and ER stress signaling pathways without any pharmacological ER stress challenge confirms that the liver is sensitive to ER stress which accompanies and contributes to most forms of liver injury and adequate GRP78 may be essential in maintaining ER homeostasis and cell health in the liver.
Global deletion of Grp78 was embryonic lethal8. However, mice with heterozygous Grp78 deficiency (Grp78W/−) survived suggesting that at least 50% of GRP78 protein is required for the early development of animals. Is GRP78 required for the liver development and normal function in adult liver? In embryos, Grp78 expression starts at time of E3 and hepatoblasts form at E8.5 when the hepatocyte-specific albumin is expressing14. In our LoxP-Cre system, the cre gene driven by the albumin promoter should express at E8.5. Assuming that Cre is fully functional and considering that the turnover time for GRP78 is three days15, more than 90% loss of GRP78 protein should occur around the time of delivery (i.e. E21). Live pups would not be delivered if at least 50% of GRP78 protein is required for survival. However, we obtained live animals with incomplete deletion of GRP78 due to variable efficiency of Cre function which has been reported16. The LGKO mice gradually lost GRP78 protein after birth and the pathological consequences were observed when greater than 50% GRP78 protein was lost. GRP78 protein levels in the liver of tLGKO (Grp78f/f Alb-CreTg/Tg) were less than 30% of WT at birth, which resulted in massive hepatic cell death and neonatal lethality. In addition, during the course of this study, a few LGKO mice died of hypoglycemia between 4–8 months after birth. Increased death rate of LGKO was observed starting 12 months after birth when the GRP78 levels in the dying LGKO were usually less than 30% of the WT littermates, suggesting that the GRP78 reduction may shorten life span. Thus at least 30% of GRP78 protein is required for liver development and greater than 50% is required for normal function of adult liver assuming that possible adverse effects by a continuous accumulation of the Cre protein are minimal. In respect to the incomplete deletion of GRP78, it is possible that Grp78 is essential for the viability of hepatocytes as is the case for HeLa cells17, forcing the outgrowth of WT and Grp78 heterozygous cells which could account for portion of GRP78 protein in total hepatocytes, rather than homogeneous reduction of Grp78 in all hepatocytes in the adult liver. It is also likely that the LGKO hepatocytes were sensitized upon substantial reduction of GRP78 causing the pathological changes without complete loss of GRP78. Nevertheless, we were able to generate viable mouse lines (LGKO) with reduced liver GRP78 expression, therefore allowing us to utilize these mice for phenotypic analysis.
Over-expression of GRP78 inhibited steatosis in the liver of obese (ob/ob) mice7. GRP78 deletion led to liver fat accumulation in this study. How ER stress regulates lipid metabolism is not fully understood. Emerging evidence indicates that each of the three branches of the UPR signaling pathways has direct molecular effects on lipid synthesis1–5. Although previous studies collectively revealed crucial roles of the UPR pathways in lipogenesis, no single animal model of ER stress led to spontaneous fatty liver under physiological conditions. The fat accumulation in our LGKO model, which is similar to situations in human NASH/NALFD, is likely to be linked to multiple mechanisms. In addition to the commonly recognized factors such as sXBP-1, active SREBPs, ATF6, Gadd34, C/EBPα, and PPARα, we found that the altered expression of MUP1, L-FABP and ADRP might also contribute to ER stress-induced steatosis in LGKO. Noticeably, direct linking of MUP, FABP, or ADRP to ER stress-caused steatosis has not been observed in other knockout mouse models of UPR. FABP and ADRP are known factors involved in lipid transport and lipogenesis18,19. MUP are secreted by the liver and excreted into the urine and recent evidence indicates that circulating MUP serve as a metabolic signal to regulate glucose and lipid metabolism20. Therefore, role of these new factors in ER stress-induced steatosis warrants further investigation.
Previous work from us and others has suggested that alcohol-induced ER stress involves increased homocysteine leading to increased SAH in the liver5,11. In the present studies, no increase in homocysteine was detected with low level oral alcohol feeding so that the enhanced ER stress and liver injury in alcohol fed LGKO probably represents the unmasking of a distinct mechanism by which alcohol induces ER stress, one that normally is largely obscured by compensatory changes which are suppressed in LGKO. Furthermore, we observed enhanced ER stress response and severe fatty liver in LGKO fed low alcohol orally in contrast to minimal effects in WT fed alcohol orally. With respect to role of ER stress in alcohol-induced liver injury, our observations imply that alcohol feeding not only enhanced ER stress but also affected ER stress signaling pathways in the LGKO. Alcohol enhanced SREBP and sXbp1 but decreased Insig 1 and ATF6 which was supported by downstream reduction of ERp57 and Derl3 and Gadd34 which appeared independent of CHOP, all of which may contribute to and/or aggravate lipid accumulation in the liver (Figure 3F). As to the question of the differential activation of Ire1α, PERK and ATF6α, we speculate that alcohol metabolites such as acetaldehyde might form adduct differentially with the ER sensors or that unknown epigenetic changes as a consequence of alcohol alter the response by the sensors.
The liver specific deletion of GRP78 also led to sensitization to liver injury by drugs such as HIV protease inhibitors (HIV PIs). HIV PIs are used in the highly active antiretroviral therapy (HAART). However, chronic HIV PIs are associated with HIV PI-induced ER stress and injury21. Considering that a significant proportion of HIV infected patients consume or even abuse alcohol, we tested effects by alcohol combined with HIV PIs on liver injury. The combination induced much severe ER stress and injury in LGKO compared to WT. PBA ameliorated both the ER stress and liver injury, confirming a direct involvement of GRP78 and ER stress in the potentiated injurious effects by alcohol and HIV PIs.
There has been no direct evidence linking ER stress to liver fibrosis/cirrhosis although cirrhotic livers exhibited partial UPR activation in the basal state and full UPR after LPS challenge22. We observed some increase in fibrosis in LGKO under basal conditions accompanied by the increased sXbp1 and CHOP which was enhanced upon CCl4 challenge. Thus, severe fibrosis developed in LGKO but not in WT with GRP78 enhancement. Acute administration of CCl4 resulted in greater increase in serum ALT and liver necrosis in LGKO than in WT, indicating that continuous augmented injury in LGKO with chronic CCl4 challenge promoted the fibrotic changes. The accelerated fibrotic changes in LGKO treated with CCl4 were associated with altered expression of CHOP and Nupr1 which are stress response factors23; Creld2 and Derl3 which are emerging mediators in the protein quality control in the ER and in regulating the onset and progression of various ER stress-associated diseases24,25; Gdf15 which is a protein belonging to TGFβ superfamily having a role in regulating inflammatory and apoptotic pathways in injured tissues and during disease processes26 In addition, αSMA and TGFβ were decreased by simultaneous injection of PBA. The evidence thus individually or collectively supports a mechanistic role of ER stress in promoting fibrotic/cirrhotic changes in the liver.
In conclusion, the loss of the key molecular chaperone-Grp78 directly disturbed ER homeostasis in the liver and caused or sensitized to a variety of acute and chronic hepatic disorders. These findings underscore the importance of UPR and GRP78 in dealing with the physiologic client protein load and viability in hepatocytes and the potential pathologic role of ER stress in the evolution of drug, toxin, and alcoholic and non-alcoholic fatty liver diseases. The LGKO mouse represents a model of impaired ER defense which unmasks an important role for ER stress in these causes of liver disease.
Supplementary Material
ACKNOWLEDGEMENTS
We thank USC Research Center for Liver Disease Cell and Tissue Imaging and Cell Culture Core and Proteomics Core and Doheny Eye Institute Specialized Microscopy for technical services and Ms. Miao Wang for helpful assistance in genotyping the Grp78 floxed mice. CJ is the principal investigator of the studies. MYL and EK are graduate student and research associate in Ji's lab. This work is supported mainly by US NIH grants R01AA018846 (CJ) and R01AA018612 (CJ), and in part by P50AA11999, P30DK48522, R01CA27607 (ASL) and R01AA014428 (NK & CJ).
Abbreviations
- ADRP
adipose differentiation-related protein
- ATF
activating transcription factor
- Biklk
Bcl2-related protein family gene
- C1q
C1q and tumor necrosis factor related protein 1
- CREBH
cyclic-AMP-responsive element-binding protein H
- Creld2
cysteine-rich with EGF-like domains 2
- ChREBP
activated carbohydrate response element-binding protein
- Derl3
derlin3
- EDEM2
mannosidase-like protein1
- FAS
fatty acid synthase
- FPP
farnesyl diphosphate synthase
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- gdf15
growth differentiation factor 15
- GST
glutathione S-transferase mu 1
- Herp1
ubiquitin-like domain member 1
- IRE
inositol-requiring kinase 1
- IL-6Ra
interleukin 6 receptor α
- IRS1
insulin receptor substrate-1
- L-FABP
liver fatty acid binding protein
- MMP-2
matrix metalloproteinase-2
- MUP
liver major urinary protein
- Nupr1
nuclear protein 1
- ORP15
oxygen-regulated protein 150
- PDI
protein disulfide isomerase
- PERK
PKR-like ER-localized eIF2a kinase
- SCD1
steroyl-coenzyme A desaturase 1
- SMA
α-smooth muscle actin
- TGFβ
transforming growth factor β
- Ube3b
ubiquitin protein ligase E3B
- UCCR
ubiquinol cytochrome C reductase
- UPR
unfolded protein response
- Usp4 and 18
ubiquitin specific peptidase 4 and 18
REFERENCES
- 1.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 3.Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132(1):24–6. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23(Suppl 1):S16–24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji C, Kaplowitz N. ER stress signaling in hepatic injury. In: Jean Francois Dufour MD, Pierre-Alain Clavien MD, editors. Signaling Pathways in Liver Diseases. 2nd edition Springer-Verlag GmbH & Co.; Heidelberg: 2010. pp. 287–304. [Google Scholar]
- 6.Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2010;23:1–7. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kammoun HL, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119(5):1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26(15):5688–97. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, et al. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci U S A. 2008;105(49):19444–9. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting serum glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 12.Shinohara M, Ji C, Kaplowitz N. Differences in betaine-homocysteine methyltransferase expression, endoplasmic reticulum stress response, and liver injury between alcohol-fed mice and rats. Hepatology. 2010;51(3):796–805. doi: 10.1002/hep.23391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 14.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322(5907):1490–4. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gülow K, Bienert D, Haas IG. BiP is feed-back regulated by control of protein translation efficiency. J Cell Sci. 2002;115(Pt 11):2443–52. doi: 10.1242/jcs.115.11.2443. [DOI] [PubMed] [Google Scholar]
- 16.Araki K, Imaizumi T, Okuyama K, Oike Y, Yamamura K. Efficiency of recombination by Cre transient expression in embryonic stem cells: comparison of various promoters. J Biochem. 1997;122(5):977–82. doi: 10.1093/oxfordjournals.jbchem.a021860. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Lu J, Zahed M, Kita K, Suzuki N. Reduction of GRP78 expression with siRNA activates unfolded protein response leading to apoptosis in HeLa cells. Arch Biochem Biophys. 2007;468(1):1–14. doi: 10.1016/j.abb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30(4):378–90. doi: 10.1055/s-0030-1267538. [DOI] [PubMed] [Google Scholar]
- 19.Imai Y, Varela GM, Jackson MB, Graham MJ, Crooke RM, Ahima RS. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132(5):1947–54. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Jiang L, Rui L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J Biol Chem. 2009;284(17):11152–9. doi: 10.1074/jbc.M900754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou H, et al. HIV protease inhibitors activate the unfolded protein response and disrupt lipid metabolism in primary hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2006;291(6):G1071–80. doi: 10.1152/ajpgi.00182.2006. [DOI] [PubMed] [Google Scholar]
- 22.Tazi KA, Bieche I, Paradis V, Guichard C, Laurendeau I, Dargere D, et al. In vivo altered unfolded protein response and apoptosis in livers from lipopolysaccharide-challenged cirrhotic rats. J Hepatol. 2007;46(6):1075–88. doi: 10.1016/j.jhep.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Goruppi S, Iovanna JL. Stress-inducible protein p8 is involved in several physiological and pathological processes. J Biol Chem. 2010;285(3):1577–81. doi: 10.1074/jbc.R109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh-hashi K, Koga H, Ikeda S, Shimada K, Hirata Y, Kiuchi K. CRELD2 is a novel endoplasmic reticulum stress-inducible gene. Biochem Biophys Res Commun. 2009;387(3):504–10. doi: 10.1016/j.bbrc.2009.07.047. [DOI] [PubMed] [Google Scholar]
- 25.Groisman B, Shenkman M, Ron E, Lederkremer GZ. Mannose trimming is required for delivery of a glycoprotein from EDEM1 to XTP3-B and to late endoplasmic reticulum-associated degradation steps. J Biol Chem. 2011;286(2):1292–300. doi: 10.1074/jbc.M110.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, Lee SJ. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol. 2000;20(10):3742–51. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.