Abstract
Schistosoma haematobium is responsible for two-thirds of the world’s 200-400 million cases of human schistosomiasis. It is a group 1 carcinogen and a leading cause of bladder cancer which occurs following years of chronic inflammation, fibrosis and hyper-proliferation in the host liver. The co-evolution of blood flukes of the genus Schistosoma and their human hosts is paradigmatic of long term parasite development, survival and maintenance in mammals. However, the contribution of host genes, especially those discrete from those of the immune system, necessary for parasite establishment and development remains poorly understood. The present study investigated the role of Metastasis-associated protein-1 gene (Mta1) product in the survival of S. haematobium. Significantly fewer S. haematobium worms and eggs were recovered from Mta1 −/− than wild type mice. Comparative analysis of cytokine profiles indicated a loss of cytokine interdependence and aberrant Th1/Th2 cytokine response in the Mta1−/− mice compared to age-matched wild type mice. By utilizing this Mta1-null mouse model, we identified a distinct, contribution of the mammalian MTA1 in establishing a productive host-parasite interaction and thus revealed a host factor critical for the optimal survival of schistosomes and successful parasitism. Moreover, MTA1 appears to play a significant role in driving inflammatory responses to schistosome egg induced hepatic granulomata reactions, and thus offers a survival cue for parasitism as well as an obligatory contribution of liver in schistosomiasis. These findings raise the possibility to develop intervention strategies targeting MTA1 to reduce the global burden of schistosomiasis, inflammation and neoplasia.
Keywords: Schistosomiasis, Liver Inflammation and Cancer, Hepato-biliary Malignancies, Hyperproliferation, Schistosoma haematobium, MTA-1
Introduction
Schistosomiasis is one of the world’s most devastating water-borne infectious diseases with 300 million or more people currently infected1 and resulting in almost 300,000 deaths annually in Africa alone2. Approximately two-thirds of the world’s schistosomiasis cases and many of the deaths result from Schistosoma haematobium infection2, an oncogenic schistosome that is associated with bladder cancer3. Schistosoma haematobium is considered a group 1 carcinogen and individuals are exposed to schistosome infection when they come in contact with water contaminated by cercariae4-6. Cercariae penetrate the skin after which they transform into schistosomula, which are also equipped with antigenic repertoire to evade the host immune response. From infection of subcutaneous tissues, schistosomula enter the circulation and travel to the lungs and then to the liver, where they achieve sexual maturity before entering into the portal venous system or the vesical venous plexus. Eggs released from the paired adults travel to the liver, intestines, and/or bladder, lodging in the tissues and producing granulomatous inflammation which can lead to fibrosis and, in the case of S. haematobium, neoplastic transformation4, 5. Like most helminth parasites, it can be predicted that schistosomes utilize diverse host factors to positively stimulate and contribute to their development and sexual maturation4.
Previous studies addressing the host-parasite interactions during schistosomiasis have focused on a subset of the immune response genes used to mount a Th1/Th2 response during infection. However, the numbers of adult worms in schistosome-infected knockout mouse models of critical immune-response genes such as IL-4, IL-6 and IL-10, which control the Th1/Th2 response, remain unaffected7-9. One interpretation of these observations is that there is an additional, yet-to-be determined, regulatory pathway(s) that accommodates host permissiveness to schistosome establishment and productive schistosome infection and parasitism. In both humans and mice, schistosomes elicit chronic inflammatory responses in the host7-9. In S. haematobium infection, the prolonged inflammatory response is thought to contribute to the development of squamous cell carcinoma10. Interestingly, granulomatous inflammation due to chronic schistosomiasis has a similar dynamic and cellular manifestation in human and murine models of infection, making mice a physiologically relevant model to study this infection4, 5, 11, 12. Further the process of dependency of schistosomes on the host factors for a successful infection is evolutionarily conserved among all species of human schistosomes including S. haematobium and S. mansoni13, both of which utilize common host mechanisms to infect its host and immune signals that appear to facilitate parasite development13.
Recent findings from this laboratory have established a novel role of Metastasis-associated protein-1 gene (Mta1) product MTA1, a chromatin bound component of the NuRD complex, in mediating the host inflammatory responses to the components of virus and bacterial infections by regulating the transcription of host-immune responsive genes14-18. Given that S. haematobium is the only member of group 1 carcinogenic helminths that develop to sexual maturity and can reproduce in mice3, 19 the key role we previously identified for overexpressed MTA1 in oncogenesis20-22 and because physiologic levels of MTA1 participate in the inflammatory response17, we hypothesized that there may be a link between S. haematobium parasitism and this unique regulator. Further, an exploration of the role of MTA1 in murine S. haematobium infections could help to unlock the links between parasitism and host inflammation. Guided and prompted by our recent findings that demonstrated a key role for MTA1 in modulating the host inflammatory response to pathogens, here we set out to investigate the role of this protein as a host co-factor for infection by S. haematobium, utilizing schistosome infections in Mta1 −/− and wild type mice as a model system.
RESULTS
MTA1 is a permissive host cofactor for Schistosoma haematobium survival
In mice, adult S. haematobium worms predominantly inhabit the portal venous system, where they mature and begin to release eggs from 10 weeks after infection. We reproducibly recovered significantly fewer worms from the Mta1−/− mice as compared with age-matched Mta1+/+ wild type (WT) mice (Figs. 1A; S1). Consistent with these results, far fewer eggs also were recovered from livers of S. haematobium infected Mta1−/− mice than age-matched WT mice (Fig. 1A). As previous studies have established that schistosome infection is accompanied by an early IgG response to Soluble Worm Antigen Preparation (SWAP)4, 5, we evaluated the serum antibody response of schistosome infected age-matched WT and Mta1−/− animals to SWAP at 2, 5 and 12 weeks post-infection (p.i.) using an indirect-ELISA for IgG against SWAP4, 5. Although levels of IgG were detected in both groups at all three time points, there was a positive correlation between the levels of IgG against SWAP and time post-infection (p.i.), i.e. IgG to SWAP increased progressively from 2 weeks through 5 weeks to 12 weeks p.i. (Fig. 2A). The median OD 492 nm for age-matched WT and Mta1-/- mice was 0.365 and 1.052, respectively, at 5-wk p.i., and the ratio for the age-matched, wild type group rose to 2.053 at 12-wk p.i. The similarity in the level of IgG against SWAP suggests of comparable infectivity for both Mta1−/− and wild type mice. Hence, the failure of S. haematobium to develop to patent infection in Mta1-/- mice was not due to an inability to establish a successful infection, but to inherent differences of worm maturation and egg deposition in the absence of MTA1 (Fig. 1B).
Figure 1.
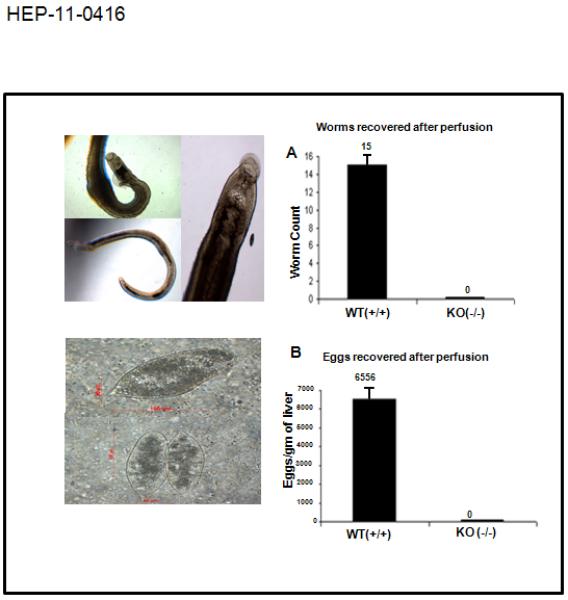
MTA-1 is a requisite cellular cofactor for productive Schistosoma haematobium infection. Representative images of adult S. haematobium worms (top left panel) and eggs (bottom left panel) recovered from age matched WT Mta-1 (+ / +) mice by portal perfusion and KOH digestion of the liver. Right panel: Bar plots for total numbers of adult worms (1A) and eggs recovered (1B) at 12 weeks after infection.
Figure 2.
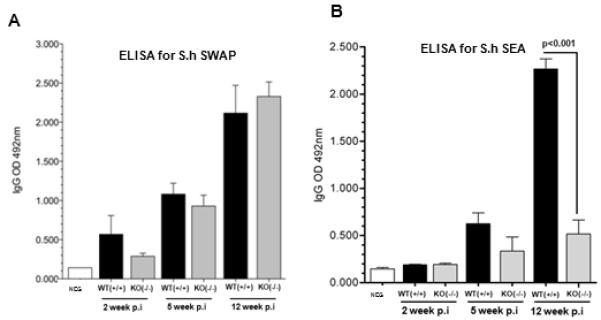
ELISA for Schistosoma haematobium SWAP (soluble adult worm antigen preparation) and SEA (soluble egg antigen) reflect equivalent initial parasite burdens in both genotypes of the host mice but higher levels of mature infections in WT Mta-1(+/+) mice. (A) Bar plot showing results from ELISA against S. haematobium worm antigen using sera collected from Mta-1 WT and −/− (= KO, knockout) mice at increasing times after infection. (B) Bar plot showing the results from ELISA against S. haematobium egg antigen using sera collected from Mta-1 WT and KO at various time points during the course of infection. Data are presented as mean ± SD.
In the mouse, the course of schistosome infection progresses through two distinct phases 4, 5, 7-9. The first is an acute-phase, which occurs 3-8 weeks after infection, when the host is exposed to the migrating immature parasites and is dominated by T helper (Th1) cellular response4, 5. As the schistosomes parasites mature in the liver and begin releasing eggs, the Th1 response is gradually diminished by the emergence of a strong Th2 response to the eggs embedded in the liver and other sites, as represented by the immune response to Schistosome Egg Antigen (SEA). As such, we measured the levels of IgG against SEA of S. haematobium using an indirect ELISA, as above. Here we observed significantly higher levels of IgG in age-matched WT mice compared Mta1−/− mice (Fig. 2B), indicating the presence of eggs in the portal system of the age-matched WT mice. These results are consistent with the finding of a far fewer worms in the portal system in the Mta1−/− compared to age-matched WT-mice. Collectively, these findings suggest that whereas the absence of Mta1 does not compromise the susceptibility to S. haematobium infection, expression of MTA1 positively influences survival and/or maturation of schistosomes in the host, and perhaps egg release and deposition as well. These observations suggest that Mta1 may represent a requisite host factor for permissiveness of schistosome infection, culminating in egg production by the adult parasites.
Pivotal role of MTA1 in immune response to S. haematobium infection
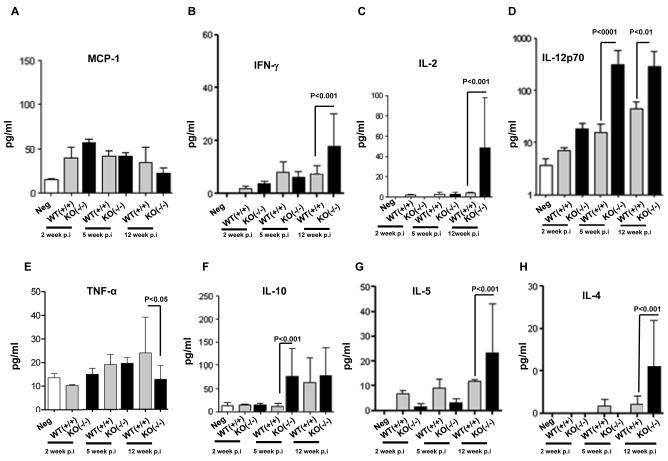
Th1 and Th2 responses are accompanied by the activation of specific cytokines IL-2, IL-12 and IFN-γ for Th1, and TNF-α, IL-4, IL-5 and IL10 for Th24, 5, 7-9, 23-25, we examined the Th1 and Th2 cytokines profiles in the context of a defective S. haematobium infection in the Mta1−/− mice. In our studies we examined systemic changes in the host immune response and towards this end; we measured the circulating cytokine concentrations in the sera of age-matched WT and Mta1−/− mice at several time points. As expected, serum Th1 cytokine levels peaked at 5-wk post-infection (p.i.) while serum Th2 cytokine levels peaked at 12 weeks p.i. (Fig 3). Serum levels of chemokine monocyte chemokine factor (MCP-1) were similar in age-matched WT and Mta1−/− mice throughout the course of infection (Fig. 3A). MCP-1 is a critical chemokine that controls parasite burden and pro-inflammatory responses that are predominant in acute phase of parasite infection9. Since levels of this mediator remained unaffected in the sera of age-matched WT and Mta1−/− mice, we can assume that there was an equal parasite burden in both genotypes as a result of infection with cercariae. Infection in age-matched WT-mice produced a balanced Th1 and Th2 response, which is evident by the lower concentrations of IFN-γ and IL-10 in sera at 12 weeks post-infection compared to Mta1−/− mice (Fig. 3B&F). The levels of the pro-inflammatory cytokine TNF-α were elevated in age-matched WT mice 5 wk p.i. compared to uninfected Mta1−/− mice (Fig 3E). These findings are consistent with the notion that a higher level of this cytokine is reflective of the acute phases of schistosome infection in murine models4, 5, 7-9, 23-25.
Figure 3.
Impact of MTA-1 status on inflammatory and Th1/Th2 cytokines in response to Schistosoma haematobium infection. Cytokine responses in age matched WT and Mta1−/− mice at 2-, 5-, and 12-wk post-infection: panel A, MCP-1; B, IFN-γ; C, IL-2; D,IL-12p70; E, TNF-alpha; F, IL-10; G, IL-5; and H, IL-4. Serum samples were used to determine the levels of inflammatory and Th1/Th2 cytokines by cytokine bead array assays. Data are presented as mean ± SD.
Among the other Th1 cytokines assayed in sera, we observed that IL-12p70, the bioactive form of IL-124, 5, 26, 27, was significantly higher in the Mta1−/− mice soon after infection and remained elevated during the course of this study (Fig. 3D). Among the Th2 cytokines evaluated, levels of IL-4 and IL-5 were significantly elevated in the Mta1−/− mice at 12 weeks p.i. while levels of the immunomodulator IL-10 were elevated early-on during the course of infection when compared to age-matched WT-mice (Fig. 3F-H). Earlier studies have demonstrated a regulatory role for IL-10 in egg-induced pathology from S. mansoni infection and in some cases this has been shown to suppress granulomatous inflammations4, 5, 7-9, 26, 27. IL-12 is a key inducer of Th1-associated inflammatory response, which provides protection against intracellular infections26, 27. The importance of IL-12 in the pathogenesis of schistosomiasis has been shown in experiments where the inclusion of SEA plus IL-12 resulted in smaller granulomas and less severe fibrosis26,27. Decreased fibrosis was associated with diminished Th2 response and increased Th1 cytokine production26, 27.
MTA1 is a host determinant of schistosome egg-induced hepatic granulomatous (HG) inflammation and hyperproliferation
In case of schistosome infection, the infective cercariae are guided by factors contributed by the host that serve as molecular cues for their development and sexual maturation of the adult blood fluke in the liver. In our studies, maturation of adult worms and egg-laying was severely compromised in Mta1−/− mice. Pathology of schistosomiasis is largely determined by egg-induced granulomatous response in the liver. Immunohistochemical analysis of liver sections from age-matched WT and Mta1−/− mice revealed significant number of granulomatous lesions in age-matched WT mice that were completely absent in Mta1−/−mice (Fig. 5). High levels of TNF-α in tandem with low levels of IFN-γ have also been shown to promote granulomatous lesions in the liver during schistosome infection7-9, 26, 27. Accordingly, higher levels of TNF-α and lower levels of IFN-γ in age-matched WT-mice mice at 5 and 12 week p.i. correlated well with an increase in hepatic granulomatous (HG) inflammations and hyperproliferation, as evident by cytokeratin19 (CK19)-positive staining and eosinophil infiltration in the liver tissues of infected mice (Figs. 4, 5).
Figure 5.
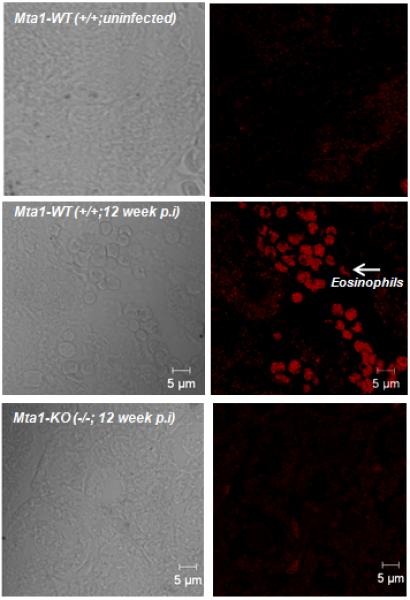
Mta1-WT mice show a higher degree of eosinophil infiltration in liver compared to age matched Mta1(−/−) mice 12 weeks post-infection. Liver tissue sections from infected WT and Mta1(−/−) mice were stained for eosinophils using EO-probe kit. Sections were scanned using Zeiss 710 confocal microscope and images were recorded using a 40× objective.
Figure 4.
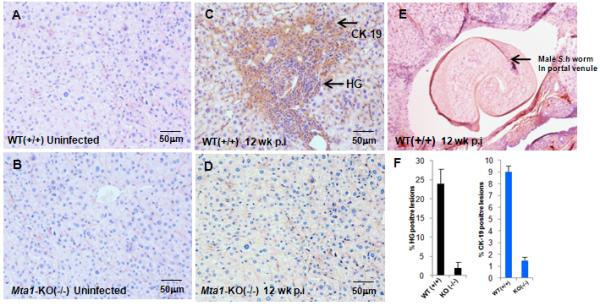
Influence of MTA-1 on formation of granulomatous inflammatory lesions in liver. Paraffin embedded liver tissue sections from age-matched WT and Mta1−/− mice at 5 and 12 weeks after exposure to Schistosoma haematobium cercariae were stained with anti-cytokeratin-19 (Ck-19) antibody followed by hematoxylin and eosin (H & E). Stained slides were scored under phase contrast microscopy for hepatic granulomatous (HG) regions. Representative liver sections from uninfected, control mice (panel A, WT Mta1, panel B, Mta1−/−), infected mice at 12 weeks (C, D) after infection. Regions showing high infiltration of polymorphonuclear cells were scored as positive for inflammation. Percentage HG positive and CK19 positive zones from 20 fields were determined and represented as bar plot (panel F). Panel E shows an adult male S. haematobium in situ in a portal venule in a WT mouse 12 weeks after exposure to cercariae (H&E stain). Images were captured under a 20 × objective.
Intrahepatic immune response and hepatic CD4+ve T cell repertoire favors successful parasitism involving a functional MTA1 pathway
Since adult schistosomes mature to sexually reproductive forms in the liver, we measured cytokine levels in the liver from age-matched WT or Mta1−/− mice by quantitative RT-PCR. Upon analysis of RNA isolated from liver at 2 and 12 weeks p.i., Mta1−/− mice showed on average a 12-fold increase in mRNA levels encoding IL-10 and IL-12 within two weeks of infection compared to age-matched WT littermates. Further, mRNA levels of some of Th1 and Th2 cytokines in both genotypes reflected the cytokine production profile observed in sera (Fig. 6A-E). Remarkably, Mta1 mRNA levels were significantly upregulated in the liver of age-matched WT-mice as the infection progressed (Fig 7A). Analysis of total hepatic CD4 expression revealed that CD4 expression in Mta1−/− mice was several-fold less in the wild type mice at 2 and 12 weeks p.i., suggesting involvement of Mta1 in the regulation of CD4 specific T cells that may facilitate infection (7B).
Figure 6.
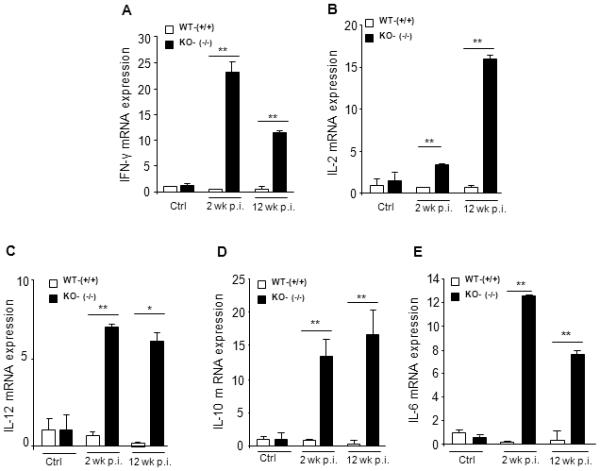
Liver cytokine expression reveals loss of cytokine interdependence in Mta1 (−/−) mice. Analysis of liver cytokine mRNA levels in Mta1-WT (+/+) and Mta1-KO (−/−) mice. 50 mg of liver tissue was used for RNA isolation and cDNA synthesis. Expression levels of Th1 and Th2 cytokine was assessed by Quantitative real-time PCR.* P <0.01 **P<0.001.
Figure 7.
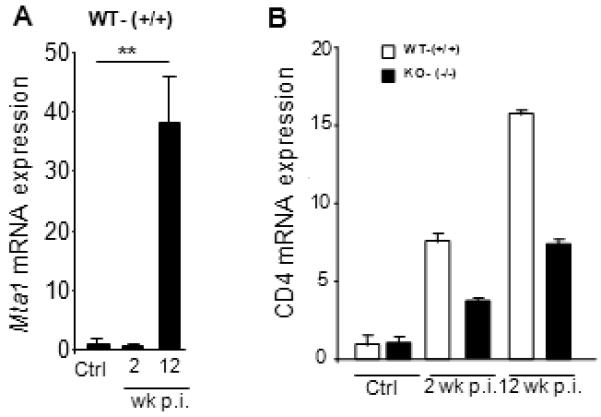
Mta1 is an early host-responsive gene following S. haematobium infection and regulates expression of CD4+ve T cell population. Expression levels of Mta1 (A), CD4 (B), was assessed by Quantitative real-time PCR.* P <0.01 **P<0.001.
DISCUSSION
Metastasis associated protein-1 gene (Mta1), a member of the NURD (nucleosome remodeling complex) has been widely associated with human cancer21, 22, 28. The Mta1 gene product is a chromatin bound coregulator involved in transcriptional regulation of genes associated with multiple cellular pathways21. Recent investigations from this laboratory have established that in addition to the oncogenic role of MTA1 overexpression29, physiological levels of MTA1 play an important role in the host inflammatory responses to viral and bacterial infections, including to the HBx core antigen of hepatitis B virus and to lipopolysaccharides, due to its role in the transcriptional regulation of host-immune response genes14-18. These findings underpin the notion, explored here, that master regulators such as MTA1, important in cancer, could also be of fundamental importance in establishing productive parasitic infections - that is, MTA1 may have an inherent role in supporting parasitism. We explored this hypothesis here using murine schistosomiasis haematobia as the model. Whereas a consistent goal in research on schistosomiasis has been to decipher the complexity and diversity of host immune responses to these complex pathogens, the contribution of host genes discrete from those of the adaptive immune system necessary for parasite establishment and development remains little understood. The present findings indicated that MTA1 is a critical factor for survival of S. haematobium (Fig. 1A) and that the absence of MTA1 leads to production of cytokines inimical to successful parasitism by schistosomes. To our knowledge, the present report is the first to ascribe a productive relationship between the expression of Mta1 and a successful infection by pathogen associated with human cancer, in this case the African blood fluke, S. haematobium.
Comparative analysis of cytokine profiles following S. haematobium infection in both genotypes indicated a loss of cytokine interdependence and aberrant cytokine production in the Mta1−/− mice when compared to WT age-matched animals (Fig 3). Of note, elevated levels of the Th1 cytokine IL-12 did not suppress IL-10 in Mta1−/− mice during the early stages of infection (Fig. 3D&F). Further, elevated levels of Th-2 cytokine IL-4 did not suppress levels of Th1 cytokine IL-2 in Mta1−/− at 12 weeks post infection (Fig. 3C&H). Age-matched WT mice exhibited a mixed Th1 and Th2 responses suggesting a strong interdependence and a balance among the cytokines, which possibly contributed to the success of the infection (Figs. 3A-H). It is noteworthy that although the levels of IL-10 were significantly elevated in the Mta1−/− mice, there was no resulting mortality (Fig. 3F). In the light of these findings, it is tempting to speculate that sustained IL-12 expression and an early Th2 response meditated by IL-5 and IL-10, as observed in the infected Mta1−/− mice, played a role in the demise of maturing worms in these mice. These observations also raise the intriguing possibility that MTA1 as a host-factor plays a causative role in S. haematobium development, by influencing expression of cytokines conducive to productive parasitism. Our studies suggest that a complex interplay between the host-factor MTA1 and the schistosome is required for the optimal persistence of the parasite in the host, without causing immune-mediated deleterious effects for the host. We speculate that the blood fluke utilizes MTA1, a master regulator of at least several, well-characterized downstream genes21 to control the host micro-environment, suggesting that Mta1 might be an early response gene to helminth parasite infection, in like fashion to exposure to LPS21 and thus, could favor parasitism by providing a key developmental cue (Fig. 7A). MTA1 is expressed in multiple organs and tissues22,30, including the skin, lungs and liver sites through which the developing schistosome migrates.
Earlier studies have utilized diverse mouse models to understand host factors in schistosomiasis. Studies that utilized S. mansoni infections in Rag1−/− mice that are deficient for B and T lymphocytes23, 31 are instructive in that they demonstrate a key role for CD4+ hepatic lymphocytes in maturation of adult schistosomes. A recent report concluded that schistosomes adopt innate immune signals for development in the host. Further, the studies demonstrated that the CD4+ T cell population was critical in regulating the mononuclear phagocyte population in liver following schistosome infection32. Here we observed far fewer worms in the portal perfusate of Mta1−/− mice, indicating that the lack of MTA1 was a predominant factor in determining parasite migration to the hepato-portal system and maturation in the liver. Detailed analysis of CD4 expression in livers of uninfected age-matched WT and Mta1−/− mice by quantitative RT-PCR revealed no differences in CD4 expression, indicating that CD4 expression did not contribute to the unsuccessful parasitism of the Mta1−/− mice. Intriguingly, CD4 expression was higher in WT mice as infection progressed suggesting that MTA1 might be involved in regulation and or specific homing of hepatic CD4+ve T cell lymphocytes in response to infection. Considering the aberrant cytokine profile observed in the Mta1−/− mice, we speculate that MTA1 is involved in maturation of a specific subset of CD4+ve T cells. These findings provide additional support to the notion that MTA1 represents a permissive stimulus or co-factor for parasite survival in the immunologically intact host.
These findings highlight a likely role of MTA1 as a master co-regulator in the pathogenesis of schistosomiasis. We hypothesize that MTA1 provides a necessary niche for the host-parasite interaction and that MTA1 plays a key role in driving granulomatous-specific inflammatory reactions in the liver. Since schistosomes can survive for many years, it is tempting to speculate that MTA1 is a permissive host regulatory factor that maintains the worms and elevates cancer causing fibrotic lesions. In addition, since expression of MTA1 is upregulated during schistosomiasis, as occurs with LPS and HBx18, and because elevated levels of MTA1 promote cancer21, 22, 28, the findings establish a rationale for prospective clinical investigations on the links between MTA1, schistosomiasis haematobia, granulomatous-specific inflammation in liver, and incidence of schistosome-associated bladder cancer10.
MATERIALS AND METHODS
Schistosoma haematobium; infection of mice
Cercariae of Schistosoma haematobium released from experimentally infected Bulinus truncatus truncatus snails were obtained from Dr. Fred A. Lewis, Biomedical Research Institute, Rockville, MD under NIH-NIAID contract HHSN272201000005I. This strain of S. haematobium is maintained in the laboratory using hamsters and B. t. truncatus snails33. Mta1 −/− and wild type mice were bred in our laboratory, as described 17,34. Mice were infected with cercariae using the percutaneous route, by immersion of the tail for one hour in 5 ml water containing ~700 cercariae. At intervals after infection, 2, 5, 12 and/or 15 weeks, mice were euthanized by overdose of Euthasol (Virbac, Fort Worth, TX). The portal vein was severed and worms recovered from the portal circulatory system by perfusion with 150 mM sodium chloride, 15 mM sodium citrate, pH 7.035, and counted. The livers were removed from the carcass, cut into several pieces, one of which was weighed then subjected to digestion in 4% KOH for 15 hours36. Subsequently, the numbers of S. haematobium eggs per gram of liver were counted using X40 objective fitted to a microscope. At necropsy, in addition to the liver, cardiac blood, spleen, lungs, bladder and small intestines were collected; part of each was fixed in buffered formalin and the rest snap frozen in liquid nitrogen. Studies with schistosome-infected mice were approved by the Institutional Animal Care and Use Committee (IACUC) of The George Washington University Medical Center.
Experimental design
We carried out two separate infections of mice with S. haematobium. Within each experiment, we included six to nine mice of each of Mta1 genotypes, wild type (+/+) and knockout (−/−). In the first, we euthanized two or three of each genotype at 2, 5 and 12 weeks after infection. In the second, all mice were euthanized at ~15 weeks after infection. In mice, adult S. haematobium worms predominantly inhabit the portal venous system, and mature and release eggs by 10 weeks after infection36, 37.
Indirect ELISA
An indirect ELISA was used to measure levels of immunoglobulin G (IgG) to the S. haematobium Soluble Adult Worm antigenic Preparation (SWAP) and to the Soluble Egg Antigen (SEA), prepared as described for S. mansoni38. A pool of positive control sera was derived from equal portions of sera each genotype at 12 weeks after infection. A pool of negative control sera was sourced from uninfected age and sex matched mice. PolySorp™ (Nalge, Nunc International, Rochester, NY) 96-well microtiter plates were coated with 100 μl/well of either 5 μg/ml of SWAP or SEA in carbonate-bicarbonate buffer, pH 9.6, sealed, and incubated overnight at 4°C. Plates were washed 3 times with PBS (pH 7.2) and blocked with 200 100 μl/well of 3% bovine serum albumin (BSA) (Sigma) diluted in PBS. Control and experimental sera were diluted 1:4,000 in PBS and 100 μl was added to each well of the microtiter plate in duplicate. The plates were sealed and incubated overnight at 4°C and then washed 3 times with PBS with 0.05% Tween 20 (PBST) at pH 7.2. A biotinylated goat anti-mouse IgG antibody (Vector Laboratories Inc.) was used at 1:5,000 in 3% BSA and PBS and applied 100 μl/ well and incubated for 90 minutes at room temperature (RT). Thereafter, plates were washed and incubated with a 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated streptavidin (GE Healthcare) in 3% BSA and PBS for 60 minutes at RT in the dark. The plates were incubated for 30 minutes with o-phenylenediamine dihydrochloride in the dark for 30 min at RT. Fifty μl of H2SO4 were added to each well to stop the reaction and the optical density (OD) at 492 nm determined (SpectraMax 340 PC reader, SOFTmax Pro software, Molecular Devices, Sunnyvale, CA).
Cytokine profiling - Cytometric Bead Arrays (CBA)
A BD™ Cytometric Bead Array (CBA) Mouse Inflammation Kit and a Mouse Th1/Th2 Cytokine Kit (BD Biosciences) were used. In brief, to detect concentrations of Interleukin (IL)-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, Monocyte Chemoattractant Protein (MCP)-1, Interferon (INF)-γ and Tumor Necrosis Factor (TNF)-α in the serum of S. haematobium-infected mice and positive and negative controls, a standard reference curve (Mouse Inflammation Standard or Mouse Th1/Th2 Cytokine Standards) provided in the kit was used to interpolate picograms per microliter levels of each cytokine. Nine-fold serial dilutions were performed with the standard to obtain a standard curve within a range of 20 - 5000 pg/ml. Each serum sample was diluted 1:2 in RMPI for a final volume of 25 μl. In parallel, RPMI was also used as a negative control. A cocktail of the beads from each cytokine was prepared with 3 μl of each bead per sample. Fifteen μl cytokine capture bead cocktail was added to samples, standards and controls. After vortexing for 10 seconds, 18 μl of the Mouse Inflammation PE Detection Reagent or Mouse Th1/Th2 PE Detection Reagent was added. Tubes were incubated at RT in the dark for 2 hours. Samples were washed with 500 μl of washing buffer and centrifuged for 7 min at 1300 rpm. After aspirating the supernatants to leave ~200 μl, samples were analyzed using a FACScan™ flow cytometer and the CBA Software (BD Biosciences). Results are expressed in pg/ml.
Immunohistochemistry
Immunohistochemistry was performed as described29. Five μm thick liver sections were cut, warmed to 60 °C, de-paraffinized in xylene and rehydrated with graded ethanol. Antigen exposure took place for 20 min in antigen retrieval solution after which endogenous peroxide activity was inactivated with 0.3% H2O2 in methanol. The sections were blocked for 20 min in normal goat serum in phosphate-buffered saline (PBS), and incubated with primary antibodies against CK-19 for 3 h. Samples were rinsed five times in washing buffer, and incubated in secondary antibody for 1 h. Samples were rinsed three times in wash buffer, and incubated in horseradish peroxidase labeled second antibody solution for 15 min. Samples were rinsed three times in wash buffer and incubated in diaminobenzidine for 5 min. Samples were rinsed three times in wash buffer and counterstained in hematoxylin for 2 min. Tissue slides were scored in a blinded fashion as coded unknown specimens by three investigators. For eosinophil staining, tissue sections were processed and stained using the EOPROBE eosinophil staining kit (SurModics In Vitro Diagnostic Products, USA). Slides were viewed with a Zeiss 710 confocal laser scanning microscope and scored in a blinded fashion by two investigators.
Quantitative real-time PCR
Quantitative real-time PCR was performed as described17. Sequences of primers are available on request.
Statistical Analysis
Differences among groups were compared by Analysis of Variance (ANOVA) and Student’s t-test. P values ≤0.05 were considered significant.
Supplementary Material
Acknowledgements
We thank Drs. Fred A. Lewis, Yung-san Liang and Allen Cheever for helpful discussions and for providing Schistosoma haematobium stages via NIH-NIAID contract HHSN272201000005I, and Dr. Stephanie Constant for assistance with cytological analysis. We acknowledge National Center for Research Resources grant support S10RR025565 to the Institutional imaging core facility for imaging studies.
This work was supported in part by National Institute of Health Grants CA98823 and CA98823-S1 and institutional new program funds (to R.K.).
REFERENCES
- 1.King CH. Parasites and poverty: The case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-saharan africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 4.Stavitsky AB. Regulation of granulomatous inflammation in experimental models of schistosomiasis. Infect Immun. 2004;72:1–12. doi: 10.1128/IAI.72.1.1-12.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 6.Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8:814–826. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann KF, Caspar P, Cheever AW, Wynn TA. IFN-gamma, IL-12, and TNF-alpha are required to maintain reduced liver pathology in mice vaccinated with schistosoma mansoni eggs and IL-12. J Immunol. 1998;161:4201–4210. [PubMed] [Google Scholar]
- 8.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: Excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann KF, Wynn TA, Dunne DW. Cytokine-mediated host responses during schistosome infections; walking the fine line between immunological control and immunopathology. Adv Parasitol. 2002;52:265–307. doi: 10.1016/s0065-308x(02)52014-5. [DOI] [PubMed] [Google Scholar]
- 10.Shiff C, Naples JM, Isharwal S, Bosompem KM, Veltri RW. Non-invasive methods to detect schistosome-based bladder cancer: Is the association sufficient for epidemiological use? Trans R Soc Trop Med Hyg. 2010;104:3–5. doi: 10.1016/j.trstmh.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Cheever AW, Duvall RH, Hallack TA., Jr Hepatic fibrosis in schistosoma haematobium-infected mice. Trans R Soc Trop Med Hyg. 1983;77:673–679. doi: 10.1016/0035-9203(83)90202-x. [DOI] [PubMed] [Google Scholar]
- 12.Dean DA, Murrell KD, Xu ST, Mangold BL. Immunization of mice with ultraviolet-irradiated schistosoma mansoni cercariae: A re-evaluation. Am J Trop Med Hyg. 1983;32:790–793. doi: 10.4269/ajtmh.1983.32.790. [DOI] [PubMed] [Google Scholar]
- 13.Lamb EW, Crow ET, Lim KC, Liang YS, Lewis FA, Davies SJ. Conservation of CD4+ T cell-dependent developmental mechanisms in the blood fluke pathogens of humans. Int J Parasitol. 2007;37:405–415. doi: 10.1016/j.ijpara.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bui-Nguyen TM, Pakala SB, Sirigiri DR, Martin E, Murad F, Kumar R. Stimulation of inducible nitric oxide by hepatitis B virus transactivator protein HBx requires MTA1 coregulator. J Biol Chem. 2010;285:6980–6986. doi: 10.1074/jbc.M109.065987. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Bui-Nguyen TM, Pakala SB, Sirigiri RD, Xia W, Hung MC, Sarin SK, Kumar V, et al. NF-kappaB signaling mediates the induction of MTA1 by hepatitis B virus transactivator protein HBx. Oncogene. 2010;29:1179–1189. doi: 10.1038/onc.2009.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghanta KS, Pakala SB, Reddy SD, Li DQ, Nair SS, Kumar R. MTA1 coregulation of transglutaminase 2 expression and function during inflammatory response. J Biol Chem. 2010 Mar 4;286:7132–8. doi: 10.1074/jbc.M110.199273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pakala SB, Bui-Nguyen TM, Reddy SD, Li DQ, Peng S, Rayala SK, Behringer RR, et al. Regulation of NF-kappaB circuitry by a component of the nucleosome remodeling and deacetylase complex controls inflammatory response homeostasis. J Biol Chem. 2010;285:23590–23597. doi: 10.1074/jbc.M110.139469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Pakala SB, Reddy SD, Bui-Nguyen TM, Rangparia SS, Bommana A, Kumar R. MTA1 coregulator regulates LPS response via MyD88-dependent signaling. J Biol Chem. 2010;285:32787–32792. doi: 10.1074/jbc.M110.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, et al. A review of human carcinogens--part B: Biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 20.Denslow SA, Wade PA. The human mi-2/NuRD complex and gene regulation. Oncogene. 2007;26:5433–5438. doi: 10.1038/sj.onc.1210611. [DOI] [PubMed] [Google Scholar]
- 21.Manavathi B, Kumar R. Metastasis tumor antigens, an emerging family of multifaceted master coregulators. J Biol Chem. 2007;282:1529–1533. doi: 10.1074/jbc.R600029200. [DOI] [PubMed] [Google Scholar]
- 22.Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, Vadlamudi RK, et al. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol. 2001;3:30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
- 23.Davies SJ, Lim KC, Blank RB, Kim JH, Lucas KD, Hernandez DC, Sedgwick JD, et al. Involvement of TNF in limiting liver pathology and promoting parasite survival during schistosome infection. Int J Parasitol. 2004;34:27–36. doi: 10.1016/j.ijpara.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 26.Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, Sher A. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 27.Kalinski P, Smits HH, Schuitemaker JH, Vieira PL, van Eijk M, de Jong EC, Wierenga EA, et al. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: Reversal of polarized Th2 phenotype by dendritic cells. J Immunol. 2000;165:1877–1881. doi: 10.4049/jimmunol.165.4.1877. [DOI] [PubMed] [Google Scholar]
- 28.Bowen NJ, Fujita N, Kajita M, Wade PA. Mi-2/NuRD: Multiple complexes for many purposes. Biochim Biophys Acta. 2004;1677:52–57. doi: 10.1016/j.bbaexp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Ohshiro K, Rayala SK, Wigerup C, Pakala SB, Natha RS, Gururaj AE, Molli PR, et al. Acetylation-dependent oncogenic activity of metastasis-associated protein 1 co-regulator. EMBO Rep. 2010;11:691–697. doi: 10.1038/embor.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toh Y, Nicolson GL. The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clin Exp Metastasis. 2009;26:215–27. doi: 10.1007/s10585-008-9233-8. [DOI] [PubMed] [Google Scholar]
- 31.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294:1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 32.Lamb EW, Walls CD, Pesce JT, Riner DK, Maynard SK, Crow ET, Wynn TA, et al. Blood fluke exploitation of non-cognate CD4+ T cell help to facilitate parasite development. PLoS Pathog. 2010;6:e1000892. doi: 10.1371/journal.ppat.1000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis FA, Liang YS, Raghavan N, Knight M. The NIH-NIAID schistosomiasis resource center. PLoS Negl Trop Dis. 2008;2:e267. doi: 10.1371/journal.pntd.0000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manavathi B, Peng S, Rayala SK, Talukder AH, Wang MH, Wang RA, Balasenthil S, et al. Repression of Six3 by a corepressor regulates rhodopsin expression. Proc Natl Acad Sci U S A. 2007;104:13128–13133. doi: 10.1073/pnas.0705878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann VH, Morales ME, Rinaldi G, Brindley PJ. Culture for genetic manipulation of developmental stages of Schistosoma mansoni. Parasitology. 2010;137:451–462. doi: 10.1017/S0031182009991211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheever AW. The intensity of experimental schistosome infections modulates hepatic pathology. Am J Trop Med Hyg. 1986;35:124–133. doi: 10.4269/ajtmh.1986.35.124. [DOI] [PubMed] [Google Scholar]
- 37.Agnew AM, Murare HM, Doenhoff MJ. Immune attrition of adult schistosomes. Parasite Immunol. 1993;15:261–271. doi: 10.1111/j.1365-3024.1993.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 38.Colley DG, Todd CW, Lewis FA, Goodgame RW. Immune responses during human schistosomiasis mansoni. VI. in vitro nonspecific suppression of phytohemagglutinin responsiveness induced by exposure to certain schistosomal preparations. J Immunol. 1979;122:1447–1453. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.