Abstract
Background
Concurrent sexual partnerships are believed to play an important role in HIV transmission in sub-Saharan Africa, but the contributions of concurrency to HIV and STI spread depend on the details of infectious periods and relationship patterns. To contribute to the understanding of sexual partnership patterns in this region, we estimated partnership lengths, temporal gaps between partners, and periods of overlap across partners at an STI clinic in Lilongwe, Malawi.
Methods
Participants underwent physical examinations and HIV tests, and responded to questionnaires about demographics and risk behaviors, including detailed questions about a maximum of 3 sexual partners in the previous 2 months. We calculated partnership length as the time between the first and most recent sexual contact with a partner, and gap length as the time between the most recent contact with one partner and the first contact with the next. We defined concurrent and consecutive partnerships as gap length≤0 days and gap length>0 days, respectively.
Results
The study population (n=183) had a mean partnership length of 858 days (median=176 days). Eighty-six percent reported 0 or 1 partner, 5% reported multiple consecutive partnerships, and 9% reported concurrency. Gaps between consecutive partnerships were short (mean=21 days), and overlaps across concurrent partners tended to be long (mean=246 days).
Conclusions
Multiple sexual partnerships were uncommon, and partnerships were long on average. Among those reporting multiple recent partners, both long-term concurrency and narrowly spaced consecutive partnerships could present substantial risk for efficient transmission of HIV and classical STIs.
Keywords: Transmission, concurrency, partnership length, gap length, overlap
Introduction
Transmission of sexually transmitted infections (STIs), including HIV, depends on behavioral and biological factors. When sexual partnerships are completely separated by “gaps” in time, an infected index case can transmit infection to only one person while a partnership remains intact. Furthermore, an individual's earlier partners are at no risk of acquiring infections introduced by the individual's subsequent partners. When partnerships are concurrent (overlapping in time), neither of these limitations is present, and HIV/STI transmission can be amplified along pathways of interconnected partners. However, the details of these partnership patterns are important, because the transmission event is constrained by biological rules unique to each pathogen. For example, consecutive partnerships can be effectively concurrent if an earlier partner introduces an STI and the “gap” between partnerships is shorter than the infectious period for that pathogen.1, 2 Partnership lengths are also important, as longer partnerships may present a greater number of transmission opportunities. The combination of “gap length” and partnership duration helps to determine the rate of epidemic spread.3
The role of sexual partner concurrency in explaining the severe HIV epidemic in sub-Saharan Africa has been debated vigorously.4-8 Mathematical models have suggested the potential for concurrency to magnify transmission,9-12 and some investigators have reported that concurrency is common in sub-Saharan Africa.13, 14 However, studies estimating the empirical association between concurrency and HIV have had mixed results,15-17 partly due to the considerable variation in operational definitions of concurrency.7, 8 To address some of these issues, the UNAIDS Reference Group on Estimates, Modelling, and Projections recently issued a consensus definition for concurrent sexual partnerships,18 an action that will facilitate comparison of concurrency across settings. While this standard measure is immensely useful, concurrency takes many different forms,19 and a detailed understanding of the predominant partnership patterns in a particular setting is critical for designing interventions to prevent HIV and other STIs.
In this study, we examined sexual partnership patterns in an STI clinic population in Lilongwe, Malawi. We conducted descriptive analyses of partnership durations, gaps between consecutive partners, and overlaps across concurrent partners, as well as correlates of consecutive partnerships and concurrency. Our findings identify ways in which specific contact patterns may contribute to the efficient spread of HIV and other STIs in sub-Saharan Africa.
Materials and Methods
Study Design, Setting, and Population
We performed a secondary analysis of data collected at the baseline and one-week follow-up visits in a study of acute HIV detection strategies and longitudinal HIV viral dynamics conducted at Kamuzu Central Hospital STI Clinic in Lilongwe, Malawi (2003-2004). The original study's sample size was based on the power required to identify symptoms associated with acute HIV infection and detect clinically relevant differences in viral load between acutely and chronically HIV-infected participants. This secondary analysis follows several previous papers focusing on the primary study endpoints.20-22 As described previously,20-22 study screening began with two parallel rapid HIV antibody tests, followed by HIV p24 and batched HIV RNA testing in all individuals with negative or discordant antibody results. Individuals with positive p24 results were invited to enroll in the longitudinal study. For each enrolled p24-positive patient, three p24-negative controls were screened for enrollment, along with one HIV-antibody-positive patient (matched by sex).
Data Collection
Trained clinic staff administered a face-to-face questionnaire and performed a physical examination (including genital examination) at baseline. The questionnaire included items about demographics and recent risk behaviors. One week later, a follow-up questionnaire sought the following information on up to 3 sexual partners contacted in the prior two months: partner type (spouse or co-habiting boyfriend/girlfriend; non-cohabiting boyfriend/girlfriend; transactional partner; or casual acquaintance), number of months since the first sexual contact, and number of days since the most recent sexual contact. The questionnaire did not assess whether partnerships were expected to continue beyond the time of the interview.
Data were recorded on paper forms and double-entered into electronic databases. We consulted the original paper forms to reconcile discrepancies between electronic entries.
Data Analysis
We used Stata 9.2 (StataCorp, College Station, Texas, USA) for analyses with generalized estimating equations (GEE), and SAS 9.1 (SAS Institute, Cary, North Carolina, USA) for all other analyses.
HIV Status Determination
Patients with negative or discordant antibody results and detectable HIV RNA were considered to have acute HIV infection (AHI). Patients with negative or discordant antibody tests and undetectable HIV RNA were classified as HIV-negative. Participants with positive antibody results were classified as having chronic (post-acute) HIV infection (CHI).
Probability of Selection into the Study
Because participants were not sampled at random, it was necessary to account for the participant selection process before drawing inferences about the entire clinic population. To account for selection based on sex and HIV test results (described under “Study Design, Setting, and Population” above), we calculated participants' inverse probabilities of selection as the reciprocal of: the number of enrolled participants of a given sex and HIV status, divided by the estimated number of patients of that sex and HIV status visiting the clinic during the study period. For example, thirty-seven HIV-negative women were enrolled in our study, and an estimated 2171 HIV-negative women attended the clinic in the twenty months during which the study took place. The corresponding probability of selection for an HIV-negative woman was 37/2171 = 0.017 and the corresponding inverse probability of selection was 1/0.017 = 58.8. The estimated numbers of patients attending the clinic during the study period (2171 in this example) were based on administrative data collected separately from the study protocol.
Partnership Lengths
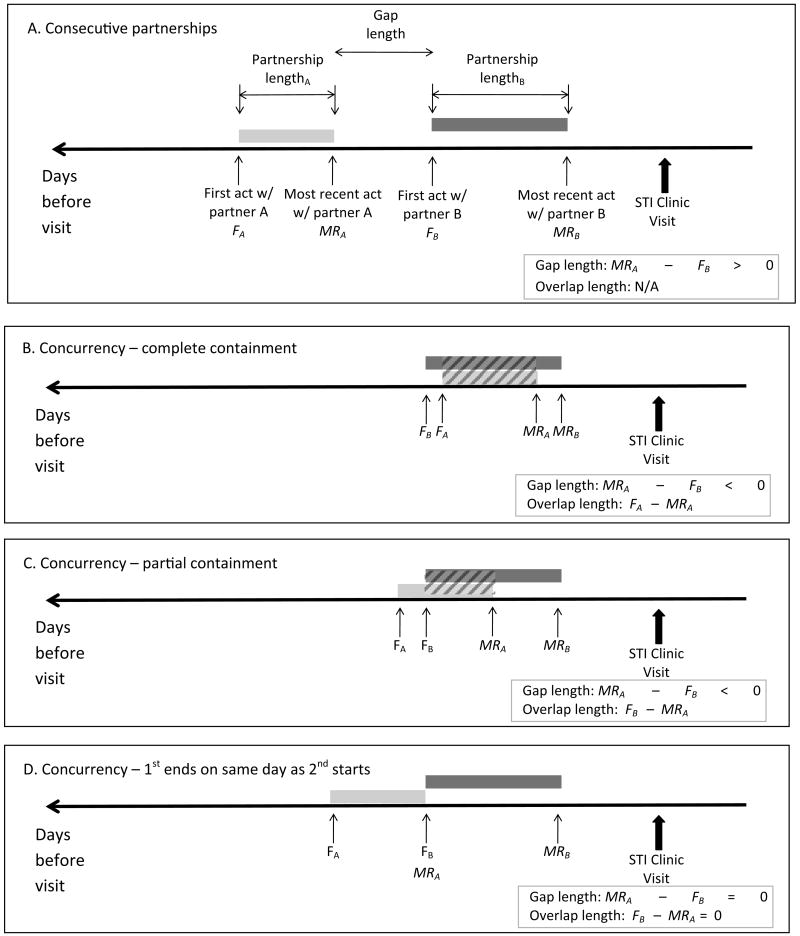
To calculate the length of each reported partnership, we first converted the time since first sex into days by multiplying the reported number of months (M) prior to the visit by 30. We then calculated the partnership length as the number of days between the first and most recent sexual contact with the partner (Figure 1A). Partnerships defined by a single contact were assigned a length of zero days.
Figure 1. Measuring partnership lengths, gap lengths, and overlap lengths.
We calculated the partnership length for each reported partnership as the time between the first sexual contact (Fpartner) and the most recent sexual contact (MRpartner) with that partner (Figure 1A). For participants reporting two partners in the prior two months, we calculated the gap length between partners A and B as MRA– FB. Because times of first and most recent sex were reported as (or converted to) the number of days before the clinic visit, earlier events have larger values. Therefore, positive gap lengths correspond to situations of consecutive partnerships (Figure 1A). Figures 1B and 1C illustrate situations of concurrency in which the most recent contact with partner A (MRA) occurred after the first contact with partner B (FB). In these situations, FB > MRA and the gap length <0. In Figure 1B, one partnership is entirely contained within another, and the overlap length (diagonally hatched bar) is equal to the partnership length of the subsumed partnership. In Figure 1C, overlap is only partial; the overlap length is equal to FB– MRA. Figure 1D illustrates a situation of concurrency in which the most recent contact with partner A (MRA) occurred on the same day as the first contact with partner B (FB). In these situations, FB = MRA; therefore, the gap length =0 = the overlap length. For participants reporting a third partner in the prior two months (partner C), the gap lengths between – and overlap lengths across – partners B and C were calculated analogously.
We estimated mean partnership lengths and corresponding 95% confidence intervals with negative binomial regression, using GEE to account for the possibility of multiple partnerships per participant. We used inverse-probability-of-selection weighting and an exchangeable working correlation matrix. This matrix type assumes constant correlation between any two partnership lengths for each individual reporting multiple partnerships, and maximizes statistical efficiency when there is no evidence for a more complex correlation structure. We estimated partnership lengths according to participants' sex, age (18-24, 25-29, 30+ years), marital/cohabitation status (unmarried and not cohabiting vs. married/cohabiting), partnership pattern in the prior 2 months (0 or 1 partner, multiple concurrent partnerships, multiple consecutive partnerships – defined below), travel in the prior 2 months (any vs. none), transactional sex in the prior 2 months (any vs. none), and baseline HIV status (negative, AHI, CHI), genital ulcer disease (GUD) status (GUD vs. no GUD), and urethral discharge status (discharge vs. no discharge, males only). We also examined partnership lengths according to participants' classifications of partner type (spouse/live-in partner, non-cohabiting boy/girlfriend, casual acquaintance, or transactional partner).
Because extreme values can influence mean partnership lengths, we calculated median partnership lengths and 25th and 75th percentiles as complementary measures of partnership length distributions, again using inverse-probability-of-selection weighting.
To compare partnership lengths across categories of participant and partnership characteristics, we used GEE to calculate partnership length differences as the weighted mean partnership length in a comparison group minus the corresponding value in a referent group, and partnership length ratios as the former divided by the latter. Given the skewed distribution of partnership lengths, we also calculated the difference in weighted median partnership lengths and conducted GEE analyses of ranked partnership lengths as complementary measures.
Gap and overlap lengths
Among those reporting contact with 2 or more partners in the prior two months, we calculated the “gap length” between each set of partners as the number of days since the most recent sexual contact with the less-recently-contacted partner minus the number of days since the first sex with the more-recently-contacted partner (Figure 1). Positive gap lengths characterized consecutive partnerships (Figure 1A). Zero or negative gap lengths characterized concurrency (Figures 1B-1D). Any partnership pattern (0 or 1 partner, multiple consecutive partners, multiple concurrent partners) could include partners of any of the following participant-reported types: spouse or cohabiting boyfriend/girlfriend, non-cohabiting boyfriend/girlfriend, transactional partner, or casual acquaintance. We therefore described partnerships with positive gaps as “consecutive” rather than “serially monogamous” to avoid any connotation that partnerships meeting this definition were necessarily stable and long-term.
Among those with negative gap lengths (concurrency), we calculated the overlap length across each set of partners in one of two ways (Figures 1B and 1C), depending on whether one partnership entirely contained another. If one partnership ended and another began on the same day, the overlap length was zero (Figure 1D).
Among those with positive gap lengths (consecutive partnerships), we calculated the mean gap length using the same GEE approach used for mean partnership lengths. We used analogous methods to calculate mean overlap lengths among those with zero or negative gap lengths (concurrency). There were too few participants reporting multiple partners to compare gap and overlap lengths across categories of participant and partnership characteristics.
Partnership Patterns
We calculated the proportion of participants in each of the following categories, based on reported behavior in the prior two months: 0 or 1 partner, multiple consecutive partners, or multiple concurrent partners. These categories were mutually exclusive, as participants reporting any concurrent partnerships were classified as having multiple concurrent partners, and participants reporting only consecutive partnerships were classified as having multiple consecutive partnerships. To assess associations of partnership patterns with participant and partnership characteristics, we conducted two rounds of weighted multinomial logistic regression: one with “0 or 1 partner” as the referent, and one with “multiple consecutive partners” as the referent. After observing that nearly all females reported 0 or 1 partner, we repeated these analyses for males only.
Results
Population demographics and HIV status
A total of 183 participants were eligible for these analyses. All results reported below take selection weights into account to adjust for the non-random sampling of participants (see Methods). The population was predominantly female and married/cohabiting (Table 1). The mean age (95% CI) was 27.0 (25.9, 28.1) years and the median age (interquartile range) was 25 (22-30) years. Participant age did not differ by sex.
Table 1. Population characteristics.
| N (weighted* %) | |
|---|---|
| Overall | 183 (100.0) |
| Sex | |
| Female | 50 (56.9) |
| Male | 133 (43.1) |
| Age | |
| 18-24 years | 86 (44.4) |
| 25-29 years | 54 (29.4) |
| 30+ years | 43 (26.2) |
| Marital/cohabitation status | |
| Married/cohabiting | 111 (70.8) |
| Unmarried and not cohabiting | 72 (29.2) |
| Travel in last 2 months | |
| No | 125 (74.7) |
| Yes | 57 (25.3) |
| Transactional sex in last 2 months | |
| No | 117 (72.2) |
| Yes | 65 (27.8) |
| HIV status | |
| HIV-negative | 130 (58.0) |
| CHI | 37 (41.7) |
| AHI | 16 (0.3) |
| Current GUD status | |
| No GUD | 123 (76.0) |
| GUD | 60 (24.0) |
| Current discharge status (males) | |
| No urethral discharge | 72 (52.7) |
| Urethral discharge | 61 (47.3) |
GUD = genital ulcer disease; CHI = chronic (post-acute) HIV infection; AHI = acute HIV infection
Percentages are weighted by participants' inverse probabilities of selection into the study (see Methods)
Partnership Lengths
Overall, the mean partnership length was 858 days and the median was 176 days (Table 2). As assessed by GEE analysis of partnership lengths in days, partnerships were statistically significantly longer among those who were female, older, married/cohabiting, or free of urethral discharge (males); or who reported just one partner, no travel, or no transactional sex in the prior two months (Table 2). Partnerships that participants classified as being with spouses/cohabiting partners were statistically significantly longer (mean partnership length=1424 days) than partnerships reported to be with non-cohabiting girlfriends/boyfriends (216 days), transactional partners (38 days), or casual acquaintances (14 days). Partnerships were longer, on average, among those who were HIV-negative or free of GUD, but these differences were not statistically significant.
Table 2. Partnership lengths among those with at least 1 partner in the prior two months, in the overall population and by participant and partnership characteristics.
| Characteristic | Patients N | Partnerships N | Weighted* median PL in days (IQR) | Weighted* mean PL in days (95% CI) | PLD in days (95% CI) | PLR (95% CI) | Difference in weighted* median PL (days) |
|---|---|---|---|---|---|---|---|
| Overall | 161 | 204 | 176 (30, 898) | 858 (630, 1169) | --- | --- | --- |
| Sex | |||||||
| Female | 42 | 46 | 646 (147, 1780) | 1126 (748, 1696) | 0. | 1. | 0. |
| Male | 119 | 158 | 48 (0, 357) | 557 (374, 827) | -569 (-1081, -59) ‡ | 0.5 (0.3, 0.9) ‡ | -598‡ |
| Age | |||||||
| 18-24 years | 75 | 94 | 152 (35, 657) | 405 (280, 584) | 0. | 1. | 0. |
| 25-29 years | 47 | 57 | 771 (16, 1780) | 1029 (706, 1500) | 624 (209, 1040) ‡ | 2.5 (1.5, 4.3) ‡ | 619 |
| 30+ years | 39 | 53 | 173 (30, 2369) | 1525 (877, 2651) | 1120 (264, 1976) ‡ | 3.8 (1.9, 7.3) ‡ | 21 |
| Marital/cohabitation status | |||||||
| Married/cohabiting | 101 | 127 | 625 (120,1673) | 1091 (797, 1493) | 0. | 1. | 0. |
| Unmarried and not cohabiting | 60 | 77 | 27 (0, 140) | 197 (78, 495) | -894(-1281,-506)‡ | 0.2 (0.1, 0.5) ‡ | -598‡ |
| Partner type | |||||||
| Spouse / live-in partner | 80† | 81 | 777 (353, 1799) | 1424 (1043, 1945) | 0. | 1. | 0. |
| Non-cohabiting girl/boyfriend | 73† | 88 | 40 (21, 174) | 216 (108, 431) | -1208 (-1686, -731) ‡ | 0.2 (0.1, 0.3) ‡ | -737‡ |
| Transactional partner | 6† | 7 | 0 (0, 0) | 38 (6, 262) | -1386 (-1837, -936) ‡ | 0.03 (0.004, 0.2) ‡ | -777‡ |
| Casual acquaintance | 23† | 26 | 0 (0, 10) | 14 (4, 49) | -1410 (-1855, -965) ‡ | 0.01 (0.003, 0.04) ‡ | -777‡ |
| Partnership pattern | |||||||
| 1 partner | 122 | 122 | 358 (113, 1673) | 1006 (720, 1407) | 0. | 1. | 0. |
| Multiple consecutive§ | 16 | 34 | 0 (0, 9) | 140 (54, 362) | -866 (-1229, -504) ‡ | 0.1 (0.05, 0.4) ‡ | -358‡ |
| Multiple concurrent§ | 19 | 44 | 159 (30, 556) | 635 (312, 1291) | -371 (-935, 191) | 0.6 (0.3, 1.4) | -199 |
| Travel in last 2 months | |||||||
| No | 106 | 133 | 419 (80, 1128) | 1033 (735, 1452) | 0. | 1. | 0. |
| Yes | 54 | 69 | 69 (11, 616) | 428 (250, 732) | -605 (-1025, -185) ‡ | 0.4 (0.2, 0.8) ‡ | -350‡ |
| Transactional sex in last 2 months | |||||||
| No | 98 | 114 | 625 (60, 1431) | 1105 (790, 1548) | 0. | 1. | 0. |
| Yes | 62 | 89 | 106 (8, 186) | 398 (198, 802) | -707 (-1172, -242) ‡ | 0.4 (0.2, 0.8) ‡ | -519‡ |
| HIV status | |||||||
| HIV-negative | 112 | 142 | 152 (21, 1012) | 935 (615, 1424) | 0. | 1. | 0. |
| CHI | 34 | 39 | 556 (120, 777) | 753 (490, 1158) | -182 (-692, 327) | 0.8 (0.4, 1.5) | 404 |
| AHI | 15 | 23 | 15 (0, 346) | 436 (202, 942) | -499 (-1016, 18) | 0.5 (0.2, 1.1) | -137 |
| Current GUD status | |||||||
| No GUD | 107 | 128 | 346 (39, 898) | 882 (614, 1267) | 0. | 1. | 0. |
| GUD | 54 | 76 | 130 (0, 712) | 786 (437, 1413) | -96 (-657, 465) | 0.9 (0.4, 1.8) | -216 |
| Current discharge status (males) | |||||||
| No urethral discharge | 61 | 84 | 117 (0, 1128) | 868 (565, 1334) | 0. | 1. | 0. |
| Urethral Discharge | 58 | 74 | 30 (0, 138) | 252 (111, 574) | -616 (-1043, -189) ‡ | 0.3 (0.1, 0.7) ‡ | -87‡ |
Partnership lengths are weighted by participants' inverse probabilities of selection into the study (see Methods).
Numbers sum to more than 161 because participants could have more than one type of partner.
Statistically significant at α=0.05. Statistical significance of PLD and PLR was assessed by GEE analysis of partnership lengths in days. Statistical significance of differences in weighted median partnership lengths was assessed by GEE analysis of partnership length ranks.
Categories were mutually exclusive, as participants reporting any concurrent partnerships were classified as having multiple concurrent partners, and participants reporting only consecutive partnerships were classified as having multiple consecutive partnerships.
CHI = chronic (post-acute) HIV infection; AHI = acute HIV infection; GUD = genital ulcer disease; PL = partnership length; PLD = partnership length difference, calculated with generalized estimating equations as the difference in weighted mean partnership lengths; PLR = partnership length ratio, calculated with generalized estimating equations as the ratio of weighted mean partnership lengths; IQR = interquartile range
For every participant and partnership characteristic, the order of mean partnership lengths across sub-groups was the same as the order of medians (Table 2), with two exceptions. While the mean partnership length increased directly with age, the median was considerably higher in the middle age group (25-29 years) than in the other groups, and partnership length ranks did not differ significantly by age. Additionally, while the mean partnership length was greatest for HIV-negative participants (935 days) and intermediate for CHI patients (753 days), the corresponding medians were reversed (152 days and 556 days, respectively). These results reflect the relatively greater presence of extremely long partnerships among older and HIV-negative patients, elevating the mean to a greater extent in these groups.
Partnership patterns, gap lengths, and overlap lengths
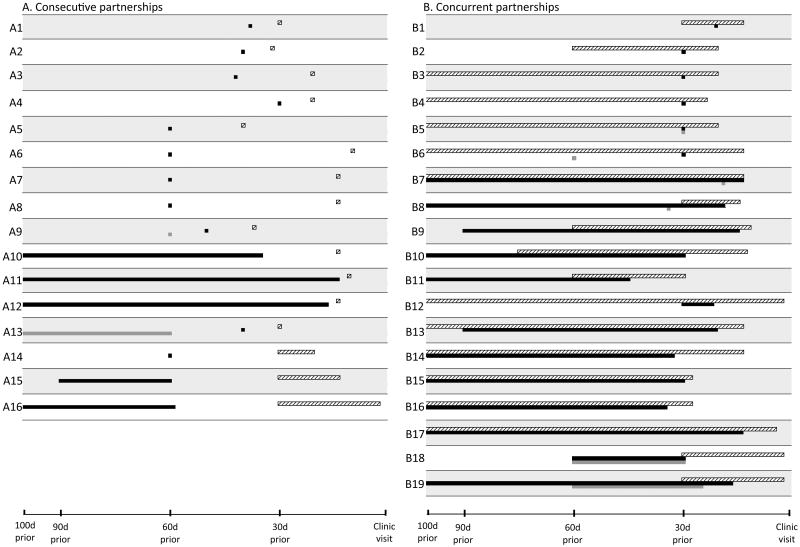
Overall, 86% of the population reported 0 or 1 partner in the previous two months: 77% reported one partner and 9% reported zero partners. Sixteen persons (weighted percent=5%) reported multiple consecutive partnerships; and nineteen (weighted percent=9%) reported multiple concurrent partnerships (Table 3). Many of the consecutive partnerships were short (Figure 2A), with 27% comprising only a single contact. Gap lengths among participants reporting consecutive partnerships were also short, with a mean (95% CI) of 21 (13, 29) days and a maximum of 50 days. By contrast, partnership lengths among those with concurrency were long (Figure 2B), with long periods of overlap (mean = 246 days). Among those reporting consecutive or concurrent partnerships, we observed four basic patterns: consecutive partnerships comprising one-off contacts only (Figure 2A rows A1-A9), consecutive partnerships with at least one partnership of duration >1 week (Figure 2A rows A10-A16), sporadic concurrency only (Figure 2B rows B1-B6), and longer-term concurrency with or without additional sporadic concurrency (Figure 2B rows B7-B19).
Table 3. Association of partnership patterns with participant characteristics, overall and among males only.
| N (weighted* %)† | Weighted* odds ratios (whole population) | Weighted* odds ratios (males only) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 or 1 partner | Multiple consecutive | Multiple concurrent | Multiple consecutive vs. 0 or 1 partner | Multiple concurrent vs. 0 or 1 partner | Multiple concurrent vs. multiple consecutive | Multiple consecutive vs. 0 or 1 partner | Multiple concurrent vs. 0 or 1 partner | Multiple concurrent vs. multiple consecutive | |
| Overall | 144 (86.5) | 16 (4.6) | 19 (8.9) | N/A | N/A | N/A | N/A | N/A | N/A |
| Sex | |||||||||
| Female | 46 (92.5) | 1 (1.5) | 2 (6.0) | 1. | 1. | 1. | N/A | N/A | N/A |
| Male | 98 (78.5) | 15 (8.8) | 17 (12.7) | 6.8 (5.1, 9.0) ‡ | 2.5 (2.1, 3.0) ‡ | 0.4 (0.3, 0.5) ‡ | N/A | N/A | N/A |
| Age | |||||||||
| 18-24 years | 70 (85.1) | 7 (3.3) | 8 (11.6) | 1. | 1. | 1. | 1. | 1. | 1. |
| 25-29 years | 44 (90.2) | 6 (7.8) | 3 (2.0) | 2.2 (1.7, 2.9) ‡ | 0.2 (0.1, 0.3) ‡ | 0.07 (0.05, 0.1) ‡ | 1.5 (1.1, 2.0) ‡ | 0.5 (0.3, 0.7) ‡ | 0.4(0.2, 0.5) ‡ |
| 30+ years | 30 (84.4) | 3 (3.4) | 8 (12.2) | 1.1 (0.8, 1.5) | 1.1 (0.9, 1.3) | 1.0 (0.7, 1.5) | 1.2 (0.9, 1.8) | 3.6 (2.8, 4.6) ‡ | 2.9 (2.0, 4.3) ‡ |
| Marital/cohabitation status | |||||||||
| Married/cohabiting | 87 (87.8) | 9 (3.3) | 12 (8.9) | 1. | 1. | 1. | 1. | 1. | 1. |
| Unmarried and not cohabiting | 57 (83.3) | 7 (7.9) | 7 (8.8) | 2.5 (2.0, 3.2) ‡ | 1.0 (0.9, 1.3) | 0.4 (0.3, 0.5) ‡ | 0.7(0.5, 0.9) ‡ | 0.5(0.4, 0.6) ‡ | 0.7 (0.5, 1.0) |
| Travel in last 2 months | |||||||||
| No | 99 (88.1) | 8 (3.6) | 14 (8.3) | 1. | 1. | 1. | 1. | 1. | 1. |
| Yes | 45 (82.7) | 7 (6.8) | 5 (10.5) | 2.0 (1.6, 2.6) ‡ | 1.4 (1.1, 1.6) ‡ | 0.7 (0.5, 0.9) ‡ | 1.3 (1.0, 1.7)‡ | 0.7 (0.5, 0.9)‡ | 0.5 (0.3, 0.7) ‡ |
| Transactional sex in last 2 months | |||||||||
| No | 101 (89.2) | 7 (3.3) | 7 (7.5) | 1. | 1. | 1. | 1. | 1. | 1. |
| Yes | 42 (78.0) | 9 (8.7) | 12 (13.3) | 3.0 (2.4, 3.8) ‡ | 2.0 (1.7, 2.4) ‡ | 0.7 (0.5, 0.9) ‡ | 2.5 (1.9, 3.3) ‡ | 1.5 (1.2, 1.9) ‡ | 0.6 (0.4, 0.9) ‡ |
| HIV status | |||||||||
| HIV-negative | 102 (85.5) | 14 (8.0) | 11 (6.5) | 1. | 1. | 1. | 1. | 1. | 1. |
| CHI | 31 (87.9) | 0 (0.0) | 5 (12.1) | N/A§ | 1.8 (1.5, 2.2) ‡ | N/A§ | N/A§ | 1.2 (1.0, 1.5) | N/A§ |
| Acute HIV | 11 (70.7) | 2 (11.7) | 3 (17.6) | 1.8 (0.5, 6.9) | 3.3 (1.0, 10.3) ‡ | 1.9 (0.4, 9.3) | 1.3 (0.3, 5.3) | 2.6 (0.8, 8.5) | 2.0 (0.4, 9.9) |
| Current GUD status | |||||||||
| No GUD | 104 (89.1) | 9 (3.4) | 9 (7.5) | 1. | 1. | 1. | 1. | 1. | 1. |
| GUD | 40 (77.9) | 7 (8.8) | 10 (13.3) | 3.0 (2.4, 3.8) ‡ | 2.0 (1.7, 2.4) ‡ | 0.7 (0.5, 0.9) ‡ | 0.8 (0.6, 1.1) | 2.3 (1.9, 2.9) ‡ | 2.9 (2.0, 4.0) ‡ |
| Current discharge status (males) | |||||||||
| No urethral discharge | 53 (80.1) | 8 (7.8) | 9 (12.1) | N/A | N/A | N/A | 1. | 1. | 1. |
| Urethral discharge | 45 (76.6) | 7 (10.0) | 8 (13.4) | N/A | N/A | N/A | 1.3 (1.0, 1.7) ‡ | 1.2 (0.9, 1.5) | 0.9 (0.6, 1.2) |
Reported values are weighted by participants' inverse probabilities of selection into the study (see Methods)
Row percents
Statistically significant at α=0.05
Estimates accounting for zero cells from weighted multinomial logistic regression are unavailable.
Note: Omitted from this table are 4 participants reporting ≥2 partners who did not have sufficient information to categorize their patterns as consecutive or concurrent.
CHI = chronic HIV infection, AHI = acute HIV infection, GUD = genital ulcer disease
Figure 2. Patterns of consecutive and concurrent partnerships among those reporting multiple partners.
Partnership patterns are shown for each of the 35 participants whose patterns in the two months prior to the STI clinic visit could be categorized as either exclusively consecutive (Figure 2A) or having one or more sets of concurrent partners (Figure 2B). For ease of presentation, the time axis is left-truncated at 100 days prior to the STI clinic visit. Each numbered row represents the partnership pattern for one participant. Within each row, the horizontal white, black, and dark gray bars represent the partners contacted most recently, second-most-recently, and third-most-recently, respectively. The four participants who reported 2 or more partners but could not be classified as having consecutive vs. concurrent partnerships (due to missing data) are excluded.
In the overall population, consecutive partnerships (vs. 0 or 1 partner) were associated with male sex, being unmarried/not cohabiting, reporting travel or transactional sex in the prior two months, and having GUD (Table 3). Participants aged 25-29 years were also more likely to report consecutive partnerships (vs. 0 or 1 partner) than younger or older participants. These same patterns held when we restricted our analyses to males, with two exceptions: males who were unmarried/not cohabiting or who had GUD were less likely to report consecutive partners (vs. 0 or 1 partner). Males with urethral discharge were more likely to report consecutive partners than 0 or 1 partner.
Most factors associated with consecutive partnerships were also associated with an increased odds of concurrency (vs. 0 or 1 partner) in the overall population (Table 3); however, the strength of the positive association was somewhat weaker (but still statistically significant) for male sex, reporting travel in the prior 2 months, reporting transactional sex in the prior 2 months, and having GUD. In other words, participants in each of these categories were less likely to report concurrent than consecutive partnerships. For those who were unmarried and not cohabiting, and for those age 25-29 years, the association with concurrency was attenuated or in the opposite direction from the corresponding association with consecutive partnerships. In contrast to the results in the overall population, males aged 30+ and males with GUD were more likely to report concurrency than consecutive partnerships.
Detailed comparisons of partnership patterns by HIV status were hindered by small numbers, but those with AHI or CHI were more likely to report concurrency than 0 or 1 partner. Those with AHI were also more likely to report consecutive partnerships (vs. 0 or 1 partner), but the measure was imprecise. In general, AHI was associated with multiple recent partnerships (odds ratio with “concurrent” and “consecutive” categories collapsed: 2.5, 95% CI = 1.0 – 6.3; result not shown in Table 3).
Discussion
We have presented a descriptive analysis of sexual partnership patterns – including durations of partnerships, gaps, and overlaps – among STI clinic patients in Lilongwe, Malawi. We found that most partnerships were long and monogamous, that concurrent partnerships were infrequent but tended to have long periods of overlap, and that consecutive partnerships had short intervening gaps that could facilitate rapid HIV/STI spread. These results highlight the variability of sexual partnership patterns, and provide detailed information about determinants of HIV/STI transmission in a semi-urban, sub-Saharan African population.
Most participants in the “high-risk” STI clinic population that we studied reported only 1 partner in the two months prior to their clinic visits. This result underscores the fact that the number of recent partners is not the only determinant of STI/HIV acquisition (as nearly all participants presented with STIs), and highlights the importance of partner characteristics (such as partners' patterns of contact with others) in determining an individual's STI risk.23 The idea that HIV/STI acquisition risk depends not only on one's own behavior, but on the behavior of one's partners (and one's partners' partners), has been recognized as an important transmission prevention message in some sub-Saharan African settings.24, 25 Our results indicate the importance of including this message in Malawian prevention campaigns.
Recent partnerships in our study were long on average, and partnership lengths differed predictably across subgroups. The mean partnership lengths fall within the range of values obtained in other African settings, where estimates have varied from 3.2 months for Tanzanian males' non-marital partners 26 to 239 months for Ugandan spouses.12 Notably, the mean overlap in concurrent partnerships was also long, as has been observed elsewhere in sub-Saharan Africa.14 We note that none of the concurrent partnerships reported in this population was polygamous, consistent with the relatively low levels of polygamy reported in population-based surveys from Malawi;27 however, the patterns of concurrency among our study participants could be similar to polygamy from a transmission standpoint. Polygamy is one type of long-term concurrency that may have a relatively benign effect on HIV/STI spread, as it can trap infection in small, isolated network components.24, 28
The patterns of consecutive partnerships observed in this study could lead to transmission amplification similar to or greater than that expected with concurrency, as the mean gap (21 days) among those reporting consecutive partnerships was shorter than the infectious period of many STIs,29 including sero-negative acute HIV infection.30 Although gap lengths among African populations have not been previously characterized, short gaps between consecutive partnerships have been observed in the US.1, 2, 31 In our study, all gaps were ≤50 days, a result that is related to constraints in our measurement methods: because the most recent contact with each partner must have occurred within the sampling window of the prior two months, each gap had to be fully contained within that time frame. Despite this limitation in estimating mean gap lengths, the occurrence of numerous consecutive relationships in rapid succession suggests considerable transmission potential.
Those who were male or unmarried, or who had traveled or had transactional sex recently, were more likely to report multiple consecutive or (to a lesser extent) concurrent partnerships (vs. having 0 or 1 partner). Elsewhere in sub-Saharan Africa, elevated levels of concurrency among these same subgroups have been observed,32, 33 although definitions of concurrency have varied. In fact, some definitions have simply required the existence of multiple partnerships in a given interval, without directly assessing whether there was any overlap.7 Many of the partnerships that we considered to be “consecutive” would have been classified as “concurrent” using this less precise definition.
Although this study was conducted in an STI clinic population, and therefore focused on individuals whose recent behavior resulted in STI/HIV acquisition, this study was not designed to determine which specific behaviors led to infection in these participants. Essentially all participants had some STI symptoms, leaving no “STI-free” group to serve as a referent for evaluating various behaviors as risk factors for participants' STI acquisition. Additionally, these data are cross-sectional, so causality between participants' behaviors and HIV/STI acquisition cannot be confirmed. Instead, the associations among partnership patterns (consecutive, concurrent) and HIV, GUD, or urethral discharge are best interpreted with respect to the risk of onward transmission from these infected individuals.34, 35 In our population, the greater odds of having multiple recent partners among those with GUD or urethral discharge suggests considerable potential for onward transmission, whether by concurrency or back-to-back consecutive partnerships. These results suggest that prevention messages should address both concurrency and rapid partner change in this setting.
HIV transmission deserves special consideration. Because the infectious period for HIV is lifelong after infection acquisition, short gaps between partnerships would seem to be of only minor importance to HIV transmission. However, the transmissibility of HIV is most highly concentrated during the first weeks or months of infection, and can also be elevated during periods of STI coinfection.36-39 Therefore, the short gaps observed in this study would certainly create great risk for onward transmission of HIV. The increased likelihood of concurrency among those with CHI, and of multiple recent partnerships in general among those with AHI, suggests substantial HIV transmission risk from these individuals.
As with all sexual behavior studies, the necessary reliance on self-reported data may have introduced recall error, which could bias results in either direction, and social desirability bias, which would likely deflate the estimated proportions engaging in multiple partnerships. Additionally, we did not have information about whether partnerships continued beyond the time of interview, a common problem in studies of partnership dynamics2, 3, 31 that can result in underestimated lengths and misclassified patterns. Despite this possibility of bias in our estimated means and medians, the partnership length differences and ratios reported in Table 2 provide unbiased comparisons between groups if certain conditions hold. Specifically, if partnership lengths were censored by the same absolute amount in each category, then the partnership length difference is a valid estimate of the absolute difference between groups. If partnership lengths were instead censored by the same relative amount, then the partnership length ratio is a valid estimate of the relative difference between groups. Furthermore, our method of sampling a fixed number of partners (3) over a fixed time period (2 months) may have led to a sample biased toward longer partnerships and/or individuals with fewer numbers of partners;40 however, any resulting bias is likely to have been small, as <5% of the population reported 3 partners. Finally, we note that our results are based on a relatively small number of participants. As a result, we were unable to conduct multivariable analyses, and the small number of participants reporting multiple partners precluded stratification of gap and overlap lengths by participant and partnership characteristics. Nevertheless, our results represent some of the first estimates of partnership, gap, and overlap lengths in sub-Saharan Africa, contributing to our limited understanding of partnership patterns in this region.
When information is available to assess whether partnerships are ongoing, Kaplan-Meier survival analysis can be used to account for censored observations in estimating partnership lengths.40 Additionally, studies can be designed to minimize the effects of sampling schemes with fixed time windows and numbers of partners.40 While our secondary analysis was constrained by the data collection methods of its parent study, future studies can produce improved estimates by carefully ascertaining whether partnerships are likely to continue, conducting Kaplan-Meier analyses to account for censoring, and considering the effects of the sampling window and the number of partners on whom information is assessed.
A lack of empirical data linking specific sexual partnership patterns to HIV transmission lies at the heart of the debate about the role of concurrency in spreading HIV. To address this issue, the UNAIDS Reference Group on Estimates, Modelling, and Projections recently issued a consensus definition of concurrency: “overlapping sexual partnerships in which sexual intercourse with one partner occurs between two acts of intercourse with another.”18 While this suggestion will facilitate comparisons of this particular measure across settings, this limited definition is unlikely to capture the rich and variable characteristics of sexual partnerships. We have described additional features of sexual partnerships, including relationship durations, gap lengths, and overlap lengths, in an STI clinic population in Lilongwe, Malawi. Additional descriptions of these characteristics are essential, as a detailed understanding of sexual behaviors in a given context is necessary for the optimal design of prevention interventions. Without improved descriptions of the predominant sexual partnership patterns in various settings, we will be limited in our understanding of HIV/STI transmission dynamics throughout the world.
Acknowledgments
This research was funded by the National Institutes of Health (5 T32 AI070114, R01 MH068686, R01 AI083059-01, R37 DK049381) and the University of North Carolina Center for AIDS Research (5 P30 AI504105).
Footnotes
These data were presented in part at the 18th Meeting of the International Society for Sexually Transmitted Disease Research, London, England, June 28 – July 1, 2009.
There are no conflicts of interest.
References
- 1.Foxman B, Newman M, Percha B, et al. Measures of sexual partnerships: lengths, gaps, overlaps, and sexually transmitted infection. Sex Transm Dis. 2006;33:209–214. doi: 10.1097/01.olq.0000191318.95873.8a. [DOI] [PubMed] [Google Scholar]
- 2.Kraut-Becher JR, Aral SO. Gap length: an important factor in sexually transmitted disease transmission. Sex Transm Dis. 2003;30:221–225. doi: 10.1097/00007435-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Chen MI, Ghani AC, Edmunds J. Mind the gap: the role of time between sex with two consecutive partners on the transmission dynamics of gonorrhea. Sex Transm Dis. 2008;35:435–444. doi: 10.1097/OLQ.0b013e3181612d33. [DOI] [PubMed] [Google Scholar]
- 4.Lurie M, Rosenthal S, Williams B. Concurrency driving the African HIV epidemics: where is the evidence? Lancet. 2009;374:1420. doi: 10.1016/S0140-6736(09)61860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein H. The mathematics of concurrent partnerships and HIV: a commentary on Lurie and Rosenthal, 2009. AIDS Behav. 14:29–30. doi: 10.1007/s10461-009-9627-x. discussion 34-27. [DOI] [PubMed] [Google Scholar]
- 6.Lurie MN, Rosenthal S. The Concurrency Hypothesis in Sub-Saharan Africa: Convincing Empirical Evidence is Still Lacking. Response to Mah and Halperin, Epstein, and Morris. AIDS Behav. 2010;14:34. doi: 10.1007/s10461-009-9640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lurie MN, Rosenthal S. Concurrent partnerships as a driver of the HIV Epidemic in sub-Saharan Africa? The evidence is limited. AIDS Behav. 14:17–24. doi: 10.1007/s10461-009-9583-5. discussion 25-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS Behav. 2010;14:11–16. doi: 10.1007/s10461-008-9433-x. dicussion 34-17. [DOI] [PubMed] [Google Scholar]
- 9.Watts CH, May RM. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math Biosci. 1992;108:89–104. doi: 10.1016/0025-5564(92)90006-i. [DOI] [PubMed] [Google Scholar]
- 10.Kretzschmar M, Morris M. Measures of concurrency in networks and the spread of infectious disease. Math Biosci. 1996;133:165–195. doi: 10.1016/0025-5564(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 11.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Morris M, Kretzschmar M. A microsimulation study of the effect of concurrent partnerships on the spread of HIV in Uganda. Mathematical Population Studies. 2000;2000:109–133. [Google Scholar]
- 13.Halperin DT, Epstein H. Concurrent sexual partnerships help to explain Africa's high HIV prevalence: implications for prevention. Lancet. 2004;364:4–6. doi: 10.1016/S0140-6736(04)16606-3. [DOI] [PubMed] [Google Scholar]
- 14.Wellings K, Collumbien M, Slaymaker E, et al. Sexual behaviour in context: a global perspective. Lancet. 2006;368:1706–1728. doi: 10.1016/S0140-6736(06)69479-8. [DOI] [PubMed] [Google Scholar]
- 15.Lagarde E, Auvert B, Carael M, et al. Concurrent sexual partnerships and HIV prevalence in five urban communities of sub-Saharan Africa. AIDS. 2001;15:877–884. doi: 10.1097/00002030-200105040-00008. [DOI] [PubMed] [Google Scholar]
- 16.Guwatudde D, Wabwire-Mangen F, Eller LA, et al. Relatively low HIV infection rates in rural Uganda, but with high potential for a rise: a cohort study in Kayunga District, Uganda. PLoS One. 2009;4:e4145. doi: 10.1371/journal.pone.0004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mermin J, Musinguzi J, Opio A, et al. Risk factors for recent HIV infection in Uganda. JAMA. 2008;300:540–549. doi: 10.1001/jama.300.5.540. [DOI] [PubMed] [Google Scholar]
- 18.UNAIDS . Lancet. Vol. 375. HIV: consensus indicators are needed for concurrency; pp. 621–622. [DOI] [PubMed] [Google Scholar]
- 19.Gorbach PM, Stoner BP, Aral SO, et al. “It takes a village”: understanding concurrent sexual partnerships in Seattle, Washington. Sex Transm Dis. 2002;29:453–462. doi: 10.1097/00007435-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiscus SA, Pilcher CD, Miller WC, et al. Rapid, real-time detection of acute HIV infection in patients in Africa. J Infect Dis. 2007;195:416–424. doi: 10.1086/510755. [DOI] [PubMed] [Google Scholar]
- 22.Powers KA, Miller WC, Pilcher CD, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. AIDS. 2007;21:2237–2242. doi: 10.1097/QAD.0b013e3282f08b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorbach PM, Drumright LN, Holmes KK. Discord, discordance, and concurrency: comparing individual and partnership-level analyses of new partnerships of young adults at risk of sexually transmitted infections. Sex Transm Dis. 2005;32:7–12. doi: 10.1097/01.olq.0000148302.81575.fc. [DOI] [PubMed] [Google Scholar]
- 24.Kretzschmar M, White RG, Carael M. Concurrency is more complex than it seems. AIDS. 2010;24:313–315. doi: 10.1097/QAD.0b013e328333eb9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scrutinize http://www.scrutinize.org.za.
- 26.Nnko S, Boerma JT, Urassa M, et al. Secretive females or swaggering males? An assessment of the quality of sexual partnership reporting in rural Tanzania. Soc Sci Med. 2004;59:299–310. doi: 10.1016/j.socscimed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Malawi Demographic and Health Survey. Calverton, Maryland: 2004. [Google Scholar]
- 28.Reniers G, Watkins S. Polygyny and the spread of HIV in sub-Saharan Africa: a case of benign concurrency. AIDS. 2010;24:299–307. doi: 10.1097/QAD.0b013e328333af03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunham RC, Plummer FA. A general model of sexually transmitted disease epidemiology and its implications for control. Med Clin North Am. 1990;74:1339–1352. doi: 10.1016/s0025-7125(16)30484-9. [DOI] [PubMed] [Google Scholar]
- 30.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 31.Katz BP, Fortenberry JD, Tu W, et al. Sexual behavior among adolescent women at high risk for sexually transmitted infections. Sex Transm Dis. 2001;28:247–251. doi: 10.1097/00007435-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Sandoy IF, Dzekedzeke K, Fylkesnes K. Prevalence and correlates of concurrent sexual partnerships in Zambia. AIDS Behav. 2010;14:59–71. doi: 10.1007/s10461-008-9472-3. [DOI] [PubMed] [Google Scholar]
- 33.Harrison A, Cleland J, Frohlich J. Young people's sexual partnerships in KwaZulu-Natal, South Africa: patterns, contextual influences, and HIV risk. Stud Fam Plann. 2008;39:295–308. doi: 10.1111/j.1728-4465.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris M. Concurrent partnerships and syphilis persistence: new thoughts on an old puzzle. Sex Transm Dis. 2001;28:504–507. doi: 10.1097/00007435-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Aral SO. Partner concurrency and the STD/HIV Epidemic. Curr Infect Dis Rep. 2010 doi: 10.1007/s11908-010-0087-2. [DOI] [PubMed] [Google Scholar]
- 36.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 Transmission, by Stage of Infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 37.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 38.Pilcher CD, Tien HC, Eron JJ, Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 39.Powers KA, Poole C, Pettifor AE, et al. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burington B, Hughes JP, Whittington WL, et al. Estimating duration in partnership studies: issues, methods and examples. Sex Transm Infect. 2010;86:84–89. doi: 10.1136/sti.2009.037960. [DOI] [PMC free article] [PubMed] [Google Scholar]