Summary
Chronic activation of the hypothalamic-pituitary adrenal (HPA) system is a risk factor for a variety of physical and mental disorders, and yet the complexity of the system has made it difficult to define the role of genetic and environmental factors in producing long term individual differences in HPA activity. Cortisol levels in hair have been suggested as a marker of total HPA activation over a period of several months. This study takes advantage of a pedigreed nonhuman primate colony to investigate genetic and environmental influences on hair cortisol levels before and after an environmental change. A sample of 226 adult female vervet monkeys (age 3–18) living in multigenerational, matrilineal social groups at the Vervet Research Colony were sampled in a stable low stress baseline environment and 6 months after the entire colony was moved to a new facility with more frequent handling and group disturbances (higher stress environment). Variance components analysis using the extended colony pedigree was applied to determine heritability of hair cortisol levels in the two environments. Bivariate genetic correlation assessed degree of overlap in genes influencing hair cortisol levels in the low and higher stress environments. The results showed that levels of cortisol in hair of female vervets increased significantly from the baseline to the post-move environment. Hair cortisol levels were heritable in both environments (h2 = 0.31), and there was a high genetic correlation across environments (rhoG = 0.79), indicating substantial overlap in the genes affecting HPA activity in low and higher stress environments. This is the first study to demonstrate that the level of cortisol in hair is a heritable trait. It shows the utility of hair cortisol as a marker for HPA activation, and a useful tool for identifying genetic influences on long term individual differences in HPA activity. The results provide support for an additive model of the effects of genes and environment on this measure of long term HPA activity.
Keywords: HPA axis, hair cortisol, heritability, genetic correlation, environmental stress, nonhuman primate, vervet monkey
1. Introduction
An increase in cortisol is an important component of the body's adaptive response to an acute challenge, but chronic activation of the hypothalamic-pituitary-adrenal axis can be a risk factor for a variety of human diseases (Mc Ewen, 2004; Cohen et al., 2007). Environmental factors will influence the release of cortisol, but there are also large individual differences in cortisol reactivity that may lead to differential risk for depression, cardiovascular disease and immune disorders (Silberg et al., 1999; Jennings et al., 2004; Wright et al., 2005; Kudielka and Wust, 2010; Essex et al., 2010). It is important to define the role of genetic influences and their relationship to environmental stressors for a better understanding of the processes involved.
Attempts to assess the genetic factors that influence variation in HPA activation have been thwarted somewhat by the complexity of the system. Serum cortisol levels vary widely and systematically throughout the day, and are also highly sensitive to context (Kudielka et al., 2009). Cortisol levels tend to increase to a peak approximately 30–45 minutes after awakening, known as the cortisol awakening response (Wust et al., 2000), and then decline throughout the day, with a rise in response to acute stressors. The search for genetic influences on the stress response system has found relatively consistent evidence for the heritability of the cortisol awakening response in children and adults, particularly when local environmental influences are controlled (Wust et al., 2000; Bartles et al., 2003; Kupper et al., 2005; Ouellet-Morin et al., 2009; Franz et al., 2010). Results for heritability of afternoon cortisol levels and cortisol reactivity to an acute stressor are less consistent, however. Most studies failed to find heritability for afternoon cortisol levels (Wust et al., 2000; Kupper et al., 2005), or report heritable afternoon levels in one context but not another (Franz et al., 2010). Steptoe et al. (2009) reported heritability of reactivity to an experimental stressor in children, but other studies have failed to find consistent genetic effects on cortisol response to an acute stressor (Kirschbaum et al., 1992; Wust et al., 2005; Ouellet-Morin et al., 2008).
Part of the inconsistency in finding genetic influences on HPA axis activity may be due to the sensitivity of the HPA response to the excitement and anticipation of the experimental procedures, or to variation in collection methods, time of day, diet, exercise or uncontrolled environmental stressors (Kudielka et al., 2009). Most research in this area uses plasma or saliva to assess cortisol levels, and both reflect changes in HPA activity that occurred within minutes before collection. In the past few years, several studies have explored the feasibility of using hair samples as a long-term integrated measure of HPA axis activity and an objective measure of chronic stress (Sauve et al., 2007; Gow et al., 2010). Research with rhesus monkeys showed that cortisol levels in hair correlate positively with salivary cortisol levels, and increase in response to the stress of being moved to a different environment (Davenport et al., 2006). In humans, hair cortisol levels were higher in patients with chronic stress from pain (van Uum et al., 2008), in hospitalized infants (Yamada et al., 2007), and in the long term unemployed (Dettenborn et al., 2010). Cortisol levels in hair discriminate hypercortisolemic Cushing's disease patients from controls, and reflect changes in disease course and response to treatment (Thomson et al., 2010). Hair cortisol is also sensitive to the typical increase in cortisol in late pregnancy (Kirschbaum et al., 2009; Kramer et al., 2009) Case control studies showed that myocardial infarction (MI) patients had higher hair cortisol content than control subjects (Pereg et al., 2010) suggesting that chronic stress may have been a contributing factor leading to the development of the acute MI. Together, these studies show that cortisol levels in hair can be used to measure endogenous cortisol levels over a period of several months in humans.
While most of the above studies focused on environmental effects, hair cortisol can also be a useful tool in measuring genetic influences on trait-like individual differences in HPA response. Here we report levels of cortisol in hair in a large extended pedigree of vervet monkeys sampled before and after relocation from a low stress to a higher stress environment. The vervet pedigree allows determination of the heritability and genetic correlation of hair cortisol in both environments, using variance components analysis (Almasy & Blangero, 1998). The genetic correlation measures the degree of overlap in the genetic influences in the two environments, thus providing the opportunity to determine if the higher stress environment uncovered genetic influences that were undetected in the baseline setting (Mahaney et al., 1999; Martin et al., 2010). A genetic correlation of 1.0 would indicate the same genes contribute to the variance in both environments, while a low genetic correlation would be consistent with the gene-environment hypothesis that the effects of risk genes are more evident in a high stress environment (Caspi & Moffit, 2006).
2. Methods
2.1 Subjects
Subjects were 226 adult female vervet monkeys (Chlorocebus aethiops sabaeus) (age 3–18 years) living in 16 multigenerational, matrilineal social groups at the Vervet Research Colony (VRC). All subjects were born at the VRC and raised in social groups that were managed to reflect the natural social composition of vervet groups in the wild. Infants and juveniles were raised by their mothers; female offspring remained with their mothers and female kin as adults, while males were removed from the natal group at adolescence and introduced into new groups as adults for breeding.
2.2 Low and higher stress environments
The VRC was originally established in 1975 with vervets captured from St. Kitts, West Indies. The original VRC facility was bounded by a high fence in a quiet park-like setting on the grounds of the Veterans Administration Greater Los Angeles Healthcare System, Sepulveda campus. The monkeys were housed in outdoor enclosures varying in size from 30–117 square meters of ground area (mean = 61 m2), with adjacent indoor shelters. Each outdoor corral had one or two large platforms and multiple perches, climbing structures and enrichment devices. Care was provided by a small and consistent staff of animal technicians. In the six months prior to the baseline sample collection (Dec 2007–Jan 2008), the only disturbances were for routine cage cleaning, home group behavioral tests, and infrequent clinical interventions.
In Jan–Feb 2008, the entire colony was transported across country to a new facility at Wake Forest University Primate Center. During the quarantine period in Feb–April 2008, the monkeys were captured and anesthetized 5 times at two week intervals. The facility, husbandry procedures and animal care staff were unfamiliar, and the rate of internal and external disturbances was increased compared to the baseline environment. Adult female and immature monkeys remained in the same social group after the move, but 11 of the 16 groups had new breeding adult males introduced in the new environment in Jan–Feb 2008.
Both facilities were fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AALAC), and the colony was managed in accordance with the Guide for Care and Use of Laboratory Animals (NIH, 1996) and The Psychological Well-Being of Nonhuman Primates (ILAR, 1998) before and after the move. All relevant procedures were approved by the Institutional Animal Care and Use committees of UCLA, the Department of Veterans Affairs, and Wake Forest University Health Sciences.
2.3 The VRC pedigree
The colony management practice of maintaining females in stable matrilines and transferring males between groups has produced one large, complex, extended pedigree spanning eight generations. In the vervet polygamous mating system, the highest ranking male typically fathers more than half of the offspring, resulting in a large number of individuals related through the paternal line. The pedigree was constructed using a panel of 14 highly polymorphic microsatellite markers to determine paternity and verify maternity (Newman et al., 2002). For the 226 subjects in this study, all had genetically verified mothers and fathers, except for 10 subjects who were assigned a dummy father. The number and types of relative pairs in this sample include 44 parent-offspring, 119 full and half siblings, 9 grandparent-grandoffspring dyads, 545 avuncular or grand avuncular relationships and 33,543 cousins (half, full, 1st, 2nd, 3rd, once or twice removed).
2.4 Hair cortisol collection and assay
Hair samples were collected in the stable baseline environment, and 6 months after relocation to the Wake Forest University Primate Center. Baseline hair samples were collected in California when the animals were anesthetized for the annual veterinary examination between December 2007 and January 2008. Post move samples were collected between July – August 2008 during the triannual TB test and veterinary examination at Wake Forest. For both collections, all members of a social group were run into a capture tunnel and anesthetized with 8–10 mg/kg ketamine hydrochloride. Using electric hair clippers, the full length of hair (average length 5.6 + 0.7 cm) from a 4 × 4 cm area was shaved from the center of the back between the shoulder blades, taking care not to damage the skin. The hair had fully re-grown when the post-move sample was collected from the same region, 27–35 weeks later (25–29 weeks following the move to the Wake Forest). The hair for each individual was wrapped in aluminum foil, placed in individual plastic bags, and stored in a dark, temperature controlled environment until overnight shipment to Colorado for analysis.
Hair cortisol analysis followed the method of Davenport et al. (2006). In brief, entire hair sample was washed two times in 5 ml 99% isopropyl alcohol and allowed to dry for 4 or more days under a hood, after which a portion of the washed hair was ground in a ball mill (Retsch MM200) at 25 hz for 15 min. Fifty mg of the powdered hair was extracted overnight in 1ml 100% HPLC grade methanol, and 0.6 ml of the extraction medium was dried under a nitrogen stream at 38°C for 45 min. The dried samples were reconstituted in 0.4 ml assay buffer from the enzyme immunoassay (EIA) (Expanded range, high sensitivity salivary cortisol EIA, #3001, Salimetrics LLC). Twenty five microliters of the reconstituted samples in assay buffer was plated in duplicate to the wells of the microtiter plate and assayed according to manufacturer's instructions (Salimetrics, LLC). Plates were read on a Biotek microtiter plate reader at 450 nm. Standard curves were determined using Gen Five software from which the unknowns were estimated. In order to establish assay variability, a pool of human hair was ground, frozen at −70°C, and then extracted at the time of each assay and added to each plate with unknown samples. Any duplicates with a CV greater than 10% were rerun in triplicate and the median value was reported. Within and between assay coefficients of variability were 4.0 and 9.1% respectively.
2.5 Statistical analysis
Cortisol levels (pg/mg) from the pre-move sample, the post-move sample, and the pre-post change scores (post-move – pre-move levels) were examined for distributional qualities and covariates, using SPSS 17.0. Age, reproductive status, dominance rank, clinical events, new male introduction and transfer date were screened as possible covariates. Paired t-test was used to compare hair cortisol values in the baseline and post-move environments, with repeated measures analysis of variance to control for effects of covariates. Raw cortisol data were log transformed to meet the distributional assumptions of the statistical genetics analyses.
Heritability and genetic correlations were estimated by variance components models using SOLAR (Sequential Oligogenic Linkage Analysis Routines, version 4.1.5), a computer package designed for variance component and linkage analysis in pedigrees of arbitrary size and complexity (Almasy & Blangero 1998). The variance component analysis included an additive genetic component (matrix of genetic relationships among all subject pairs times the proportion of phenotypic variance attributable to genetic variation), plus the shared environmental effects (matrix of shared environmental variables times the proportion of variation attributable to those shared environmental effects), plus a term for the unique environment variation and error. Genetic contributions to the phenotypic covariance were estimated using relatedness information from the full pedigree. In this analysis, we measured the effects of shared environments (c2) by incorporating a matrix identifying individuals that were raised by the same mother. This creates a parameter which corresponds to the fraction of the variance associated with the effect of a common maternal environment. Final models included covariates only if their contribution to the variance met a threshold value of p ≤ 0.05 using log likelihood ratio tests. Values of p ≤ 0.05 were considered significant.
Bivariate analysis was performed to estimate the genetic correlation between hair cortisol values in the baseline and post move environments, using SOLAR. For this analysis, the phenotypic variance-covariance matrix is partitioned into the additive-genetic and environmental variance-covariance matrices using the full pedigree information. The additive genetic correlation (rhoG) estimates the additive effects of shared genes on the covariance of hair cortisol levels in the two environments. A genetic correlation significantly different from zero indicates the two measures share susceptibility genes. A genetic correlation of one indicates that all susceptibility genes are shared (Mahaney et al., 1999).
3. Results
3.1 Demographic covariates
Baseline environment. Hair cortisol levels in the baseline environment were not significantly affected by age within the age range (3–18) tested here (r = 0.08, df = 224, ns), and there were no significant effects of female dominance rank (rank coded as high, middle, low: r = −0.01, df = 224, ns). Only 2 of the females in the baseline sample were detectably pregnant and 5 had given birth within the past 3 months. None of these 7 females delivered within 1 month of sample collection, and they did not differ from the remaining females in baseline hair cortisol level (t = 0.09, df = 224, ns). None of the adult females in the baseline sample had experienced experimental procedures or significant clinical interventions in the three months prior to sample collection.
Post-move environment and pre-post change scores. A number of females were pregnant or had recently delivered an infant when they were sampled in the post move environment, and there was a significant positive correlation of hair cortisol levels with gestation month (r = 0.24, df=224, p<0.001). The duration of pregnancy for vervet females is approximately 5.5 months, and the period of cortisol accumulation in hair for the females who were sampled within 1 month before or after delivery probably included most or all of the final third of pregnancy. Post hoc tests indicated that females sampled within 1 month of delivery had significantly higher hair cortisol levels compared to non-pregnant females and females sampled earlier in pregnancy (delivered within 1 month: n = 71, mean = 71.7; delivered later or not pregnant: n = 155, mean = 64.8, t=2.99, df=224, p < 0.01). Pre-post change scores were significantly affected by pregnancy status (t = 4.05, df=224, p < 0.01) and by age, with the trend of younger females showing a greater increase in cortisol levels than older females (r = −.18, df = 224, p < 0.01). None of the other variables screened as covariates (dominance rank, new male introduction, clinical events) were significantly related to post move cortisol or pre-post change scores in this adult female sample.
3.2 Effects of environmental change on hair cortisol level
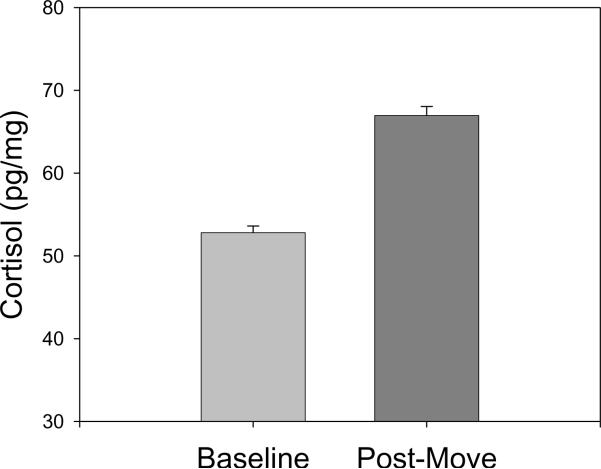
Figure 1 shows the mean (+s.e.) of hair cortisol levels for adult females sampled before and 6 months after the move from the low stress baseline environment to the higher stress post-move environment. There was a 27% mean increase in hair cortisol from before to after the move (paired t = 12.89, df = 225, p<0.001). Fifteen percent of the females had equal or lower hair cortisol levels in the post move sample, while 85% had higher hair cortisol levels in the new environment. The increase in hair cortisol levels in the post-move environment remained significant after controlling for pregnancy status and age (F(1,224) = 47.5, p<.01).
Figure 1.
Mean (+ se) hair cortisol level in the baseline environment and 6 months after relocation to the higher stress environment.
While cortisol levels increased in the post-move environment, the pre- and post-move scores were significantly correlated (r = 0.41, df = 224, p < 0.01), indicating a degree of continuity across environments (Figure 2). The pre-post correlation increased slightly when pregnancy status and age were controlled (r = 0.45, df = 222, p < 0.001).
Figure 2.
Phenotypic correlation of individual differences in log transformed hair cortisol in the baseline and post move environments.
3.3 Genetic influences on hair cortisol levels
Table 1 shows the heritability and maternal contributions for pre-move and post-move cortisol levels and pre-post change scores, with the bivariate correlation for cortisol levels between environments. The variance components analysis provided support for the hypothesis that there are significant genetic contributions to variation in hair cortisol levels in the baseline environment (h2 = 0.31, p<0.01) (Table 1). In the higher stress post-move environment, pregnancy status was a significant covariate accounting for 4% of the variance, and the genetic contribution to post-move hair cortisol level was similar to the baseline value (h2 = 0.31, p<0.01). Pregnancy status and age accounted for 10% of the variance in the pre-post change scores, and the heritability did not reach statistical significance (h2 = 0.19, p=0.11). There were no significant maternal effects for any of the three cortisol measures.
Table 1.
Heritability and genetic correlation (rhoG) of hair cortisol levels in the baseline and post move environments for 226 vervet monkey females from an extended pedigree.
| Variable | Genetic Component | Maternal Component | Covariates | Bivariate correlation | |||
|---|---|---|---|---|---|---|---|
| h2 (se) | p | c2(se) | p | (% of variance) | rhoG (se) | p | |
| Baseline cortisol | 0.31 (.13) | =.001 | 0.07 (.10) | ns | - | Baseline and Post move 0.79 (.33) | p from 0 = .04 p from 1, ns |
| Post move cortisol | 0.31 (.14) | =.008 | 0.00 | ns | Pregnancy status (4%) | ||
| Pre-post change | 0.19 (.17) | ns | 0.06 (.10) | ns | Pregnancy status, age (10%) | ||
The bivariate analysis produced a genetic correlation (rhoG) between the baseline and post-move hair cortisol levels of 0.79. This value was significantly greater than 0 (p<0.05) and not significantly different from 1.0, and is consistent with substantial overlap in the genes that influence variation in cortisol in both environments (Table 1).
4. Discussion
This study provides the first evidence for significant genetic contributions to individual differences in hair cortisol levels under baseline conditions and under conditions of significant environmental challenge. Individual differences in basal HPA activity and reactivity are notoriously difficult to measure, given the sensitivity of the system to time of day, diet, exercise, and sample collection procedures (Kudielka et al., 2009; Kudielka and Wust, 2010). The results of this study are consistent with evidence for heritability of the cortisol awakening response (Bartles et al., 2003; Wust et al., 2000; Kupper et al., 2005; Ouellet et al., 2009; Franz et al., 2010) and suggest that hair cortisol may provide an effective integrated measure of long-term HPA activity that can be useful in the search for genetic influences on stress response pathways.
The results of this study also replicated the effects of a major environmental stressor on mean cortisol levels assessed in hair, and add to the growing body of evidence that hair cortisol is an effective and simply collected marker for long term activity of the HPA system in response to persistent environmental challenge. A similar increase in hair cortisol levels was found following relocation to a new environment in rhesus monkeys (Davenport et al., 2006; 2008) and the stress of chronic pain, neonatal hospitalization and long term unemployment has been reflected in hair cortisol in humans (VanUum et al., 2008; Yamada et al., 2007; Dettenborn et al., 2010). The present study also confirmed the sensitivity of vervet hair cortisol to increases in circulating cortisol in late pregnancy found in human studies (Kirschbaum et al., 2009; D'Anna et al., in review).
We do not have a precise estimate of hair growth rates or the time course of cortisol accumulation in vervet hair. In their study of rhesus monkeys, Davenport et al. (2006) shaved sections of hair 3–4 months prior to new hair collection, thus allowing them to specify the time window for the increase in hair cortisol following relocation to new housing more accurately. They also found no significant difference in cortisol levels between the proximal and distal portions of the hair shaft. The vervet hair samples, reported here, showed significantly higher levels of cortisol in hair sampled 25–29 weeks after the cross country transport, and approximately 4 months after the end of the quarantine period. Our methodology does not allow us to determine whether the higher post-move cortisol levels included these more intense stressors, or whether they simply measured response to the ongoing environmental disturbances following quarantine. The finding of higher levels of cortisol in hair of vervet females sampled in late pregnancy suggests the possible weighting of the prior 1–2 months in hair cortisol accumulation.
The results of the present study indicated a relatively high degree of overlap in the genetic influences on hair cortisol levels in both low and higher stress environments, and did not provide evidence for a major role for gene-environment interactions. There has been considerable interest in gene-environment interactions to explain inconsistency in candidate gene studies of risk for psychopathology (Rutter, 2009). Most studies of gene-environment interactions have focused on extreme early adversity as a risk factor that interacts with genetic vulnerability to produce depression or other biobehavioral disorders (Caspi and Moffit, 2006; Suomi, 2007). In research on cortisol reactivity, studies with human infants and nonhuman primates have provided preliminary evidence for interactions of different genetic polymorphisms with early life stress (Barr et al., 2004; Chen et al., 2010; Luijk et al., 2010), and recent stressful life events were found to interact with the serotonin transporter promoter polymorphism in cortisol response to an experimental psychosocial stressor (Alexander et al., 2009).
The early adversity hypothesis could not be tested in the present study as none of the subjects had experienced mother-infant separation or abusive treatment during early development. The environments studied here were within the range of mild to moderate levels of stressor exposure. The monkeys were socially housed in stable matrilineal groups that were maintained after the move to the new environment, and mean levels of cortisol in hair were considerably lower than levels reported for individually housed monkeys in other studies (Davenport et al., 2006; 2008). Under these circumstances, we found evidence for an additive role of genetic influences and current environmental stress on a long term measure of HPA activity. Almost all subjects showed an increase in hair cortisol in the more challenging environment, and subjects who had relatively high cortisol levels in the baseline environment tended to have high levels in the post move environment. Individual differences in cortisol response to the change in environment were influenced by pregnancy status and female age, but we did not find evidence for a significant genetic contribution to the individual pre-post change scores. This result, combined with the relatively high genetic correlation, does not dismiss the possibility of candidate gene-environment interactions in HPA activity, but it does not provide support for a major role for gene-environment interactions under these circumstances.
Longitudinal research on other neurobehavioral phenotypes has demonstrated similar stability of genetic influences over time and across circumstances. In studies of twins, shared genetic influences across developmental stages have been reported for internalizing and externalizing behavior problems (Haberstick et al., 2005), reading difficulties (Astrom et al., 2007) and brain morphology (Pfefferbaum et al., 2004). Longitudinal bivariate analysis of body mass index (BMI) over a 25 year period showed significant increases over time, with a high genetic correlation suggesting that the same genes influenced variation in BMI at both time points (Martin et al., 2010). These longitudinal studies are consistent with our current findings of relative consistency in the genetic influences on hair cortisol levels before and after a major environmental change.
In summary, the results of this study are important in several respects. They are the first demonstration of significant genetic contributions to individual differences in hair cortisol levels. They support the hypothesis that hair cortisol is a marker for increased HPA activity in response to persistent environmental stressors. They also show substantial overlap in the genes that influence hair cortisol levels in low and higher stress environments, a finding that is consistent with an additive model of genetic and environmental influences on long term HPA activity. These results support the value of hair cortisol as a useful measure for identifying genetic factors that add to chronic environmental stress in creating increased risk for psychiatric, immunological and cardiometabolic disorders.
Acknowledgements
The authors would like to thank Susan Service for her role in the construction of the vervet pedigree, and Anthony Comuzzie for valuable consultation on the statistical genetics methods. We would like thank Jill Byrnit and Glenvile Morton for assistance with hair collection, and Africa Armendariz and Crystal Natvig for their expert assistance in processing and analyzing the hair samples for this study. We also thank Steven Shapiro for the loan of the ball grinder for processing initial samples in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: Implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom RL, Wadsworth SJ, DeFries JC. Etiology of the stability of reading difficulties: the longitudinal twin study of reading disabilities. Twin Res. Hum. Genet. 2007;10:434–439. doi: 10.1375/twin.10.3.434. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwand M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol. Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bartles M, de Geus EJC, Kirschbaum C, Sluyter F, Bloomsma DI. Heritability of daytime cortisol levels in children. Behavior Genetics. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Chen G-L, Novak MA, Meyer JS, Kelly BJ, Vallender EJ, Miller GM. The effect of rearing experience and TPH2 genotype on HPA axis function and aggression in rhesus monkeys: A retrospective analysis. Horm. Behav. 2010;57:184–191. doi: 10.1016/j.yhbeh.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. J.A.M.A. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Lutz CK, Tienfenbacher S, Novak MA, Meyer JS. A rhesus monkey model of self-injuty: Effects of relocation stress on behavior and neuroendocrine function. Biol. Psychiatry. 2008;63:990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettenborn L, Tietze A, Brucker F, Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.04.006. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am. J. Psychiatry. 2010;167:40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CE, York TP, Eaves L, Mendoza SP, Hauger RL, Hellhammer DH, Jacobsen KC, Levine S, Lupien SJ, Lyons MJ, Prom-Wormley E, Xian H, Kremen WS. Genetic and environmental influences on cortisol regulation across days and contexts in middle-aged men. Behav. Genet. 2010;40:467–479. doi: 10.1007/s10519-010-9352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow R, Thomson S, Rieder M, Van Uun S, Koren G. An assessment of cortisol in hair and its clinical applications. Forensic Sci. Int. 2010;196:32–37. doi: 10.1016/j.forsciint.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Schmitz S, Young SE, Hewitt JK. Contributions of genes and environments to stability and change in externalizing and internalizing problems during elementary and middle school. Behav. Genet. 2005;35:381–396. doi: 10.1007/s10519-004-1747-5. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production: Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J. Clin. Endocrinol. Metab. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am. J. Epidemiol. 2009;169:1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Wust S. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituatary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Kupper N, de Geus EJC, van den Berg M, Kirschbaum C, Bloomsma DI, Willemsen G. Familial influences on basal cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Luijk MPCM, Velders FP, Tharner A, van IJzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VWV, Hofman A, Verhulst FC, Tiemeier H. FKBP5 and resistant attachment predict cortisol reactivity in infants: Gene-environment interaction. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.04.012. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Mahaney MC, Blangero J, Rainwater DL, Mott GE, Comuzzie AG, MacCluer JW, VandeBerg JL. Pleiotropy and genotype by diet interaction in a baboon model for atherosclerosis: A multivariate quantitative genetic analysis of HDL subfractions in two dietary environments. Arterioscler. Thromb. Vasc. Biol. 1999;19:1134–1141. doi: 10.1161/01.atv.19.4.1134. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Woo JG, Morrison JA. Evidence of shared genetic effects between pre- and postobesity epidemic BMI levels. Obesity. 2010;18:1378–1382. doi: 10.1038/oby.2009.394. [DOI] [PubMed] [Google Scholar]
- Mc Ewen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the psychopathology of psychiatric disorders. Ann. N.Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Newman TK, Fairbanks LA, Pollack D, Rogers J. Effectiveness of human microsatellite loci for assessing paternity in a captive colony of vervets (Chlorocebus aethiops sabaeus) Amer. J. Primatol. 2002;56:237–243. doi: 10.1002/ajp.1078. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Boivin M, Dionne G, Lupien SJ, Arsenault L, Barr RG, Perusse D, Tremblay RE. Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Arch. Gen. Psychiatry. 2008;65:211–218. doi: 10.1001/archgenpsychiatry.2007.27. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Dionne G, Perusse D, Lupien SJ, Arseneault L, Barr RG, Tremblay RE, Boivin M. Daytime cortisol secretion in 6-month-old twins: Genetic and environmental contributions as a function of early family adversity. Biol. Psychiatry. 2009;65:409–416. doi: 10.1016/j.biopsych.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, Koren G. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress. 2010 doi: 10.3109/10253890.2010.511352. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullican EV, Carmelli D. Morphological changes in aging brain structures are differently affected by time-linked environmental influences despite strong genetic stability. Neurobiol. Aging. 2004;25:175–183. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Rutter M. Gene-environment interaction: Biologically valid pathways of artifact? Arch. Gen. Psychiatry. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- Sauve B, Koren G, Walshe S, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Invest. Med. 2007;30:E183–191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Carbonneau R, Murrelle L, Foley D, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Arch. Gen. Psychiatry. 1999;56:225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Steptoe A, van Jaarsveld CHM, Semmler C, Plomin R, Wardle J. Heritability of daytime cortisol levels and cortisol reactivity in children. Psychoneuroendocrinology. 2009;34:273–280. doi: 10.1016/j.psyneuen.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann. N.Y. Acad. Sci. 2007;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SH, Van Uum SHM. Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp. Clin. Endocrinol. Diabetes. 2010;118:133–138. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Uum SH, Sauve B, Fraser LA, Morley-Forster P, Paul TL, Koren G, Van Uum SHM. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11:483–8. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr. Opin. Allergy Clin. Immunol. 2005;5:23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and free cortisol response to awakening. Psychoneuroencocrinology. 2000;25:707–725. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko IS, van Rossum EFC, Kroper JW, Hellhammer DH. Habituation of cortisol responses to repeated psychosocial stress: Further characterization and impact of genetic factors. Psychoneuroendocrinology. 2005;30:199–211. doi: 10.1016/j.psyneuen.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007;92:42–49. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]