Abstract
Background
Plasma concentrations of several protease inhibitors are decreased during pregnancy. Few data are available describing atazanavir exposure during pregnancy, especially when used in combination with tenofovir, whose coadministration with atazanavir results in decreased atazanavir exposure.
Design
IMPAACT 1026s is an on-going, prospective, non-blinded study of antiretroviral pharmacokinetics in HIV-infected pregnant women that included two cohorts receiving atazanavir/ritonavir 300mg/100mg once daily during the third trimester through 6-12 weeks postpartum either with or without tenofovir.
Methods
Intensive steady-state 24-hour pharmacokinetic profiles were performed during the third trimester and at 6-12 weeks postpartum. Atazanavir was measured by reverse-phase HPLC with a detection limit of 0.13 mcg/mL. Pharmacokinetic targets were the estimated 10th percentile atazanavir AUC (29.4 mcg*hr/mL) in non-pregnant historical controls taking the standard dose (mean AUC=57 mcg*hr/mL) and a trough concentration of 0.15 mcg/mL, the concentration target used in therapeutic drug monitoring programs. Infant bilirubin concentrations were measured at 24-48 hours and 4-6 days after birth.
Results
Atazanavir pharmacokinetic data were available for 38 women (18 without tenofovir, 20 with tenofovir. Median atazanavir AUC was reduced during the third trimester compared to postpartum for subjects not receiving tenofovir (41.9 vs 57.9 mcg*hr/mL, p=.02) and for subjects receiving tenofovir (28.8 vs. 39.6 mcg*hr/mL, p=.04). During the third trimester, AUC was below the target in 33% (6/18) of women not receiving tenofovir and 55% (11/20) of women receiving tenofovir. Trough concentration was below the target in 6% (1/18) of women not receiving tenofovir and 15% (3/20) of women receiving tenofovir. The median (range) ratio of cord blood/maternal atazanavir concentration in 29 paired samples was 0.18 (0 - 0.45). No excessive infant bilirubin concentrations were observed.
Conclusions
Atazanavir exposure is reduced by pregnancy and by concomitant tenofovir use. A dose increase of atazanavir/ritonavir to 400mg/100mg may be necessary in pregnant women to ensure atazanavir exposure equivalent to that seen in nonpregnant adults, especially for pregnant women who are antiretroviral-experienced and/or who are also receiving tenofovir.
Keywords: atazanavir, tenofovir, pharmacokinetics, pregnancy, HIV, mother to child transmission
Introduction
Antiretroviral agents are commonly administered to HIV-infected pregnant women to prevent mother-to-child HIV transmission and to maintain maternal health.1 Current US Public Health Service guidelines on the management of HIV-infected women during pregnancy recommend use of a combination regimen consisting of two nucleoside reverse transcriptase inhibitors and either one protease inhibitor or one non-nucleoside reverse transcriptase inhibitor.2 The combination of atazanavir and ritonavir administered once daily is a popular second line protease inhibitor regimen used during pregnancy, often in combination with tenofovir and emtricitabine to create a complete once-a-day highly active antiretroviral therapy (HAART) regimen. Currently, nearly 25% of HIV infected pregnant women cared for at our network sites receive atazanavir while 50% receive lopinavir.
Previous studies of the pharmacokinetics in pregnant women of several protease inhibitors, including indinavir, lopinavir, nelfinavir and saquinavir, have demonstrated reduced plasma protease inhibitor concentrations during pregnancy.3-8 Two small studies of atazanavir exposure during pregnancy have been inconsistent. One showed a decrease in atazanavir exposure during pregnancy when compared to postpartum and the other did not. 9-10 Although coadministration of tenofovir and atazanavir is common and has been shown to result in a roughly 25% reduction in plasma atazanavir concentrations in nonpregnant adults, no data are available describing atazanavir exposure during pregnancy when used in combination with tenofovir.11-12 The goal of this study was to describe atazanavir pharmacokinetics during pregnancy, both with and without concomitant tenofovir use.
Methods
International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network Protocol 1026s is a multi-center, prospective study to evaluate the pharmacokinetics of antiretrovirals among pregnant HIV-infected women. This report includes women receiving atazanavir 300 mg and ritonavir 100 mg once daily. P1026s is a sub-study of P1025, a prospective cohort study of HIV-infected pregnant women receiving care at IMPAACT sites.
Eligibility criteria for this atazanavir arm of P1026s were: enrollment in IMPAACT P1025 and initiation of standard dose atazanavir/ritonavir (300mg/100mg once daily) as part of clinical care before the beginning of the 35th week of gestation. Exclusion criteria were: concurrent use of medications known to interfere with the absorption, metabolism or clearance of atazanavir or ritonavir, multiple gestation pregnancy, and clinical or laboratory toxicity that, in the opinion of the site investigator, would likely require a change in the medication regimen during the study. Local institutional review boards approved the protocol at all participating sites and signed informed consent was obtained from all subjects prior to participation. Subjects continued to take their prescribed medications throughout the course of their pregnancies. The choice of additional antiretrovirals was determined by the subject's physician, who prescribed all medications and remained responsible for her clinical management throughout the study. Women continued on study until the completion of postpartum pharmacokinetic sampling.
For women enrolling during the second trimester of pregnancy, atazanavir pharmacokinetics were determined in real time between 20 and 26 weeks gestation and repeated between 30 and 36 weeks gestation. Women enrolling in the third trimester had pharmacokinetic sampling performed between 30 and 36 weeks gestation. Pharmacokinetic sampling was repeated between 6 and 12 weeks postpartum. Atazanavir area under the concentration versus time curve (AUC0-24) was calculated for each woman and compared to the atazanavir AUC0-24 in non-pregnant adult populations.12 Each subject's physician was notified of the subject's plasma concentrations and AUC0-24 within two weeks of sampling. If the AUC0-24 was below the 10th percentile in non-pregnant adult populations (29.4 mcg*hr/mL), the physician was offered the option of discussing the results and possible dose modifications with a study team pharmacologist.
Clinical and laboratory monitoring
HIV-related laboratory testing was performed as part of the parent study (P1025) and as part of routine clinical care. Maternal data from P1025 accessed for this analysis were: maternal age, ethnicity, weight, concomitant medications, CD4 and plasma viral load assay results. Plasma viral load assays were done locally and had lower limits of detection ranging from less than 20 copies/mL to less than 400 copies/mL. Maternal clinical and laboratory toxicities were assessed through clinical evaluations (history and physical examination) and laboratory assays (ALT, AST, creatinine, BUN, albumin, bilirubin, hemoglobin) on each pharmacokinetic sampling day and at delivery. Infant data from P1025 included birth weight, gestational age at birth, and HIV infection status. Infants received physical examinations and serum bilirubin determinations at 24-48 hours and 4-6 days after delivery. The study team reviewed toxicity reports on monthly conference calls, although the subject's physician was responsible for toxicity management. The Division of AIDS (DAIDS)/NIAID Toxicity Table for Grading Severity of Adult Adverse Experiences was used to report adverse events for study subjects.13 All toxicities were followed through resolution.
Sample collection
Subjects were stable on their antiretroviral regimen for at least two weeks prior to pharmacokinetic sampling. Eight plasma samples were drawn at the second trimester, third trimester and at the postpartum pharmacokinetic evaluation visits, starting immediately before an oral atazanavir dose and at 1, 2, 4, 6, 8, 12 and 24 hours post-dose. Atazanavir was given as an observed dose after a light meal. Other information collected included the time of the two prior doses, the two most recent meals and maternal height and weight. A single maternal plasma sample and an umbilical cord sample after the cord was clamped were collected at delivery.
Drug assays
Atazanavir and ritonavir were measured by the University of California, San Diego (UCSD) Pediatric Clinical Pharmacology Laboratory using a validated, reversed-phase multiplex high performance liquid chromatography (HPLC) method.14 The lower limit of quantitation (LLOQ) was 0.047 mcg/mL for atazanavir and 0.094 mcg/mL for ritonavir. The interassay coefficient of variation (CV) was 8.8% at the LLOQ for atazanavir and 17% for ritonavir and ranged from 2.7% to 4.6% CV and 5.5% to 9.1% CV respectively for low, middle and high controls. Overall recovery from plasma was 102% for atazanavir and 117.3% for ritonavir. The UCSD laboratory has been enrolled in the AIDS Clinical Trials Group Quality Assurance/Quality Control (QA/QC) Proficiency Testing Program since 2001, which performs standardized inter-laboratory testing twice a year.15
Pharmacokinetic analyses
The pre-dose concentration (Cpre-dose), maximum plasma concentration (Cmax), corresponding time (Tmax), minimum plasma concentration (Cmin), and 24 hour post-dose concentration (C24h) were determined by direct inspection. For concentrations below the assay limit of detection, a value of one-half of the detection limit (0.024 mcg/mL for atazanavir, 0.047 mcg/mL for ritonavir) was used in summary calculations. Presence of an absorption lag was defined as a 1-hour post-dose concentration lower than the pre-dose concentration. AUC0-24 during the dose interval (from time 0 to 24 hours post-dose) for atazanavir and ritonavir were estimated using the trapezoidal rule. Apparent clearance (CL/F) from plasma was calculated as dose divided by AUC0-24. The terminal slope of the curve (λz) was estimated from the last two measurable and declining concentrations between 8 and 24 hours post-dose. Half-life was calculated as dose divided by λz, and apparent volume of distribution (Vd/F) was determined by CL/F divided by λz. Vd/F and CL/F were also estimated using a one-compartment model with first-order absorption and elimination (ADVAN2 TRANS2) at steady-state in the software program NONMEM®, version VI (ICON Development Solutions, Ellicott City, MD). Pharmacokinetic parameters derived from each approach were compared to assess potential limitations of each methodology. NONMEM was also used to perform Monte Carlo simulations to estimate trough concentrations in 1000 patients taking a 400 mg/100 mg atazanavir/ritonavir dose during pregnancy with and without tenofovir. The median clearance, volume and absorption rate parameter estimates along with their coefficients of variation obtained from the one-compartment analysis were used in the simulations, with a 15% residual error.
Statistical analyses
Target enrollment was at least 25 women with evaluable third trimester atazanavir pharmacokinetics in each arm (with or without tenofovir). To prevent ongoing enrollment of subjects receiving inadequate dosing, enrollment was to be stopped early if six study subjects had third trimester atazanavir AUC0-24 below the estimated 10th percentile for the non-pregnant historical controls (29.4 mcg*hr/mL). The statistical rationale for this early stopping criterion has been previously described.5
Atazanavir pharmacokinetic parameters during the third trimester and postpartum were compared at the within-subject level using 90% confidence limits for the geometric mean ratio of AUC0-24 and Cmax. When the true geometric mean of the ratio (the antilog of the true mean of the log ratios) of the pharmacokinetic parameters for pregnant and non-pregnant conditions has a value of 1, this indicates equal geometric mean pharmacokinetic parameters for the pregnant and non-pregnant conditions. If the 90% confidence intervals (CIs) are entirely outside the limits (0.8 and 1.25), the pharmacokinetic parameters for the pregnant and non-pregnant conditions are considered different. If, on the other hand, the 90% confidence limits are entirely within the limits (0.8, 1.25), the parameters are considered equivalent. If the 90% confidence interval overlaps with (0.8, 1.25), these data alone do not support any conclusions. Wilcoxon signed-rank test was used to assess the difference between third trimester and postpartum pharmacokinetic parameters for each arm and to assess the difference between subjects not receiving tenofovir and those receiving tenofovir during the third trimester and postpartum. McNemar's exact test was used to compare the number of women exhibiting an absorption lag during the third trimester and postpartum. Descriptive statistics were calculated for pharmacokinetic parameters of interest during each study period.
Results
Subject characteristics and outcomes
A total of 38 women were enrolled, of whom 18 did not receive concomitant tenofovir. Pharmacokinetic sampling was completed between November 2004 and May 2009. The clinical characteristics of the subjects and their pregnancy outcomes are presented in Table 1. Atazanavir and ritonavir were well tolerated by the subjects. Grade 3 or 4 toxicities were noted in 16 subjects, including hyperbilirubinemia in 14 (7 in each arm), anemia in one, and elevated liver function tests in two.
Table 1. Characteristics and pregnancy outcomes of the study population.
| Without Tenofovir | With Tenofovir | |||
|---|---|---|---|---|
| Characteristic | N (%) | Median (range) | N (%) | Median (range) |
| Age at delivery (years) | 18 | 27.0 (18.1 – 42.0) | 20 | 32.0 (22.2 – 45.1) |
| Weight at delivery (kg) | 15 | 83.0 (65.9 – 133.7) | 16 | 82.0 (56.7 – 159.1) |
| Weight postpartum (kg) | 14 | 75.7 (53.1- 123.5) | 19 | 82.2 (58.5 – 156.7) |
| CD4+ at delivery (cells/μL) | 16 | 461.5 (242-1444) | 20 | 472.5 (53 – 1494) |
| Race/ethnicity | ||||
| Black | 12 (67%) | 8 (40%) | ||
| Hispanic | 6 (33%) | 7 (35%) | ||
| White | ----- | 3 (15%) | ||
| Other | ----- | 2 (10%) | ||
| Concomitant antiretrovirals* | ||||
| Zidovudine + lamivudine | 12 (63%) | 3 (15%) | ||
| Zidovudine + lamivudine + abacavir | 3 (16%) | ----- | ||
| Lamivudine + abacavir | 3 (16%) | 1 (5%) | ||
| Emtricitabine | ----- | 16 (80%) | ||
| third trimester plasma HIV-1 RNA (copies/mL) | 18 | <62.5 (<50 - 19314) | 19 | <50 (<20 – 1630) |
| Undetectable (<20, 50, 75 or 400) | 13 (72%)** | 15 (79%)§ | ||
| Detectable | 5 (28%) | 4 (21%) | ||
| Delivery plasma HIV-1 RNA (copies/mL) | 16 | <62.5 (<50 - 29933) | 19 | <75 (<20 – 116) |
| Undetectable (<20, 50, 75 or 400) | 11 (69%)† | 17 (89%)‡ | ||
| Detectable | 5 (31%) | 2 (11%) | ||
| Postpartum plasma HIV-1 RNA (copies/mL) | 11 | <50 (,50 – 29933) | 14 | <75 (<20 - <400) |
| Undetectable (<20, 50, 75 or 400) | 8 (73%)+ | 14 (100%)++ | ||
| Detectable | 3 (27%) | 0 | ||
| Pregnancy outcomes | ||||
| Gestational age at delivery (weeks) | 18 | 38.6 (36.3 – 41) | 20 | 39.0 (35.7 – 41.4) |
| Birth weight (grams) | 18 | 2917 (2320 – 4125) | 20 | 2970 (2160 – 4260) |
Other concomitant medications for atazanavir with tenofovir subjects included: didanosine, lamivudine, nelfinavir and fosamprenavir
9 subjects were < 50, 2 subjects were < 75, 2 subjects were <400
1 subject was <20, 10 subjects were <50, 4 subjects were <75
8 subjects were <50, 3 subjects were < 75
1subject was <20, 8 subjects were <50, 5 subjects were <75, 3 subjects were <400
6 subjects <50, 2 subjects <75
1 subject was <20, 5 subjects were <50, 4 subjects were <75, 4 subjects were <400
Plasma viral load during the third trimester was undetectable in 13 of 18 subjects not receiving tenofovir and in 15 of 19 subjects receiving tenofovir, and was not available for one subject. Plasma viral load at delivery was undetectable in 11 of 16 women not receiving tenofovir and 17 of 19 subjects receiving tenofovir and was not available for three subjects. Thirty-seven infants are HIV-uninfected and infection status is indeterminate for one infant, who was HIV PCR negative at birth and 5 weeks of age but was subsequently lost to follow up. No excessive infant bilirubin concentrations were observed. The highest infant bilirubin concentrations were 8.8 gm/dL on day of life 1-2 and 7.8 gm/dL on day of life 4-8.
Atazanavir and ritonavir exposure
Atazanavir and ritonavir concentration-time plots are presented in Figures 1-2SDC and the pharmacokinetic parameters are presented in Tables 2 and 3. Among subjects not receiving tenofovir, lags in atazanavir absorption were noted in 1 of 1 (100%), 7 of 18 (39%), and 3 of 13 (23%) subjects in the second trimester, third trimester, and postpartum, respectively (Table 2). Among subjects receiving tenofovir, lags in atazanavir absorption were noted in 1 of 4 (25%), 9 of 20 (45%), and 5 of 19 (26%) subjects in the second trimester, third trimester, and postpartum, respectively (Table 3). The frequency of absorption lag between third trimester and postpartum was not significantly different for either arm. AUC0-24 was significantly reduced during the third trimester compared to postpartum for women not receiving tenofovir (41.9 vs 57.9 mcg*hr/mL, p=.02) and for those receiving tenofovir (28.8 vs. 39.6 mcg*hr/mL, p=.04). The geometric mean and 90% confidence intervals of the ratio of third trimester to postpartum atazanavir pharmacokinetic parameters are presented in Table 4 SDC.
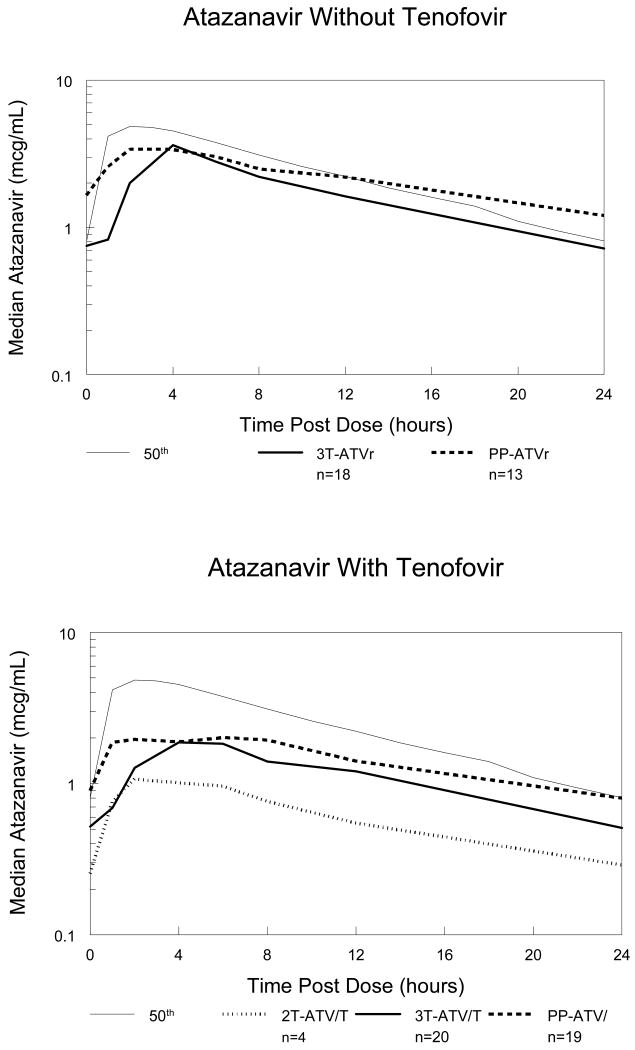
Figure 1. Median atazanavir concentrations during pregnancy and postpartum.
Median atazanavir concentration-time curves for atazanavir without tenofovir subjects (top graph) and atazanavir with tenofovir subjects (bottom graph). The thin solid line represents the expected (50th percentile) concentration-time profile in non-pregnant adults.
Table 2. Atazanavir without tenofovir subjects: Median (interquartile range) atazanavir and ritonavir non-compartmental pharmacokinetic parameters.
| Second Trimester n=1 |
Third Trimester n=18 |
Postpartum n=13 |
||
|---|---|---|---|---|
| Atazanavir | AUC0-24 (mcg*hr/mL) | 88.2 | 41.9 (27.4 – 60.8)* | 57.9 (47.1 – 64.8) |
| Cpre-dose (mcg/mL) | 3.6 | 0.8 (0.35 – 1.1)* | 1.7 (1.3 – 2.5) | |
| Cmax (mcg/mL) | 9.1 | 3.6 (2.8 – 5.1) | 4.1 (3 – 5.8) | |
| Tmax (hr) | 4 | 4 (2.5 – 4) | 2 (1 – 4) | |
| C24h (mcg/mL) | 2.0 | 0.7 (0.5 – 1.1)* | 1.2 (1.1 – 2) | |
| Cmin (mcg/mL) | 2.0 | 0.7 (0.4 – 0.9)* | 1.2 (1.1 – 1.8) | |
| CL/F (L/hr) | 3.4 | 7.2 (4.9 – 11)* | 5.2 (4.6 – 6.4) | |
| Vd/F (L) | 119 | 112 (92 – 151) | 153 (102 – 167) | |
| t½ (hr) | 24.3 | 10.9 (8.6 – 12.8)* | 15.3 (12 – 22) | |
| Ritonavir | Absorption Lag, n (%) | 1 (100%) | 7 (39%) | 3 (23%) |
| AUC0-24 (mcg*hr/mL) | 14.7 | 5.7 (4.1 – 7.9)* | 13.1 (10.8 – 17) | |
| Cpre-dose (mcg/mL) | 0.6 | <0.094 (<0.094 – <0.094)* | 0.2 (<0.094 – 0.3) | |
| Cmax (mcg/mL) | 2.0 | 0.8 (0.6 – 0.9)* | 1.8 (1.1 – 2.3) | |
| Tmax (hr) | 6 | 4 (2 – 6) | 4 (2 – 6) | |
| C24h (mcg/mL) | <0.094 | <0.094 (<0.094 – <0.094)* | <0.094 (<0.094 – 0.1) | |
| Cmin (mcg/mL) | <0.094 | <0.094 (<0.094– <0.094)* | <0.094 (<0.094– 0.1) | |
| CL/F (L/hr) | 6.8 | 17.6 (12.6 – 24.6)* | 7.7 (5.9 – 9.3) | |
| Vd/F (L) | 34.2 | 110 (82 – 146)* | 47 (40 – 71) | |
| t½ (hr) | 3.5 | 4.8 (3.8 – 5.8) | 5.0 (3.3 – 5.8) |
p<0.05, third trimester compared to postpartum;
AUC0-24 = area under the plasma concentration-time curve;
Cpre-dose = pre-dose concentration;
Cmax = maximum concentration;
Tmax = time post-dose of maximum concentration;
C24h = 24-hour post-dose concentration;
Cmin = minimum concentration;
Tmin = time post-dose of minimum concentration;
CL/F = oral clearance;
Vd/F = apparent volume of distribution;
t½ = half-life
Table 3. Atazanavir with tenofovir subjects: Median (interquartile range) atazanavir and ritonavir non-compartmental pharmacokinetic parameters.
| Second Trimester n=4 |
Third Trimester n= 20 |
Postpartum n=19 |
||
|---|---|---|---|---|
| Atazanavir | AUC0-24 (mcg*hr/mL) | 14.5 (11.3 – 21) | 28.8 (15.4 – 34.5)* | 39.6 (21 – 54.9) |
| Cpre-dose (mcg/mL) | 0.3 (0.2 – 0.5) | 0.5 (0.2 – 0.7)* | 0.9 (0.3 – 1.4) | |
| Cmax (mcg/mL) | 1.2 (0.8 – 1.7) | 2.5 (1.6 – 3)* | 4.1 (1.7 – 4.5) | |
| Tmax (hr) | 4 (3.5 – 4.5) | 4 (2 – 6) | 4 (2 – 6) | |
| C24h (mcg/mL) | 0.3 (0.2 – 0.4) | 0.5 (0.4 – 0.7)* | 0.8 (0.6 – 1.2) | |
| Cmin (mcg/mL) | 0.2 (0.2 – 0.3) | 0.4 (0.2 – 0.6)* | 0.6 (0.3 – 1) | |
| CL/F (L/hr) | 21.3 (15.7 – 27.4) | 10.4 (8.7 – 19.7)* | 7.6 (5.5 – 14.3) | |
| Vd/F (L) | 76 (53 – 139) | 160 (117 – 445) | 161 (83 – 396) | |
| t½ (hr) | 2.6 (1.7 – 3.9) | 9.7 (8.7 – 11.5)* | 14.7 (10.1 – 21.7) | |
| Ritonavir | Absorption lag, n(%) | 1 (25%) | 9 (45%) | 5 (26%) |
| AUC0-24 (mcg*hr/mL) | 3.1 (1.8 – 6.6) | 4.3 (2.5 – 6.7)* | 11.8 (7.9 – 15.5) | |
| Cpre-dose (mcg/mL) | <0.094 (<0.094 – 0.1) | <0.094 (<0.094 – <0.094)* | 0.1 (<0.094 – 0.2) | |
| Cmax (mcg/mL) | 0.4 (0.3 – 0.9) | 0.5 (0.2 – 0.8)* | 1.2 (0.6 – 1.9) | |
| Tmax (hr) | 2.5 (1 – 6) | 4 (4 – 6) | 4 (2 – 4) | |
| C24h (mcg/mL) | <0.094 (<0.094 – 0.1) | <0.094 (<0.094 – 0.1)* | 0.1 (0.1 – 0.2) | |
| Cmin (mcg/mL) | <0.094 (<0.094 – 0.1) | <0.094 (<0.094 – <0.094)* | <0.094 (<0.094 – 0.1) | |
| CL/F (L/hr) | 38.5 (19.1 – 57.7) | 22.9 (14.8 – 38.3)* | 10.5 (6.6 – 16.5) | |
| Vd/F (L) | 110 (83 – 137) | 135 (1.2 – 267) | 111 (57 – 195) | |
| t½ (hr) | 5.1 (5.0 – 5.1) | 5.4 (3.5 – 8.2) | 7.8 (5.2 – 10.7) |
p<0.05, third trimester compared to postpartum by Wilcoxon rank sum test;
AUC0-24 = area under the plasma concentration-time curve;
Cpre-dose = pre-dose concentration;
Cmax = maximum concentration;
Tmax = time post-dose of maximum concentration;
C24h = 24-hour post-dose concentration;
Cmin = minimum concentration;
Tmin = time post-dose of minimum concentration;
CL/F = oral clearance;
Vd/F = apparent volume of distribution;
t½ = half-life
The target atazanavir AUC0-24 during pregnancy was at least 29.4 mcg*hr/mL, the estimated 10th percentile AUC0-24 based on available data from non-pregnant adults.12 Among the women not receiving tenofovir, the AUC0-24 target was met by the one subject studied in the second trimester but was not met by 6 of 18 (33%) in the third trimester and 1 of 13 (8%) at 6-12 weeks postpartum. Among the women who also received tenofovir, the AUC0-24 target was not met by 3 of 4 (75%) in the second trimester, 9 of 20 (45%) in the third trimester and 7 of 19 (37%) postpartum. In women receiving tenofovir, atazanavir AUC0-24 was lower during the third trimester (p=0.04) and postpartum (p=.055) when compared to women not taking tenofovir.
Atazanavir concentration 24 hours after the dose fell below 0.15 mcg/mL, the standard atazanavir trough concentration target for treatment-naïve adults in therapeutic drug monitoring programs, in 1 of 18 (6%) third trimester subjects who did not receive tenofovir and 3 of 20 (15%) third trimester subjects who also received tenofovir.16 Median atazanavir C24h was significantly reduced during the third trimester compared to postpartum both for women not receiving tenofovir (0.7 vs. 1.2 mcg/mL, p=.002) and for those receiving tenofovir (0.5 vs. 0.8 mcg/mL, p=.0008). When women receiving tenofovir were compared to those who were not, atazanavir C24h was lower postpartum (p=0.024) but not during the third trimester (p=0.18). Monte Carlo simulation of 1000 pregnant patients taking an increased dose of 400 mg atazanavir and 100 mg ritonavir without tenofovir during the third trimester resulted in 40 of 1000 (4%) trough concentrations less than 0.15 mcg/mL. For a corresponding simulation of 1000 subjects who were taking concomitant tenofovir, 69 of 1000 (7%) trough concentrations were below 0.15 mcg/mL.
The one-compartment analysis showed most atazanavir pharmacokinetic parameters similar to the non-compartmental analysis. The one-compartment median (interquartile range; coefficient of variation) second trimester, third trimester and postpartum CL/F values in women not on tenofovir were 3.1 L/hr, 6.7 L/hr (4.8 – 9 L/hr; 40%), and 4.9 L/hr (4 – 6 L/hr; 49%), respectively. The corresponding Vd/F estimated values were 84 L, 90 L (83 – 101 L; 20%), and 113 L (89 – 124 L; 53%). For women taking tenofovir, the CL/F values for second trimester, third trimester and postpartum were 14.5 L/hr (11.9 – 16.4 L/hr; 32%), 9.9 L/hr (7.4 – 11.8 L/hr; 33%) and 7.2 L/hr (5.3 – 10.2 L/hr; 47%), respectively. The corresponding Vd/F estimated values were 133 L (125 – 151 L; 18%), 127 L (100 – 161 L; 30%), and 129 L (86 – 161 L; 39%).
Ritonavir AUC0-24, C0, Cmax and C24 were decreased and Cl/F increased during the 3rd trimester compared to postpartum in both groups. (Figure 2 SDC). However, ritonavir pharmacokinetics were difficult to estimate in some subjects because of the high frequency of undetectable concentrations. Ritonavir concentration was below the assay limit of detection in 57 of 251 (23%) samples from women not on tenofovir and 92 of 326 (28%) samples from women receiving tenofovir. Two subjects not on tenofovir had undetectable ritonavir concentrations in every sample after a witnessed dose, one at the third trimester and the other at the postpartum evaluation. One subject on tenofovir had undetectable ritonavir concentrations in every sample after a witnessed dose at both the second and the third trimester evaluations, while the ritonavir concentrations were detectable in every sample in this same woman at the postpartum visit.
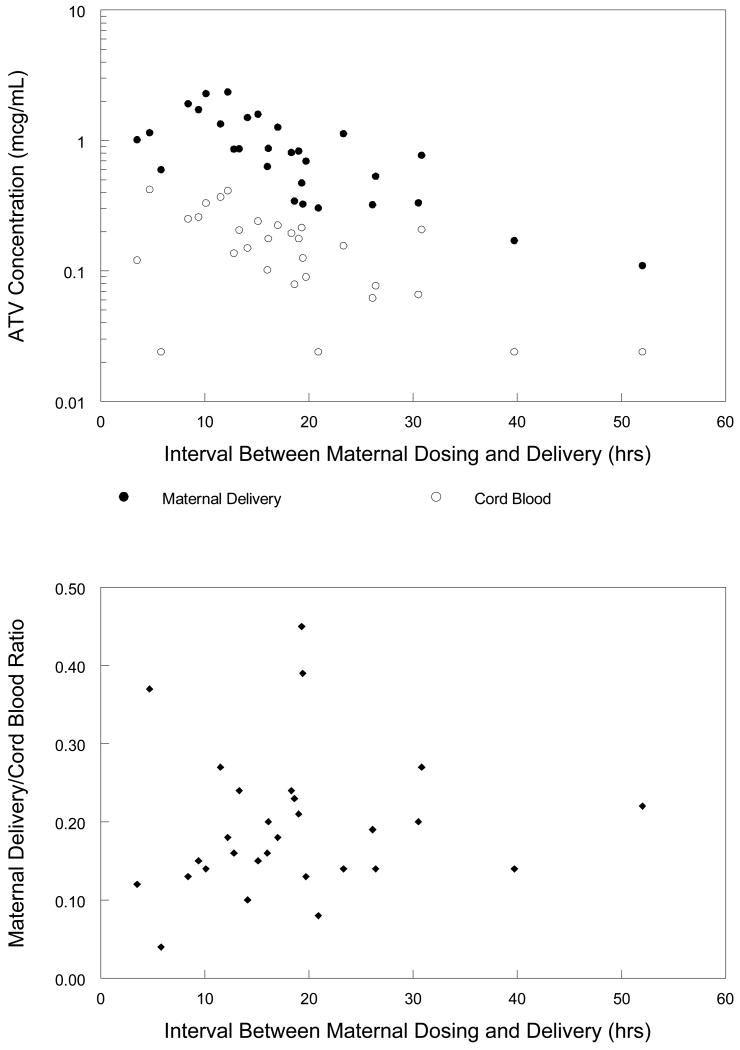
Maternal delivery and cord blood samples were collected from 35 mother-infant pairs. Maternal delivery atazanavir concentration was above the assay limit of detection in samples from 29 subjects. In these subjects, median (range) atazanavir concentration in cord blood was 0.16 (<0.047 – 0.42) mcg/mL and in maternal delivery plasma was 0.83 (0.11 - 2.36) mcg/mL. The median (range) ratio of the atazanavir concentration in cord blood to that in maternal delivery plasma was 0.18 (0.04 – 0.45). Maternal delivery and cord blood atazanavir concentrations and their ratio are plotted as a function of the time interval between maternal dosing and delivery in figure 3.
Figure 3. Maternal delivery and cord blood atazanavir concentrations.
Maternal delivery atazanavir concentrations, cord blood atazanavir concentrations and their ratio plotted against the interval between maternal dosing and delivery. Filled circles represent maternal plasma atazanavir concentration at delivery, open circle represent cord blood atazanavir concentration, filled diamonds represent the ratio of cord blood to maternal delivery atazanavir concentration.
Discussion
Previous studies of the pharmacokinetics during pregnancy of several protease inhibitors have demonstrated lower plasma concentrations with standard dosing during pregnancy than in nonpregnant adults. AUC of unboosted indinavir was 68% lower during pregnancy compared to postpartum, although when indinavir is boosted with ritonavir, trough concentrations during pregnancy appear adequate.17 Saquinavir AUC, Cmin and Cmax with use of a ritonavir boosted regimen (saquinavir 1200 mg/ritonavir 100 mg once daily) were reduced during pregnancy compared to nonpregnant women, but adequate saquinavir trough concentrations were achieved in 93% of pregnant subjects.18 We have previously shown that lopinavir AUC is reduced by 50% when administered during the third trimester as capsules at standard dosing (lopinavir 400 mg/ritonavir 100 mg twice daily) and that administration during the third trimester of an increased dose as either capsules (lopinavir 533 mg/ritonavir 133 mg twice daily) or tablets (lopinavir 600 mg/ritonavir 150 mg twice daily) results in lopinavir AUC equivalent to that seen in nonpregnant adults with standard dosing.5, 19-20 Similarly, several studies have shown that nelfinavir AUC and Cmin are reduced during pregnancy and an increased dose of nelfinavir in pregnancy is currently under study. 6, 21-22
Two previous studies are available that describe atazanavir pharmacokinetics in pregnant women. Ripamonti et al, showed no difference in atazanavir AUC and Cmin in 17 pregnant women receiving standard dosing with atazanavir and ritonavir during the third trimester and again postpartum.9 In contrast, Eley et al, studied 12 women in the third trimester and again postpartum, and found a decrease in atazanavir AUC and Cmin during the third trimester compared to either postpartum or to a reference population of nonpregnant adults.10 In both of these studies, geometric mean atazanavir AUC was low during the third trimester (28.5 mcg*hr/mL and 26.6 mcg*hr/mL, respectively, compared to 46.1 mcg*hr/mL in nonpregnant HIV infected adults). These studies differ in that mean postpartum atazanavir AUC in the Ripamonti study was 30.5 mcg*hr/mL, no different from that observed during pregnancy, while in the Eley study atazanavir AUC increased postpartum to 57.2 mcg*hr/mL, similar to that observed in nonpregnant subjects not receiving tenofovir in our and other studies.9-10, 12
We undertook this study because of these conflicting results and because no prior data existed describing atazanavir pharmacokinetics in pregnant women also receiving tenofovir, which reduces atazanavir exposure by 25% in nonpregnant adults.11-12 While zidovudine and lamivudine remain the most common nucleosides used as part of HAART regimens in pregnant women, use of tenofovir and emtricitabine with atazanavir and ritonavir as a once a day dosing regimen during pregnancy is becoming more common.23 In our study, we have shown that median atazanavir AUC0-24 and Cmin are reduced by 30-34% during pregnancy compared to postpartum and are reduced both during pregnancy and postpartum by an additional 25% when coadministered with tenofovir. The magnitude of the reduction in atazanavir concentrations with tenofovir coadministration in our subjects during pregnancy and postpartum is consistent with that seen in nonpregnant adults. 11-12
The relationship between atazanavir pharmacokinetic parameters and virologic response has been evaluated in several studies. In an early study, atazanavir AUC was a predictor of viral suppression.24 Subsequent studies in protease-inhibitor experienced patients have shown that the atazanavir genotypic inhibitory quotient, calculated by dividing the atazanavir trough concentration by the number of resistance mutations present, correlates best with virologic response.25-27 HIV resistance testing is not available for the subjects in this study, but 15% of the women who received atazanavir with tenofovir failed to meet the trough concentration target of 0.15 mcg/mL used in therapeutic drug monitoring programs.16
Our study has several limitations. Since we used an opportunistic design where a requirement for enrollment was treatment with atazanavir as part of ongoing clinical care, our population was heterogeneous in terms of HIV disease state, history of antiretroviral exposure and duration of atazanavir use at entry. While the reduction in ritonavir exposure seen in our patients during the 3rd trimester was comparable to that previously reported in pregnant women receiving ritonavir to boost lopinavir and saquinavir, the large number of undetectable ritonavir concentrations made estimation of ritonavir pharmacokinetic parameters unreliable in some subjects.3, 5, 8, 28 Our data are inadequate to explain the mechanism for the reduction in atazanavir exposure during pregnancy. Pregnancy may reduce atazanavir exposure by a direct effect on atazanavir disposition, by an indirect effect through reduction of ritonavir exposure and its inhibition of atazanavir metabolism, or by a combination of both mechanisms.
Our data demonstrate that atazanavir exposure is reduced during the second and third trimesters of pregnancy compared to postpartum and is further reduced by concomitant tenofovir use. Until more is known about the relationship between atazanavir plasma concentrations and virologic response, a reasonable goal for atazanavir dosing during pregnancy is to achieve plasma exposure in pregnant women equivalent to that seen in nonpregnant adults treated with standard doses. Several dosing options are available to increase atazanavir plasma concentrations. Given the high frequency of undetectable ritonavir concentrations and the large magnitude of ritonavir pharmacokinetic changes seen in our subjects, the dose of the ritonavir booster could be increased. However, the ritonavir concentration necessary to provide maximal enzyme inhibition during pregnancy is completely unknown and the poor tolerability of ritonavir makes increasing the dose unattractive to patients and providers. Another alternative would be to change the dose interval to every 12 hours from every 24 hours. While this would achieve higher trough concentrations, it is likely also to result in decreased patient adherence. A third option, and the one we chose to investigate in our simulation and is currently being studied in a new arm of this protocol, is to increase the atazanavir dose from 300 mg to 400 mg. Most dose increase strategies typically increase doses by a maximum of 50%. We chose a dose increase of 33% to 400 mg in order to be conservative since protease inhibitors often demonstrate non-linearity in drug exposure with dose increases and since atazanavir is readily available in 200 mg capsules.
Supplementary Material
Acknowledgments
We thank the subjects who enrolled in this trial. In addition to the authors, members of the IMPAACT 1026 protocol team include: Francesca Aweeka, PharmD, Emily Barr, CPNP, CNM, MSN, Kenneth D. Braun, Jr, BA, Jennifer Bryant, Kathleen A. Medvik, BS, MT, Amy Jennings.
Sources of Funding: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under NIAID cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by NIAID and by the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network (contract number N01-DK-9-001/HHSN267200800001C).
Footnotes
Presented in part at the 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Canada, February, 2009, abstract number 941.
References
- 1.Shapiro D, T R, Pollack H, et al. Mother-to-child HIV transmission risk according to antiretroviral therapy, mode of delivery and viral load in 2895 US women (PACTG 367). Paper presented at: Program and Abstracts of the 11th Conference on Retroviruses and Opportunistic Infections; Feb. 2004; San Francisco, CA. Abstract 99. [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. [May 24, 2010];2010 May 24;:1–117. Available at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf.
- 3.Mirochnick M, Best BM, Stek AM, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008 Dec 15;49(5):485–491. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unadkat JD, Wara DW, Hughes MD, et al. Pharmacokinetics and safety of indinavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2007 Feb;51(2):783–786. doi: 10.1128/AAC.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006 Oct 3;20(15):1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 6.Read JS, Best BM, Stek AM, et al. Pharmacokinetics of new 625 mg nelfinavir formulation during pregnancy and postpartum. HIV Medicine. 2008 Nov;9(10):875–882. doi: 10.1111/j.1468-1293.2008.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vithayasai V, Moyle GJ, Supajatura V, et al. Safety and efficacy of saquinavir soft-gelatin capsules + zidovudine + optional lamivudine in pregnancy and prevention of vertical HIV transmission. J Acquir Immune Defic Syndr. 2002 Aug 1;30(4):410–412. doi: 10.1097/00042560-200208010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Best BM, Stek AM, Mirochnick M, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010 Aug 1;54(4):381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ripamonti D, Cattaneo D, Maggiolo F, et al. Atazanavir plus low-dose ritonavir in pregnancy: pharmacokinetics and placental transfer. AIDS. 2007 Nov 30;21(18):2409–2415. doi: 10.1097/QAD.0b013e32825a69d1. [DOI] [PubMed] [Google Scholar]
- 10.Eley T, Vandeloise E, Child M, Conradie F, Zorrilla C, Josipovic D, Wang Y, Yang R, McGrath D, Bertz R, the Atazanavir 182 Pregnancy Study Group Steady State Pharmacokinetics and Safety of Atazanavir after Treatment with ATV 300 mg Once Daily/Ritonavir 100 mg Once Daily + ZDV/3TC during the Third Trimester in HIV+ Women. 15th Conference on Retoviruses and Opportunistic Infections; February 3-6, 2008; Boston, MA. Abstract 624. [Google Scholar]
- 11.Taburet AM, Piketty C, Chazallon C, et al. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004 Jun;48(6):2091–2096. doi: 10.1128/AAC.48.6.2091-2096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyataz pacakage insert. Bristol-Myers Squibb Co.; Princeton, NJ: Apr, 2009. [August 6, 2009]. http://packageinserts.bms.com/pi/pi_reyataz.pdf. [Google Scholar]
- 13.The Division of AIDS (DAIDS) Standardized Toxicity Table for Grading Severity of Adult Adverse Experiences. 1992 August; Available from http://rcc.tech-res-intl.com.
- 14.Droste JA, Verweij-Van Wissen CP, Burger DM. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther Drug Monit. 2003 Jun;25(3):393–399. doi: 10.1097/00007691-200306000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Holland DT, DiFrancesco R, Stone J, Hamzeh F, Connor JD, Morse GD. Quality assurance program for clinical measurement of antiretrovirals: AIDS clinical trials group proficiency testing program for pediatric and adult pharmacology laboratories. Antimicrob Agents Chemother. 2004 Mar;48(3):824–831. doi: 10.1128/AAC.48.3.824-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panel on Antiretroviral Guidelines for Adults and Adolescents . Department of Health and Human Services; Dec 1, 2009. [May 5, 2010]. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents; pp. 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 17.Ghosn J, De Montgolfier I, Cornelie C, et al. Antiretroviral therapy with a twice-daily regimen containing 400 milligrams of indinavir and 100 milligrams of ritonavir in human immunodeficiency virus type 1-infected women during pregnancy. Antimicrob Agents Chemother. 2008 Apr;52(4):1542–1544. doi: 10.1128/AAC.01301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Cortes LF, Ruiz-Valderas R, Rivero A, et al. Efficacy of low-dose boosted saquinavir once daily plus nucleoside reverse transcriptase inhibitors in pregnant HIV-1-infected women with a therapeutic drug monitoring strategy. Ther Drug Monit. 2007 Apr;29(2):171–176. doi: 10.1097/FTD.0b013e31803bb54e. [DOI] [PubMed] [Google Scholar]
- 19.Mirochnick M, Best BM, Stek AM, et al. Lopinavir Exposure With an Increased Dose During Pregnancy. J Acquir Immune Defic Syndr. 2008 Nov 4; doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Best B, Stek A, Hu C, Burchett S, Rossi S, Smith E, Sheeran E, Read J, Capparelli E, Mirochnick M, for the PACTG/IMPAACT P1026S team High-dose lopinavir and standard-dose emtricitabine pharmacokinetics during pregnancy and postpartum. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. February 3-8, 2008; Abstract 629. [Google Scholar]
- 21.Bryson YJ, Mirochnick M, Stek A, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: Pediatric AIDS Clinical Trials Group (PACTG) Protocol 353. HIV Clin Trials. 2008 Mar-Apr;9(2):115–125. doi: 10.1310/hct0902-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villani P, Floridia M, Pirillo MF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br J Clin Pharmacol. 2006 Sep;62(3):309–315. doi: 10.1111/j.1365-2125.2006.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baroncelli S, Tamburrini E, Ravizza M, et al. Antiretroviral treatment in pregnancy: a six-year perspective on recent trends in prescription patterns, viral load suppression, and pregnancy outcomes. AIDS Patient Care STDS. 2009 Jul;23(7):513–520. doi: 10.1089/apc.2008.0263. [DOI] [PubMed] [Google Scholar]
- 24.O'Mara E, Cirincione B, Mummaneni V, Grasela T, Grasela D. Population pharmacodynamic (PD) assessment of the safety and antiretroviral activity of atazanavir (BMS-232632). 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. 2001. [Google Scholar]
- 25.Solas C, Colson P, Ravaux I, et al. The genotypic inhibitory quotient: a predictive factor of atazanavir response in HIV-1-infected treatment-experienced patients. J Acquir Immune Defic Syndr. 2008 Jun 1;48(2):177–180. doi: 10.1097/QAI.0b013e318164226a. [DOI] [PubMed] [Google Scholar]
- 26.Cleijsen RM, van de Ende ME, Kroon FP, et al. Therapeutic drug monitoring of the HIV protease inhibitor atazanavir in clinical practice. J Antimicrob Chemother. 2007 Oct;60(4):897–900. doi: 10.1093/jac/dkm298. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrin I, Breilh D, Ragnaud JM, et al. Virological responses to atazanavir-ritonavir-based regimens: resistance-substitutions score and pharmacokinetic parameters (Reyaphar study) Antivir Ther. 2006;11(4):421–429. [PubMed] [Google Scholar]
- 28.van der Lugt J, Colbers A, Molto J, et al. The pharmacokinetics, safety and efficacy of boosted saquinavir tablets in HIV type-1-infected pregnant women. Antivir Ther. 2009;14(3):443–450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.