Abstract
Ischemia/reperfusion (I/R) injury remains a key risk factor significantly affecting morbidity and mortality after liver transplantation (LTx). B7-H1, recently identified member of the B7 family, is known to play important roles in regulating local immune responses. We hypothesized that B7-H1 plays crucial roles during innate immune responses induced by hepatic I/R injury and tested this hypothesis in the mouse LTx model using B7-H1 KO liver grafts with 24 hr cold storage. Cold I/R injury in WT to WT LTx enhanced constitutive B7-H1 expression on dendritic cells and sinusoidal endothelial cells, and promptly induced B7-H1 on hepatocytes. When B7-H1 KO liver grafts were transplanted into WT recipients, serum ALT levels and graft necrosis were significantly higher than WT to WT LTx. Augmented tissue injury in B7-H1 KO grafts was associated with increased frequencies and absolute numbers of graft CD3+ T cells, in particular CD8+ T cells. B7-H1 KO grafts had significantly lower incidences of Annexin V+ CD8+ T cells, indicating the failure to delete infiltrating CD8+ T cells. To evaluate the relative contribution of parenchymal and bone marrow-derived cell (BMDC) B7-H1 expression, chimeric liver grafts lacking B7-H1 on parenchymal cells or BMDC were generated and transplanted into WT recipients. Selective B7-H1 deficiency on parenchymal cells or BMDC resulted in similar levels of ALT and liver injury, suggesting that both parenchymal and BMDC B7-H1expression is involved in the control of liver damage. Human livers upregulated B7-H1 expression after LTx. Conclusion: The study demonstrates that graft tissue expression of B7-H1 plays critical roles in regulating inflammatory responses during LTx-induced hepatic I/R injury, and suggests that negative coregulatory signals may have an important function in hepatic innate immune responses.
Keywords: apoptosis, T cells, hepatocyte, nonparenchymal cells, dendritic cells
Introduction
Liver damage caused by ischemia/reperfusion (I/R) of liver grafts represents a highly complex cascade of events leading to hepatocellular injury after liver transplantation (LTx). These events are triggered when the liver is transiently exposed to hypoxic and hypothermic conditions and reperfused with warm and oxygenated blood. The procedure is unavoidable during transplantation surgery, and every liver graft suffers from some degree of I/R injury. Liver I/R injury represents a serious problem in LTx, significantly affecting patient and graft outcomes (1, 2). It has been reported that in a large series of living- and deceased-donor liver transplant patients, a longer cold ischemic time associates with a higher frequency of early graft failure, and a higher rate of acute cellular rejection (3). Moreover, I/R injury contributes to donor organ shortage because of the higher susceptibility of marginal livers to the ischemic insult. To date, there is no specific treatment available to prevent or reduce hepatic I/R injury and current treatment is based merely on supportive care. Thus, extensive research efforts to better understand mechanisms of hepatic I/R injury after LTx are warranted.
B7-H1 (also named as CD274 or programmed death 1 ligand [PD-L1]) is a recently-identified member of the B7 family with important regulatory functions in cell-mediated immune responses (4, 5). Together with the PD-1 receptor, B7-H1 is known to play an important role in regulating local immune responses in infection (6, 7), autoimmunity (8, 9) and alloimmunity (10-12). PD-1 is a member of the CD28 family expressed by activated CD4 and CD8 T cells, B cells and myeloid cells (13, 14). In contrast, B7-H1 is expressed by antigen-presenting cells (APC), including dendritic cells (DC), monocytes and B cells upon stimulation (15). Moreover, B7-H1 can be detected in parenchymal cells in non-lymphoid organs including hepatocytes (16). A growing number of reports suggest a crucial role of B7-H1 expression in the liver in the regulation of local immune responses. It has been reported that the interaction with B7-H1 in the liver selectively deletes activated CD8+ T cells (17). Moreover, the spontaneous acceptance of mouse liver grafts is prevented when the grafts lack B7-H1 expression, due to reduced apoptosis of graft-infiltrating host CD8+ T cells (18).
In this study we hypothesized that hepatic expression of B7-H1 plays crucial roles during innate immune responses induced by hepatic I/R injury and tested this hypothesis directly in the mouse LTx model with prolonged 24 hours cold preservation using B7-H1 KO mice. We show that lack of B7-H1 in the liver graft significantly worsens hepatic I/R injury and is associated with an increased infiltration and reduced apoptosis of CD8+ T cells in the liver graft. Using bone marrow (BM) transplantation to generate chimeric donor liver grafts, we also show that B7-H1 expression on both hepatocytes and BM-derived cells (BMDC) in liver grafts is important in regulating T cell infiltration and the extent of liver I/R injury.
Materials and Methods
Reagents
Collagenase B was purchased from Boehringer (Ridgefield, CT). Trypsin inhibitor, BSA, EDTA, and EGTA were obtained from Sigma (St Louis, MO). Ca+Mg+-free HBSS, HEPES Buffer, RPMI-1640, L-glutamine, FBS, and gentamicin were from Life Technologies (Grand Island, NY). FITC-, PE-, PE-Cy5-, PE-Cy7-, or Pacific Blue-conjugated monoclonal antibodies (mAbs) directed against mouse CD3 (145-2C11), CD4 (H129.19), CD8 (53-6.7), CD11c (HL3), CD11b (M1/70), CD31 (390), CD45 (30-F11), CD45.1 (A20), B220/CD45R (RA3-6B2), IAb β-chain (MHC class II, 25-9-17), NK1.1 (pk136), and B7-H1 (MIH5), as well as appropriate Ig isotype controls were obtained from eBioscience (San Diego, CA) or BD PharMingen (San Diego, CA).
Animals
Male 8- to 12-wk old C57BL/6 (B6, H-2b), B6.CD45.1, or GFP-transgenic mice were purchased from The Jackson Laboratory. B7-H1-deficient B6 mice (B7-H1 KO) (17) were bred from pairs kindly provided by Dr. Lieping Chen, Johns Hopkins University School of Medicine, Baltimore MD and maintained in the specific pathogen-free facility at the University of Pittsburgh School of Medicine.
LTx
The basic techniques of liver harvesting and orthotopic liver transplantation without hepatic artery reconstruction were based on the method described by Qian et al (19), with minor modifications, as described (20). Liver grafts were perfused with 1.0 ml of UW solution via the portal vein, stored in UW solution for 24 hrs at 4 °C, and implanted orthotopically by anastomosing the suprahepatic vena cava with a running 10–0 suture and the portal vein and inferior vena cava with the cuff technique. Anhepatic time averaged 19.8±1.7 min. The bile duct was connected via the ligation over the stent.
BM Chimeras
BM cells were collected from long bones of the extremities of WT or KO mice, and 2 × 107 unfractionated cells were injected intravenously into lethally (9.5 Gy) irradiated WT or KO mice via the tail vein. Animals were used as liver graft donors >2 months after BM transplantation. Additionally, replacement of BMDC in the liver was confirmed in GFP radiation chimeras.
Experimental Design
Syngenic LTx was performed using KO, WT, or chimeric animals as donors and WT B6 or B6 CD45.1 mice as recipients with 24 hr cold storage. Recipient animals were euthanized 1, 3, 6, 12 and 24 hr after reperfusion to obtain serum and liver graft samples. All procedures in this study were performed according to the guidelines of the National Institutes of Health's Guide for Care and Use of Laboratory animals and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Routine and Immunohistopathology
Liver samples were fixed in 10% formalin, embedded in paraffin, sectioned (6 μm), and stained with H&E. Grafts were also embedded in OCT, and 6 μm cryosections were stained with anti-CD3 and anti-CD11b mAb with nuclear Hoechst staining. Sections were visualized with an Olympus BX51 epifluorescence microscope, and 2-dimensional images were digitized with an Olympus/Optronics (Goleta, CA, USA) CCD camera, interfaced with MagnaFire Image Capture Software.
Real-time RT-PCR
mRNA expression was quantified by SYBR Green real-time RT-PCR using ABI PRISM 7000 Sequence Detection System (PE Applied Biosystems) as described (22) and with primers for B7-H1 (17) and other inflammatory and death-related molecules. The expression of each gene was normalized to β-actin mRNA content and calculated relative to normal liver.
Isolation of Hepatocytes and Non-parenchymal Cells (NPC)
Hepatocytes and hepatic NPC were isolated from the liver grafts by the collagenase digestion method (21), with some modifications (22). Briefly, the liver was perfused in situ via the infrahepatic inferior vena cava, initially with 20 ml of Ca+Mg+-free HBSS containing 5 mM EGTA and 10 mM HEPES, then with 100 ml of HBSS containing 0.025% collagenase B, 5 mM HEPES, 56 mg of CaCl2, and 0.005% trypsin inhibitor. NPC and parenchymal cells were liberated from removed liver grafts, and the initial cell suspension was filtered through 70 μm nylon mesh. Hepatocytes and NPC were separated by low-speed centrifugation (5 × 45 g for 5 min) and washed by high-speed centrifugation (at 150 g for 10 min).
Flow Cytometric Analysis
After NPC were treated with FcR-blocking rat anti-mouse CD16/32 mAb (2.4G2) to avoid non-specific binding, five-color flow cytometry was conducted by incubating the cells for 30 min with fluorochrome-conjugated mAbs. Intragraft T cell apoptosis was detected with the Annexin V-PE apoptosis detection kit (BD PharMingen) following the manufacturer's protocol. Flow analysis was performed using a LSR II flow cytometer (BD Biosciences).
Multiplex Quantum Dot (QD) Staining of Human Liver Allografts
Analysis of human tissue was carried out according to University of Pittsburgh Institutional Review Board protocol (PRO10110393). Formalin-fixed paraffin-embedded human liver allograft biopsy sections were obtained from 3 normal livers and 16 post-reperfusion (1-4 hrs) biopsies, and analyzed with multiplex QD-based immunofluorescent staining to evaluate B7-H1 expression on specific cell types (22). Briefly, 4 μm sections were deparaffinized, hydrated, and treated with citrated buffer antigen retrieval. Triplex or quadruplex staining was performed by sequential incubation cycles of blocking, primary antibody incubation, biotinylated secondary antibody incubation and streptavidin-coated QD incubation. For each cycle, sections were blocked with avidin and biotin block kit (Vector Laboratories Inc, Burlingame, CA) and protein block (Dako, Carpinteria, CA). Primary antibodies included rabbit anti-B7-H1 (LifeSpan Bioscience, Seattle, WA) and anti-CD11c (Abcam, Cambridge, MA), and mouse anti-CD31, CD68, HepPar1 (Dako), and CK19 (Santa Cruz Biotechnology, Santa Cruz, CA). After all antibodies were stained and Hoechst nuclear staining was applied, digital images of whole stained slides were obtained with MIRAX MIDI digital whole slide scanning systems (Carl Zeiss MicroImaging, Jena, Germany) and analyzed with Pannoramic Viewer (3D Histech, Ltd, Ramsey, NJ).
Human hepatocyte culture and hypoxia
Human hepatocytes were isolated from histologically normal livers using a three-step collagenase perfusion technique (Drs. Steven Strom and Ken Dorko, University of Pittsburgh Core Pathology Facility, Pittsburgh, PA) according to an Institutional Review Board–approved protocol. After overnight culture, hepatocytes in DMEM supplemented with 10% FBS were exposed to hypoxia (1% O2) at 37°C and harvested at 1-6 hrs for RNA isolation and RT-PCR using primers for human B7-H1 (forward: 5′-CTGTCCGCCTGCAGGGCATT-3′; reverse: 5′-AACAGCCGGGCCCTCTGTCT -3′).
Statistical Analysis
Data are represented as the mean ± SEM. Comparisons between the groups at different time points were performed by Student's “t” test or ANOVA using the Statview program (Abacus Concepts, Inc., Berkeley, CA). Differences were considered significant at a P value < 0.05.
Results
After LTx cold ischemia B7-H1 is up-regulated both in hepatic parenchymal cells and NPC
Modulation of B7-H1 expression on both hepatocytes and NPC has been shown after inflammatory stimulation (23, 24). To determine B7-H1 expression after LTx with 24 hr cold ischemia, we first performed real-time RT-PCR of whole liver graft tissues. B7-H1 mRNA levels in liver grafts promptly increased after WT to WT LTx, peaking at 6 hr (Fig. 1A). When separated hepatocytes and NPC were analyzed, B7-H1 mRNA up-regulation, although seen in both fractions, was more prominent in hepatocytes than in the NPC fraction at 3 and 6 hr after LTx (Fig. 1B). Subsequent analysis of B7-H1 protein expression by flow cytometry showed that under steady-state conditions (naïve mice), B7-H1 was expressed on CD11c+ DC and CD31+ sinusoidal endothelial cells, but not on hepatocytes (Fig. 1C). After hepatic I/R injury in WT to WT LTx, B7-H1 protein expression was up-regulated in all 3 types of liver cells (Fig. 1C). In contrast, after B7-H1 KO to WT LTx, we observed a modest up-regulation of B7-H1 only on DC, most likely due to infiltrating WT recipient DC (Fig. 1C).
Figure 1. Up-regulation of hepatic B7-H1 expression with cold I/R injury after mouse liver transplantation (LTx).
(A) B7-H1 mRNA levels in liver graft samples. Liver graft tissues were harvested 1, 3, 6 and 12 hr after WT to WT LTx with 24 hr cold ischemia and analyzed by RT-PCR (n=3). Whole liver tissue from normal mice was used as control and values are expressed as fold increases. B7-H1 mRNA increased at 3 hr and peaked at 6 hr.
(B) B7-H1 mRNA levels in hepatocytes and non-parenchymal cells (NPC). Parenchymal cells and NPC were isolated after collagenase digestion as described in the Materials and Methods, at 3 and 6 hr after WT to WT LTx with 24 hr cold ischemia and analyzed by RT-PCR (n=3). B7-H1 message was detected in both hepatocyte and NPC fractions, with a significantly higher increase after LTx in parenchymal cells (*p<0.05).
(C) B7-H1 protein expression on hepatocytes, DC and sinusoidal endothelial cells (SEC). Liver NPC and hepatocytes were isolated 6 hr after WT to WT or B7-H1 KO to WT LTx with 24 hr cold preservation and analyzed for cell surface B7-H1 expression by flow cytometry. In normal naive mice, B7-H1 was expressed on CD11c+ dendritic cells (DC) and CD31+ SEC, but not on hepatocytes. After WT to WT LTx, B7-H1 was up-regulated on all of these cell types. By contrast, in KO to WT LTx, B7-H1 was not expressed on hepatocytes or SEC, although there was slight B7-H1 positivity on DC (most likely due to infiltrating host WT DC). Data are representative of three independent experiments.
B7-H1 deficiency in the graft exacerbates transplant-induced hepatic I/R injury
To directly examine the role of hepatic graft B7-H1 expression in the pathogenesis of transplant-induced liver I/R injury, severities of hepatic injury were compared between B7-H1 KO and WT grafts transplanted into WT recipients with 24 hr cold storage. Serum ALT levels at 6 and 12 hr after LTx were 1.5- to 2-fold higher in KO to WT than in WT to WT LTx (5759±549 vs 2100±368 IU/L; p<0.05; 8750±1123 vs 5867±654 IU/L, p<0.05 respectively), indicating that the lack of graft B7-H1 expression augmented hepatic I/R injury (Fig. 2A). Histopathological analysis at 12 hr confirmed increased areas of necrosis in B7-H1 KO compared with WT grafts (Fig. 2B).
Figure 2. Hepatic injury after WT or B7-H1 KO to WT LTx with 24 hr cold preservation.
(A) Serum ALT levels at 6 and 12 hr after LTx in KO to WT and WT to WT combinations (n=3-4 for each group at each time point). Serum ALT levels were significantly higher at both 6 and 12 hr in recipients given B7-H1 KO grafts compared to the WT to WT combination (*p<0.05).
(B) Representative H&E staining of liver graft tissue harvested 12 hr after B7-H1 KO or WT to WT LTx with 24 hr cold ischemia. Arrows indicate necrotic areas. Original magnification ×200.
B7-H1-deficient grafts show increased numbers of lymphocytes after LTx compared with WT grafts
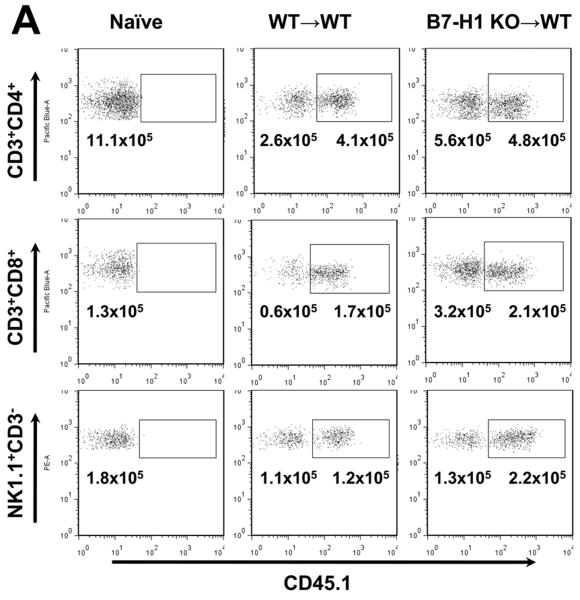
To explore the mechanisms of increased injury in liver grafts lacking B7-H1 expression, we conducted flow cytometry of hepatic NPC obtained from WT and KO grafts after 6 hr of reperfusion in WT recipients. As shown in Fig. 3A, liver CD45+CD31- cells were defined as hepatic NPC and analyzed for their phenotypes. During cold I/R injury, there was a dramatic increase of SSChigh NPC in WT to WT LTx. In contrast, KO grafts showed a notable increase of SSClow lymphoid lineage NPC. These changes reflected striking percentage increases of CD11b+ cells among hepatic NPC in WT than in KO grafts (80.7% vs 46.4%) (Fig. 3A). Likewise, increased SSClow lymphocytes in KO grafts were mostly due to higher frequencies of CD3+ T cells (Fig. 3A). Consistent with flow cytometry findings, immunofluorescence staining of liver grafts 6 hr after LTx showed more CD3+ T cells in KO grafts compared with WT grafts (Fig. 3B). Also using immunohistochemistry, more numerous CD11b+ cells were detected in WT than in KO grafts (Fig. 3B). Interestingly, CD11b+ cells typically showed focal accumulation in WT grafts (Fig. 3B, lower arrows); however, in KO grafts, they were distributed homogenously throughout the liver and did not form focal aggregates.
Figure 3. Analysis of liver non-parenchymal cells from WT or B7-H1 KO to WT LTx with 24 hr cold preservation.
(A) Flow cytometric analysis of NPC isolated from normal liver, and KO and WT grafts after 6 hr reperfusion. After gating on live CD45+CD31- cells (upper panels), liver NPC were analyzed for SSC, CD3, NK1.1, CD45R/B220, CD11c, and CD11b, and data reported as percentages of total CD45+ cells. Compared to normal livers, I/R injury in WT livers resulted in increased SSChighCD45+ cells, while KO grafts showed SSClowCD45+ lymphoid increases (second panels). The percentages of CD11b+ cells were higher in WT than in KO liver grafts at 6 hr after LTx (third panels). In contrast, the percentages of CD3+ cells were higher in KO grafts (fourth panels) due to increased SSClowCD45+ lymphoid lineage cells. Pie graphs show percentages of various lineage cells in CD45+CD31- hepatic NPC, including T cells (CD3+NK1.1-), NK cells (NK1.1+ CD3-), NKT cells (CD3+NK1.1+), B cells (B220+), DC (CD11c+), and myeloid cells (CD11b+). Data are representative of three independent experiments.
(B) Immunohistochemistry of liver grafts at 6 hr after LTx with CD3 (upper) and CD11b (lower) shows more numerous CD3+ cells (red, thin arrows) in B7-H1 KO than in WT grafts. In contrast, CD11b+ (red) cells were more frequently seen in WT than KO grafts with focal aggregation formation (thick arrows). CD11b+ cells were distributed homogenously in KO grafts. Representative image of three independent experiments. Original magnification ×200.
(C) Representative flow cytometry for NK1.1/CD3, CD3/CD8, and B220/CD11c of hepatic CD45+CD31- lymphoid NPC at 6 hrs after WT to WT and KO to WT LTx. KO liver grafts show higher percentages of T cells (CD3+NK1.1-, upper). Among CD3+ T cells, CD8+ T cells increased in KO grafts (middle). Frequencies of CD11c+ DC or B220+ B cells were not different between WT and KO grafts (lower).
(D) Absolute numbers of CD3+ T cells, as well as CD3+CD8+ and CD3+CD4+ T cells. KO grafts at 6 hrs show significantly more CD3+ T cells (NK1.1-) and CD3+CD8+ cells compared to WT grafts. NK cells and NKT cell numbers are comparable between WT and KO grafts (n=3 for each group).
CD8+ T cells are increased during cold I/R injury in B7-H1 KO grafts
As augmented I/R injury in B7-H1 KO liver grafts was associated with increased CD3+ cell infiltration, we further analyzed CD3+ and other lymphocytes in graft NPC. KO grafts had significantly higher frequencies of CD8+ T cells compared to WT grafts, while percentages of CD4+ T cells were comparable between groups. Accordingly, absolute numbers of CD3+ and CD8+ T cells were significantly higher in KO than in WT grafts (Fig. 3D). CD4+ T cells did not differ between WT and KO grafts. Frequencies and absolute numbers of other lymphoid cells, such as NK, NKT, B cells or DC, were not significantly different between KO and WT grafts.
Both donor and host CD8+ T cells are increased in B7-H1 KO grafts
To verify the host or graft origin of T cells that accumulated in KO grafts, we next analyzed liver graft NPC in WT to CD45.1 B6 LTx. Most of the CD8+ T cells in WT grafts were host-derived. KO grafts had more host CD8+ T cells than WT grafts; however, significantly more donor phenotype CD8+ T cells were found. As CD8+ cells have been shown to accumulate in the liver in naïve B7-H1 KO mice (17), these results suggest that both graft and host cells were able to survive in the B7-H1 deficient liver environment during hepatic I/R injury (Fig. 4A). The frequency of donor-type CD4+ T cells decreased after transplant, with a concomitant increase in host CD4+ T cells in WT grafts. Graft- and host NK cell frequencies did not differ significantly between WT and KO grafts (Fig. 4A).
Figure 4. Analysis of graft- and host-derived CD4 T cells, CD8 T cells and NK cells.
(A) Flow cytometric analysis of liver NPC for donor/recipient phenotype. Hepatic NPC from B7-H1 KO and WT grafts 6 hr after LTx into CD45.1 recipients were analyzed. CD3+CD4+, CD3+CD8+ and NK1.1+CD3- cells were analyzed for CD45.1 (recipient) expression. Numbers are absolute numbers in liver grafts. Data are representative of three independent experiments.
(B) T cell apoptosis was determined by flow cytometry in liver NPC. CD3+CD4+, CD3+CD8+ and NK1.1+CD3- cells were analyzed for CD45.1 and Annexin V expression. Annexin V expression by host CD45.1+ CD8, but not CD4 or NK cells was reduced in KO grafts compared with WT grafts. No differences were detected in Annexin V expression by graft CD45.1- CD4, CD8 or NK cells between KO and WT grafts. Data are representative of three independent experiments.
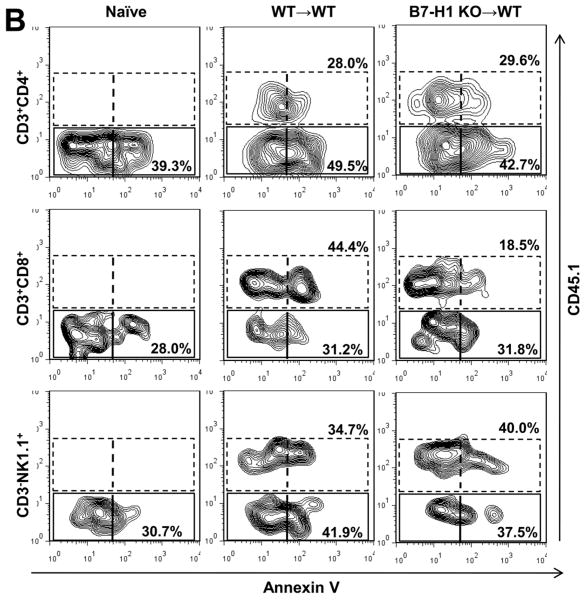
Donor CD8+ T cell apoptosis is impaired in B7-H1 KO grafts
It has been shown that B7-H1 can induce T cell apoptosis (17). We next explored the hypothesis that reduced apoptosis could be responsible for CD8+ T cell accumulation in KO grafts. To address this hypothesis, Annexin V expression by liver CD4, CD8 and NK cells after KO or WT to CD45.1 B6 LTx was examined. Appreciable numbers of CD4, CD8 and NK cells were Annexin V+ in normal livers. Annexin V expression by host CD45.1+ CD8, but not by CD4 or NK cells was reduced in KO grafts as compared with WT liver grafts. Annexin V expression by graft CD45.1- CD4, CD8 and NK cells was not different between WT and KO grafts (Fig. 4B).
B7-H1 deficient grafts show up-regulation of inflammation-related molecules
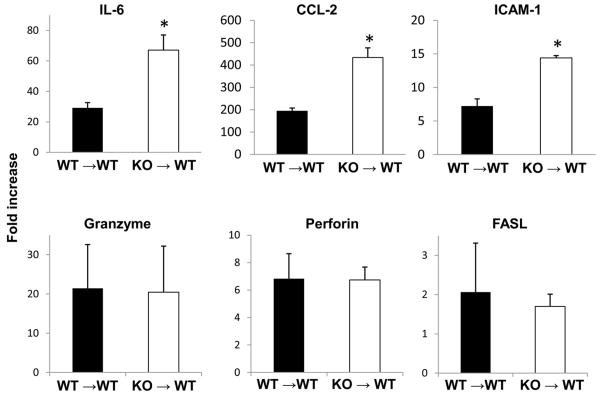
In association with severe hepatic injury in B7-H1 KO grafts, mRNA levels for IL-6, CCL-2, and ICAM were significantly higher in KO than WT grafts at 12 hrs after LTx. However, death-related molecules, such as granzyme, perforin, or FASL were not significantly different between WT and KO grafts (Fig. 5).
Figure 5. Hepatic graft mRNA levels for inflammatory molecules after WT or B7-H1 KO to WT LTx with 24 hr cold preservation.
Graft mRNA levels were determined by RT-PCR in liver graft samples obtained 12 hrs after LTx in KO to WT and WT to WT combinations. * p<0.05 vs. WT to WT LTx (n=3-4 for each group).
Expression of B7-H1 on both hepatocytes and BMDC is important in the pathogenesis of transplant-induced I/R injury
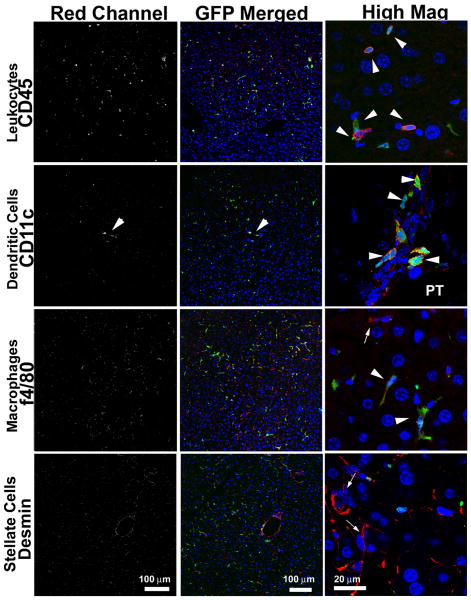
To determine the role of B7-H1 expression by hepatocytes and BMDC in the control of liver damage after transplantation, we created BM radiation chimeras using B7-H1 KO or WT BM, and generated liver grafts lacking B7-H1 exclusively in either parenchymal cells (WT/KO) or BMDC (KO/WT). These chimeric liver grafts were then transplanted into WT recipients after 24 hr cold storage. Replacement of BMDC in the liver was confirmed in B6 radiation chimera with GFP BM cells. All of CD11c+ DC and ∼60% of F4/80+ Kupffer cells were replaced with GFP+ phenotypes, while desmin+ stellate cells and parenchymal cells remained as GFP-negative phenotype (Fig. 6).
Figure 6. Replacement of BM-derived cells in the liver in radiation chimeras.
Unfractionated BM cells (2 × 107) obtained from B6 GFP-transgenic mice that constitutively express GFP were intravenously injected into 9.5 Gy whole body irradiated B6 mice. Liver samples were obtained at 30 and 75 days (n=2) after perfusion fixation with PFA for immunohistochemistry. Majority of CD45+ cells in the liver were GFP+. CD11c+ cells in the periportal area (PT) were totally replaced with GFP+ CD11c+ cells. In contrast, F4/80+ cells were composed of both GFP+ and GFP- populations. Desmin+ stellate cells, as well as parenchymal cells (hepatocytes, sinusoidal endothelial cells) remained GFP-negative phenotype.
Serum ALT levels after 12 hr reperfusion were higher compared with WT to WT liver grafts but were not significantly different between WT/KO and KO/WT grafts (Fig. 7A), suggesting that the expression of B7-H1 on both hepatocytes and BMDC regulates liver damage after LTx. Further analysis of liver NPC at 6 hr after LTx showed that the frequencies of CD3+CD8+ T cells and Annexin V expression were similar between groups (Fig. 7B).
Figure 7. Serum ALT levels and CD3+CD8+T cells in BM chimeras.
(A) For these experiments, two different types of BM chimera were set-up, as described in the Materials and Methods. KO/WT: B7-H1 KO BM into irradiated WT mice; WT/KO: WT BM into irradiated B7-H1 KO mice. Liver grafts lacking B7-H1 either on BMDC (KO/WT) or parenchymal cells (e.g. hepatocytes) (WT/KO) were transplanted two months after BM transplantation into WT recipients, and serum ALT levels were assayed 12 hr after reperfusion. No differences were detected between KO/WT and WT/KO grafts (n=3 for each group).
(B) Hepatic NPC were analyzed with flow cytometry at 6 hr after KO/WT or WT/KO to WT LTx. Frequencies of CD3+CD8+ cells, as well as percentages of Annexin V positive cells were similar between the two types of chimeric grafts.
B7-H1 was upregulated in human post-reperfusion liver grafts
To confirm the relevance of our findings in the mouse model for clinical LTx, B7-H1 upregulation was analyzed in human liver biopsy samples. B7-H1 protein expression was apparently upregulated in post-reperfusion liver graft biopsy samples, compared to normal liver samples that showed scarce B7-H1 expression (Fig. 8). In post-reperfusion biopsies, hepatocytes, particularly in the perivenular regions, became B7-H1-positive. These B7-H1 expressing hepatocytes were swollen and/or detached from hepatic column, suggesting the relation between hepatocyte injury and B7-H1 upregulation. In the sinusoids of post-reperfusion samples, B7-H1 upregulation on CD31+ endothelial cells was evident, and CD11c+CD68- DC, and CD68+ Kupffer cells also occasionally expressed B7-H1. In normal livers, rare B7-H1 expression was only seen on CD31+ endothelial cells, and other types of cells were negative for B7-H1. CK19+ biliary epithelial cells did not show B7-H1 in normal livers and post-reperfusion samples (data not shown).
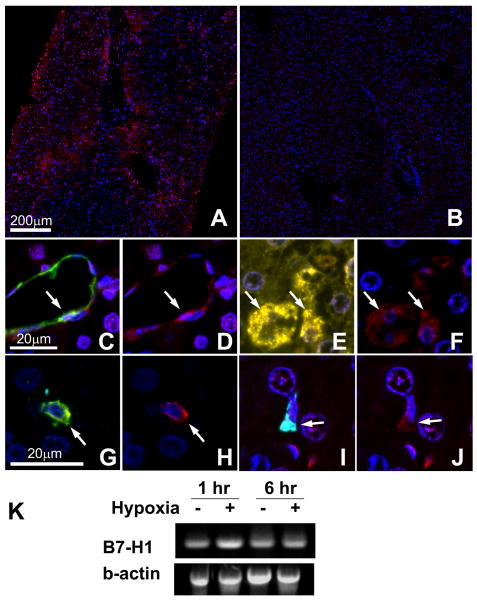
Figure 8. B7-H1 expression in human liver graft biopsy samples and cultured hepatocytes.
Liver graft biopsy samples (n=3 for normal, n=16 for post-reperfusion) were analyzed by multiplex quantum dot (QD) staining using antibody combinations of B7-H1 (red)/CD31 (endothelial cells, green)/CK19 (biliary epithelial cells, cyan)/HepPar1 (hepatocytes, yellow) or B7-H1 (red)/CD11c (DC, green)/CD68 (Kupffer cells, cyan). Representative images were selected.
(A) B7-H1 expression (red) was observed in perivenular lobular areas of post-reperfusion biopsy. (B) In contrast, normal liver samples showed only scarce B7-H1 expression largely limited to endothelial cells. (C, D) In the post-reperfusion biopsy, CD31+ endothelial cells (C, green) in the dilated sinusoids expressed B7-H1 (D, red). (E, F) HepPar1+ hepatocytes (E, yellow) in post-reperfusion biopsy specimens also showed B7-H1 expression (F, red). (G, H) CD11c+ CD68- DC (G, green) showed B7-H1 (H, red). (I, J) CD68+ CD11c- Kupffer cells (I, cyan) also showed B7-H1 expression (J, red).
(K) Primary human hepatocytes showed mRNA upregulation for B7-H1 when exposed to hypoxia. Representative image of two similar experiments.
Human primary hepatocytes showed B7-H1 mRNA upregulation as early as 1 hr after exposed to hypoxia (Fig. 8K).
Discussion
Herein, we show for the first time, in the murine LTx model, that liver graft B7-H1 expression plays a significant protective role during transplant-induced liver cold I/R injury. Hepatic cold I/R injury promptly up-regulated liver graft B7-H1 expression on hepatocytes, sinusoidal endothelial cells, and DC. The absence of hepatic B7-H1 expression in B7-H1 KO grafts was associated with a greater extent of tissue damage and also with an increased number of CD3+ T cells and a concomitant decrease of CD11b+ cells compared with WT grafts. Further analysis of liver lymphocytes showed that graft CD8+ T cells were significantly increased after LTx in KO compared with WT grafts, at least in part due to reduced apoptosis of intrahepatic CD8+ cells. In addition, evaluation of liver damage in chimeric liver grafts lacking B7-H1 on parenchymal or BMDC showed that the absence of B7-H1 expression on both hepatocytes and BMDC contributed to I/R injury after LTx.
Although B7-H1 mRNA is expressed in many cells and tissues, surface expression of this protein is more restricted. Indeed, in the liver, B7-H1 protein is expressed constitutively by DC, Kupffer cells, and sinusoidal endothelial cells, as well as by other monocyte-derived cells (17, 25). It also can be up-regulated on both NPC and hepatocytes after inflammatory stimulation (16, 23, 24). In this study, we confirm that hepatocytes do not express B7-H1 message or protein under steady-state condition, while DC and sinusoidal endothelial cells constitutively express B7-H1 protein. After LTx, B7-H1 mRNA and protein expression are strongly up-regulated on both NPC and hepatocytes, indicating that hepatic I/R injury efficiently promotes B7-H1 gene transcription and induces robust B7-H1 protein expression on liver grafts. As constitutive expression of B7-H1 on sinusoidal endothelial cells, DC, and Kupffer cells has been shown to inhibit proliferation and division of activated T cells and other leukocytes, enhanced hepatic B7-H1 expression during I/R injury might be a defense mechanism to protect the liver from further damage induced by host innate immune responses. Augmented cold I/R injury in B7-H1-deficient liver grafts in this study was associated with significantly increased frequencies and absolute numbers of graft CD8+ T cells. Likewise, B7-H1 KO mice display hepatic accumulation and impaired apoptosis in CD8+ T cells and accelerated hepatocyte damage during experimental autoimmune hepatitis (17). Recently, Morita et al (18) have shown that B7-H1 deficiency or blockade inhibits the development of spontaneous acceptance of mouse liver allografts, with reduced CD8+ T cell apoptosis. These results strongly suggest that hepatic B7-H1 expression plays crucial roles in regulating T cells and other immune cells in the liver probably for self protection from immune-mediated damage.
IFNs are important regulators of B7-H1 expression, and we have previously shown that tissue and serum IFNγ levels increase as early as 3 hr after LTx in this model (20), and it has been shown that B7-H1 can be up-regulated on DC by type II IFN (26). Further, it has been shown in humans and mice that both constitutive and IFNγ-induced B7-H1 expression is dependent on IFN regulatory factor (IRF)-1 (27). IRF-1 is a key transcription factor that regulates gene expression during inflammation and is markedly induced in liver grafts by 3 hr after transplant (20). These results suggest that IFNs might be involved in B7-H1 up-regulation during hepatic I/R injury in this model. Another possible mechanism involved in the up-regulation of B7-H1 during hepatic I/R injury is the release of danger signals, such as self-DNA and high mobility group box-1 (HMGB1) that, through interaction with specific receptors, can activate MyD88 and TNF receptor-associated factor 6 (TRAF6) which have been shown to be essential in B7-H1 expression since lack of those molecules results in a failure to up-regulate B7-H1 (28).
Accumulating evidence supports an important role of T cells in mediating both short- and long-term damage during I/R injury. Different experimental approaches that target T cell function, such as systemic immune suppression with cyclosporine or FK506 (29), T-cell-deficient (nude) mice (30, 31) and FTY720 treatment with T cell redistribution from the host peripheral blood to the lymph node compartment (32), attenuate I/R injury. Although precise mechanisms remain undetermined, T cells could alter innate immune responses via the secretion of cytokines and chemokines or through direct cell-cell interactions. It has been shown that liver parenchymal cell death in necroinflammatory liver injury results largely from CD8+ T cell-mediated killing (24). Accordingly, expression of PD-1, an immunoinhibitory receptor for B7-H1, on T cells could alter hepatic I/R injury. PD-1-deficient mice exhibit multiple autoimmune features, and PD-1 is crucial to maintain peripheral T cell tolerance (8). In this respect, Ji H et al (33) have shown in a murine warm I/R injury model, that stimulation of PD-1 with a dimeric recombinant fusion protein consisting of the extracellular domain of B7-H1 and the Fc portion of IgG improves hepatic injury by diminished hepatic T cell, neutrophil, and macrophage infiltration/activation, further suggesting an important role of B7-H1/PD-1 pathway in hepatic I/R injury.
In conclusion, this study shows that hepatic expression of B7-H1 plays a critical role in regulating inflammatory responses after LTx-induced hepatic I/R injury. Liver I/R damage in chimeric livers lacking B7-H1 on either hepatocytes or BMDC suggests that B7-H1 expression on both cell compartments is involved in regulating innate immunity. Hepatic B7-H1 expression might be crucial to protect the liver from immune-mediated damage.
Acknowledgments
The authors thank Rita M. Sico and Eizaburo Sasatomi for their superb support and Carla Forsythe for the preparation of the manuscript.
Financial support: This work was supported by the National Institutes of Health Grants P01AI081678-02 (Thomson), DK071753 (Murase). S. Ueki was supported by a Postdoctoral Fellowship grant from the Thomas E. Starzl Transplantation Institute. A. Castellaneta was supported by a Basic Science Fellowship from the American Society of Transplantation, by an American Liver Foundation Sunflowers for Holli Fellowship, and by a Starzl Transplantation Institute Young Investigator Grant.
Abbreviation
- ALT
alanine aminotransferase
- APC
antigen presenting cells
- BM
bone marrow
- BMDC
bone marrow-derived cell
- DC
dendritic cell
- I/R
ischemia/reperfusion
- LTx
liver transplantation
- NPC
non-parenchymal cells
- UW
University of Wisconsin solution
Contributor Information
Shinya Ueki, Email: ueueshin@yahoo.co.jp.
Antonino Castellaneta, Email: castellanettaa@upmc.edu.
Osamu Yoshida, Email: yoshidao@upmc.edu.
Kikumi Ozaki, Email: ozakiks@upmc.edu.
Matthew Zhang, Email: zhang.matthew@medstudent.pitt.edu.
Shoko Kimura, Email: kimuras@upmc.edu.
Kumiko Isse, Email: issek@upmc.edu.
Mark Ross, Email: mross@pitt.edu.
Lifang Shao, Email: shaol@upmc.edu.
Donna B. Stolz, Email: dstolz@pitt.edu.
Angus W. Thomson, Email: thomsonaw@upmc.edu.
Anthony J. Demetris, Email: demetrisaj@upmc.edu.
David A. Geller, Email: gellerda@upmc.edu.
Noriko Murase, Email: murase@pitt.edu.
References
- 1.Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, et al. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009;9:301–308. doi: 10.1111/j.1600-6143.2008.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu YY, Lin CW, Cheng KS, Lin C, Wang YM, Lin IT, Chou YH, et al. Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clin Exp Immunol. 2010 doi: 10.1111/j.1365-2249.2010.04217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez IB, Pasquinelli V, Jurado JO, Abbate E, Musella RM, de la Barrera SS, Garcia VE. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis. 2010;202:524–532. doi: 10.1086/654932. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 10.Dai H, Zhu H, Lei P, Yagita H, Liu J, Wen X, Zhou W, et al. Programmed death-1 signaling is essential for the skin allograft protection by alternatively activated dendritic cell infusion in mice. Transplantation. 2009;88:864–873. doi: 10.1097/TP.0b013e3181b6ea74. [DOI] [PubMed] [Google Scholar]
- 11.Ozkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O'Keefe T, Duong T, et al. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169:6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 12.Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, Okumura K, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 13.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 15.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 17.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 18.Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, Yagita H, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. 2010;10:40–46. doi: 10.1111/j.1600-6143.2009.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueki S, Dhupar R, Cardinal J, Yoshida J, Ozaki KS, Kim K, Tsung A, Murase N, Geller DA. Role of interferone regulatory factor-1 (IRF-1) in transplant induced liver ischemia-reperfusion injury. Am J Transplant. 2009;9 [Google Scholar]
- 21.Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isse K, Grama K, Abbott IM, Lesniak A, Lunz JG, Lee WM, Specht S, et al. Adding value to liver (and allograft) biopsy evaluation using a combination of multiplex quantum dot immunostaining, high-resolution whole-slide digital imaging, and automated image analysis. Clin Liver Dis. 2010;14:669–685. doi: 10.1016/j.cld.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology. 2009;50:1625–1637. doi: 10.1002/hep.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 Ligation Subverts IFN-{alpha} Production by Liver Plasmacytoid Dendritic Cells and Inhibits Their T Cell Allostimulatory Activity via B7-H1 Up-Regulation. J Immunol. 2009;183:6922–6932. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, Leonard JP. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002;9:133–145. doi: 10.1038/sj/mn/7800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, Busuttil RW, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 31.Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, Busuttil RW, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 32.Anselmo DM, Amersi FF, Shen XD, Gao F, Katori M, Lassman C, Ke B, et al. FTY720 pretreatment reduces warm hepatic ischemia reperfusion injury through inhibition of T-lymphocyte infiltration. Am J Transplant. 2002;2:843–849. doi: 10.1034/j.1600-6143.2002.20906.x. [DOI] [PubMed] [Google Scholar]
- 33.Ji H, Shen X, Gao F, Ke B, Freitas MC, Uchida Y, Busuttil RW, et al. Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology. 2010;52:1380–1389. doi: 10.1002/hep.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]