Abstract
The second messenger cyclic AMP (cAMP) can either stimulate or inhibit programmed cell death (apoptosis). Here, we review examples of cell types that show pro-apoptotic or anti-apoptotic responses to increases in cAMP. We also show that cells can have both such responses, although predominantly having one or the other. Protein kinase A (PKA)-promoted changes in phosphoylation and gene expression can mediate pro-apoptotic responses, such as in murine S49 lymphoma cells, based on evidence that mutants lacking PKA fail to undergo cAMP-promoted, mitochondria-dependent apoptosis. Mechanisms for the anti-apoptotic response to cAMP likely involve Epac (Exchange protein activated by cAMP), a cAMP-regulated effector that is a guanine nucleotide exchange factor (GEF) for the low molecular weight G-protein, Rap1. Therapeutic approaches that activate PKA-mediated pro-apoptosis or that block Epac-mediated anti-apoptotisis may provide a means to enhance cell killing, such as in certain cancers. By contrast, efforts to block PKA or stimulate Epac have the potential to be useful in diseases settings (such as heart failure) associated with cAMP-promoted apoptosis.
Keywords: apoptosis, protein kinase A, Epac, Rap1, S49 cell
Introduction
Cyclic AMP (cAMP) is a well-studied second messenger with >95,000 entries (as of December, 2010) in PubMed. Regulation of cell death is one of the actions of cAMP. Indeed, a PubMed search with the terms “cyclic AMP” and ”cell death” reveals >2000 published articles (including 160 reviews) over the past 40 years but none that review the stimulation or inhibition of cell death, in particular, apoptosis (programmed cell death) by cAMP. Moreover, components in the cAMP signaling pathway, including the cAMP effector protein kinase A (PKA), have been proposed as targets to enhance apoptosis, such as in the treatment of certain cancers (e.g., Cross, et al 2000;Lerner et al 2000). In this article we focus on publications in recent years and summarize pro-and anti-apoptotic responses to cAMP in various cell types, mechanisms for these actions and therapeutic implications of such findings.
cAMP as a regulator of cell death
Even though cAMP was discovered in the late 1950’s, the role of cAMP in regulating cell death has been studied much less intensely than other actions, such as regulation of metabolism and various physiological responses. The ability of cAMP to promote cell death was first recognized in the early 1970’s (e.g., Martorana 1971; Basile et al 1973; Pratt and Martin, 1975; Coffino et al 1975; Insel et al 1975) but the full range of cell types subject to this action is still being defined. The initial description of apoptosis occurred at about the same time (Kerr et al 1972) but only in recent years has the regulation of apoptotic cell death by cAMP been studied.
Apoptosis is one form of cell death, the two other major types being autophagy and necrosis, each of which has distinct morphological features and mechanisms. Considerable effort has been directed at distinguishing necrosis from apoptosis (e.g., Walker et al, 1988). Necrosis is a largely passive phenomenon that follows irreversible injury while apoptosis is an active process that requires numerous proteins and has two primary forms: intrinsic, mitochondrial-dependent and extrinsic, resulting from the action of membrane death receptors by extracellular agents such as Fas ligand. The intrinsic pathway involves B-cell lymphoma (Bcl)-related family proteins that have anti-apoptotic (e.g., Bcl-2, BclXL) or pro-apoptotic (e.g., Bax, Bak, BAD, BIM, BclXS) actions that can regulate mitochondrial release of cytochrome c and Smac (second mitochondria-derived activator of caspases/DIABLO (direct IAP-binding protein with low pI). Both intrinsic and extrinsic pathways involve the activation of caspases (cysteinyl aspartate-specific proteinases), enzymes that mediate cell killing. Apoptosis has physiological roles in regulating tissue size and remodeling but also occurs in pathological settings. Characteristic morphological features of apoptosis include condensation of nuclear chromatin and the cytoplasm, fragmentation of the nucleus (in association with internucleosomal cleavage of DNA) and cell budding with eversion of the plasma membrane and generation of membraneous bodies that are disposed of by adjacent cells. The distinctive internucleosomal cleavage of DNA in apoptosis generates electrophoretically observed DNA “ladders” that contrast with the random degradation of DNA that occurs with necrosis.
Few studies have assessed the impact of cAMP on necrosis (e.g., Journot et al, 1998) but there has been recent interest related to cAMP and autophagy--a mechanism by which cells deliver misfolded, ubiquinated proteins for degradation by lysosomes, in particular as a means to supply macromolecules in settings of nutrient deprivation. Its action in such settings has led to the proposal that autophagy is primarily a mechanism for cell survival rather than cell death (e.g., Levine and Yuan 2005). Numerous studies implicate cAMP in the regulation of autophagy in mammalian cells and lower eukaryotes and suggest that cellular components involved in autophagy can influence cAMP-mediated signal transduction (e.g., Holen et al, 1996; Budovskaya et al, 2005; Stephan et al, 2009; Houslay and Christian, 2010; Chin et al, 2010).
Cells that show pro-apoptotic responses to cAMP
Increases in cAMP are pro-apoptotic in numerous cell types (Table 1), including normal cells as well as malignant cells from virtually every tissue. In some cases, the apoptotic responses to cAMP analogs have been assessed whereas in other studies, endogenous cAMP concentrations have been raised by the use of hormones, neurotransmitters, the diterpene forskolin (which activates adenylyl cyclase) or cAMP phosphodiesterase (PDE) inhibitors. Cyclic AMP can also sensitize cells to the pro-apoptotic action of agents, such as DNA damage, that work via non-cAMP pathway components (e.g., Ugland et al 2008).
Table 1.
Examples of cell types in which increases in cAMP are pro-apoptotic
Some examples in Table 1 involve individual cell lines or cultured cells; for that reason, we use the term “certain” before several cell types. Of note are the substantial data indicating cAMP-promoted apoptosis in certain lymphoid cells, especially immature lymphoid cells, certain neuronal cells and cardiac myocytes (Table 1). The findings for cardiac myocytes, including the evidence that apoptosis occurs in response to the activation of β1-adrenoceptors (e.g., Singh et al 2001; Hasegawa et al 2001), are implicated in the progression of heart failure and provide a rationale for the use of β-blockers to treat patients with heart failure (McMurray 2010).
Cells that show anti-apoptotic responses to cAMP
Table 2 summarizes examples of settings in which cAMP is anti-apoptotic, including its blockade of spontaneous apoptosis and apoptosis induced by a variety of agents. Agents that elevate cAMP (e.g., endogenous hormones or neurotransmitters, forskolin, PDE inhibitors or cAMP analogs) blunt apoptosis in numerous types of neurons, epithelial cells, in pancreatic islet β-cells and many other cell types.
Table 2.
Examples of cell types in which increases in cAMP are anti-apoptotic
Can cAMP be pro-and anti-apoptotic in the same cell?
Certain cell types are listed in both Tables 1 and 2. In some cases, this reflects the use of different cell lines derived from the same tissue, perhaps reflecting differences in the capacity of particular cell types in a given tissue to undergo pro- or anti-apoptosis in response to cAMP or the use of different experimental approaches, for example, whether spontaneous apoptosis is assessed or if a pro-apoptotic agent is added prior to assessing the impact of endogenous cAMP or a cAMP analog. Cell permeable cAMP analogs that are resistant to PDE inhibition can lead to sustained PKA activation and regulation of downstream targets. Elevation of endogenous cAMP by receptor activation generally produces more transient PKA activation due to receptor desensitization and PDE-mediated degradation of cAMP. Thus, different responses, both qualitatively and quantitatively, may occur with these two means of increasing cAMP.
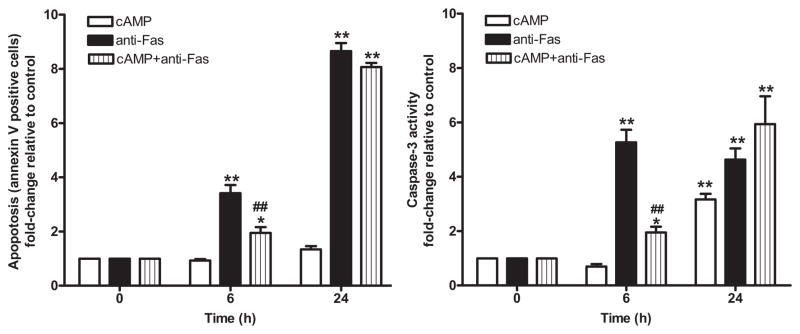
In other cases, though, investigators use similar cell types but obtain different results. Although technical differences may contribute (especially since different methods are sometimes used to assay apoptosis and isolate and grow cells or in some cases, different cell isolates are employed in different studies), we believe that there is another explanation: Individual cells may undergo both pro-apoptotic and anti-apoptotic responses to cAMP. Results in a given experiment may thus depend on the conditions under which it is conducted (e.g, Chen et al 2002). For example, murine S49 lymphoma cells, which undergo apoptosis in response to increased cAMP levels, in particular after 48–72 hr treatment (Yan et al 2000; Zhang and Insel 2001; Zhang and Insel 2004; Zambon et al 2005; Zhang et al 2008b), show a transient anti-apoptotic response to cAMP (in terms of annexin V binding to phosphatidylserine on plasma membrane and caspase 3 activity; Figure 1) if also treated with certain pro-apoptotic agents (e.g., anti-Fas in Figure 1 and ultraviolet light, data not shown). Data from other laboratories also implicate cAMP as having pro- or anti-apoptotic activity in the same cell (e.g., McEwan et al 2007; Loffler et al 2008).
Figure 1. cAMP can both stimulate and blunt apoptosis in S49 lymphoma cells.
Wild-type S49 cells were incubated with 100μM CPT-cAMP, 500ng Anti-Fas or the combination of CPT-cAMP and Anti-Fas for 6h and 24h and then assayed for apoptosis (Annexin-V binding; left panel) or caspase 3 activity (determined using a colorometric assay kit from EMD; right panel). The results shown are from 3 separate experiments. *p<0.05, ** p<0.01 compared to control (0h), ## p<0.01 compared to anti-Fas alone by t-test analysis. Note that CPT-cAMP blunts Annexin V binding and caspase 3 activity induced by anti-Fas treatment at 6 hr but this blunting is lost by 24h and that CPT-cAMP increases caspase 3 activity at 24 hr as a prelude to apoptosis (Zhang and Insel, 2004; Zhang et al, 2008b).
Mechanisms for pro-and anti-apoptotic actions of cAMP
Different molecular mechanisms mediate cAMP-promoted pro-apoptosis and anti-apoptosis. Substantial evidence indicates that PKA is pro-apoptotic through the phosphorylation of protein targets (e.g., Zambon et al, 2005; Carie and Sebti 2007; Zhang et al 2008b; Benz et al 2008). Studies in S49 lymphoma cells have shown that this pro-apoptotic effect of PKA occurs by an intrinsic, mitochondria-dependent mechanism (Coffino et al 1975; Insel et al 1975; Yan et al 2000; Zhang and Insel 2004; Zambon et al 2005; Zhang et al 2008b). The exact nature of the protein targets, in particular those that are necessary and sufficient to induce cAMP-promoted apoptosis, in wild-type S49 cells and other cell types has not yet been defined but is an area of active study.
Selective involvement of the two isozymes (I and II) of PKA may influence the role of cAMP in cell death. For example, cAMP analogs selective for the type I regulatory (R) subunit inhibit natural killer cell-mediated cytotoxicity (Torgresen et al 1997; Raskovalova et al, 2006) and expression of PKA type II R subunits can modulate apoptosis of fibroblasts (Porcellini et al 2003). An altered balance between PKARI and RII has been implicated in various cancers; manipulation of these isozymes may be a means to enhance cAMP-mediated apoptosis (Mantovani et al 2008; Bouizar et al 2010).
In addition to being pro-apoptotic, PKA can also be anti-apoptotic, perhaps via effects that alter the balance of serine/threone phosphorylation of Akt by other kinases, such as p38 kinase and phosphatidylinositol 3-kinase (PI3 kinase) (Kragsted et al 2004; Leone et al 2007; Torella et al 2009). Dynamin-related protein 1, a mitochondrial protein, is a PKA target that protects from apoptosis (Cribbs and Strack 2007). Inhibition of the PI3 kinase/Akt pathway can also occur in a PKA-independent manner (Smith et al 2005).
Epac (Exchange protein activated by cAMP) is a second effector of cAMP action that can mediate cAMP-promoted anti-apoptosis (Tiwari et al 2004; Kamrava et al 2005; Misra and Pizzo 2005; Grandoch et al 2010; Murray and Insel [unpublished]). Epac, a guanine nucleotide exchange factor (GEF) for the low-molecular weight GTP binding protein Rap1, produces PKA-independent responses (Gloerich and Bos 2010; Grandoch et al 2010). Epac-regulated targets for cAMP-promoted anti-apoptosis are not well defined. Limited data also implicate Epac in certain pro-apoptotic responses (e.g., Grandoch M, Lopez de Jesus M et al 2009).
Potential therapeutic applications of the pro-and anti-apoptotic actions of cAMP
Information related to the ability of increases in cAMP to promote or block apoptosis may provide opportunities for new therapeutic approaches. Apoptosis is desirable in some settings (e.g., killing of neoplastic cells) but harmful in others (e.g., loss of pancreatic β-cells that leads to diabetes mellitus or of neurons, cardiac myocytes or epithelial cells following injury). Thus, one would seek to enhance cAMP/PKA-promoted apoptosis in cancer but blunt such apoptosis or increase cAMP/Epac-promoted anti-apoptosis in settings to maintain tissue integrity. Agents that target PKA will likely have undesirable side effects because of its widespread expression. Alternative approaches to exploit the pro-apoptotic role of PKA are by seeking to achieve specificity in PKA signaling, for example by targeting selectively expressed or subcellularly localized PKA R or C (catalytic) subunits, A kinase anchoring proteins (AKAPs) or phosphorylation targets of PKA.
The development of Epac-directed drugs or agents directed at Epac targets is an alternative approach that may yield novel ways to alter cAMP-regulated apoptosis, especially if used together with increases in cAMP in particular cell types or cellular compartments or that would regulate partners of Epac and PKA (e.g., Gao et al 2010; Savai et al 2010; Perrino et al 2010; Patel et al 2010). Perhaps approaches that increase cAMP along with ones that inhibit Epac could provide combination therapies to supplement other types of anti-neoplastic agents (e.g., Desiniotis et al 2010), although one must be cautious since increased cAMP can blunt apoptosis by such agents in cancer cells (Naderi et al 2009; Safa et al 2010).
Conclusions
Cyclic AMP can either promote or block apoptosis in a large number of cell types. The pro-apoptotic response to cAMP occurs via PKA and its phosphoylation of target proteins while the anti-apoptotic response may occur through the actions of Epac. Increasing insight regarding the pro- and anti-apoptotic actions of cAMP has the potential to identify new therapeutic approaches that may impact on a number of clinically important disorders.
Acknowledgments
Work on this topic was supported by grants from the National Institutes of Health, Ellison Medical Foundation, American Heart Association and Lymphoma and Leukemia Society
Footnotes
Conflict of interest
There is no conflict of interest.
References
- Adissu HA, Schuller HM. Antagonistic growth regulation of cell lines derived from human lung adenocarcinomas of Clara cell and aveolar type II cell lineage: Implications for chemoprevention. Int J Oncol. 2004;24:1467–72. [PubMed] [Google Scholar]
- Altucci L, Rossin A, Hirsch O, Nebbioso A, Vitoux D, Wilhelm E, Guidez F, De Simone M, Schiavone EM, Grimwade D, Zelent A, deThe H, Gronemeyer H. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase–mediated desubordination of retinoid X receptor. Cancer Res. 2005;65:8754–65. doi: 10.1158/0008-5472.CAN-04-3569. [DOI] [PubMed] [Google Scholar]
- Al-Wadei HA, Schuller HM. Cyclic adenosine monophosphate-dependent cell type-specific modulation of mitogenic signaling by retinoids in normal and neoplastic lung cells. Cancer Detect Prev. 2006;30:403–11. doi: 10.1016/j.cdp.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Keren-Tal I, Aharoni D, Dantes A, Land-Bracha A, Rimon E, Sasson R, Hirsh L. Steroidogenesis and apoptosis in the mammalian ovary. Steroids. 2003a;68:861–7. doi: 10.1016/j.steroids.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Sasson R, Keren-Tal I, Aharoni D, Dantes A, Rimon E, Land A, Cohen T, Dor Y, Hirsh L. Alternative pathways of ovarian apoptosis: death for life. Biochem Pharmacol. 2003b;66:1355–62. doi: 10.1016/s0006-2952(03)00485-4. [DOI] [PubMed] [Google Scholar]
- Andrade da Costa BL, Kang KD, Rittenhouse KD, Osborne NN. The localization of PGE2 receptor subtypes in rat retinal cultures and the neuroprotective effect of the EP2 agonist butaprost. Neurochem Int. 2009;55:199–207. doi: 10.1016/j.neuint.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol. 2010;320:16–24. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Barlow CA, Kitiphongspattana K, Siddiqui N, Roe MW, Mossman BT, Lounsbury KM. Protein kinase A-mediated CREB phosphorylation is an oxidant-induced survival pathway in alveolar type II cells. Apoptosis. 2008;13:681–92. doi: 10.1007/s10495-008-0203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile DV, Wood HN, Braun AC. Programming of cells for death under experimental conditions: relevance to the tumor problem. Proc Nat Acad Sci USA. 1973;70:3055–9. doi: 10.1073/pnas.70.11.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz PM, Feller SM, Sickmann A, Walter U, Renne T. Prostaglandin-induced VASP phosphorylation controls alpha II-spectrin breakdown in apoptotic cells. Int Immunopharmacol. 2008;8:319–24. doi: 10.1016/j.intimp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Bouizar Z, Ragazzon B, Viou L, Hortane M, Bertherat J, Rizk-Rabin M. Cl-cAMP modifies the balance between PKAR1 and PKAR2 and modulates the cell cycle, growth and apoptosis in human adreoncortical H295R cells. J Mol Endocrinol. 2010;44:331–47. doi: 10.1677/JME-09-0120. [DOI] [PubMed] [Google Scholar]
- Brown JA, Gianino SM, Gutmann DH. Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity. J Neurosci. 2010;30:5579–89. doi: 10.1523/JNEUROSCI.3994-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation in the pancreas, gut and central nervous system. Endocrinology. 2004;145:2653–9. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- Budovskaya YV, Stephan JS, Deminoff SJ, Herman PK. An evolutionary proteomics approach identifies substrates of the cAMP-dependent protein kinase. Proc Nat Acad Sci USA. 2005;102:13933–8. doi: 10.1073/pnas.0501046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat M, Larocca L, Roca V, Hauk V, Pregi N, Nesse A, Perez Leiros C. Vasoactive intestinal peptide inhibits TNF-alpha-induced apoptotic events in acinar cells from nonobese diabetic mice submandibular glands. Arthritis Res Ther. 2009;11:R53. doi: 10.1186/ar2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carie AE, Sebti SM. A chemical biology approach identified a beta-2 adrenergic receptor agonist that causes human tumor regression by blocking the Raf-1/Mek-1/Erk pathway. Oncogene. 2007;26:3777–88. doi: 10.1038/sj.onc.1210172. [DOI] [PubMed] [Google Scholar]
- Chai B, Li JY, Zhang W, Newman E, Ammori J, Mulholland MW. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides. 2006;27:2846–57. doi: 10.1016/j.peptides.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Chan AS, Ng LW, Poon LS, Chan WW, Wong YH. Dopaminergic and adrenergic toxicities on SK-N-MC human neuroblastoma cells are mediated through G protein signaling and oxidative stress. Apoptosis. 2007;12:167–79. doi: 10.1007/s10495-006-0524-8. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Chaturvedi K, Boyadjieva N, Sarkar DK. Ethanaol induces apoptotic death of developing beta-endorphrin neurons via suppression of cyclic adenosine monophosphate production and activation of transforming growth factor beta1-linked apoptotic signaling. Mol Pharmacol. 2006;69:706–17. doi: 10.1124/mol.105.017004. [DOI] [PubMed] [Google Scholar]
- Chen HL, Demiralp B, Schneider A, Koh AJ, Silva C, Wang CY, McCauley LK. Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. J Biol Chem. 2002;277:19374–81. doi: 10.1074/jbc.M108913200. [DOI] [PubMed] [Google Scholar]
- Chen SU, Chen RJ, Shieh JY, Chou CH, Lin CW, Lu HF, Yang YS. Human chorionic gonadotropin up-regulates expression of myeloid cell leukemia-1 protein in human granulose-lutein cells: implication of corpus luteum rescue and ovarian hyperstimulation syndrome. J Clin Endocrinol Metab. 2010;95:3982–92. doi: 10.1210/jc.2009-2596. [DOI] [PubMed] [Google Scholar]
- Chen TC, Hinton DR, Zidovetzki R, Hofman FM. Up-regulation of the cAMP/PKA pathway inhibits proliferation, induces differentiation and leads to apoptosis in malignant gliomas. Lab Invest. 1998;78:165–74. [PubMed] [Google Scholar]
- Chin TY, Kao CH, Wang HY, Huang WP, Ma KH, Chueh SH. Inhibition of the mammalian target of rapamycin promotes cyclic AMP-induced differentiation of NG108–15 cells. Autophagy. 2010;6:1139–56. doi: 10.4161/auto.6.8.13564. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Kim SY, Oh JM, Juhnn YS. Stimulatory heterotrimeric G protein augments gamma ray-induced apoptosis by up-regulation of Bak expression via CREB and AP-1 in H1299 human lung cancer cells. Exp Mol Med. 2009;41:592–600. doi: 10.3858/emm.2009.41.8.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P, Bourne HR, Tomkins GM. Mechanism of lymphoma cell death induced by cyclic AMP. Am J Pathol. 1975;81:199–204. [PMC free article] [PubMed] [Google Scholar]
- Conran N, Almeida CB, Lanaro C, Ferreira RP, Traina F, Saad ST, Costa FF. Inhibition of caspase-dependent spontaneous apoptosis via a cAMP-protein kinase A dependent pathway in neutrophils from sickle cell disease patients. Br J Haematol. 2007;139:148–58. doi: 10.1111/j.1365-2141.2007.06748.x. [DOI] [PubMed] [Google Scholar]
- Cornu M, Modi H, Kawamori D, Kulkarni RN, Joffraud M, Thorens B. Glucagon-like peptide-1 increases beta-cell glucose competence and proliferation by translational induction of insulin-like growth factor-1 receptor expression. J Biol Chem. 2010;285:10538–45. doi: 10.1074/jbc.M109.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem. 2010;113:649–60. doi: 10.1111/j.1471-4159.2010.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–44. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine kinases and apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- Dai H, Zhang Z, Zhu Y, Shen Y, Hu W, Huang Y, Luo J, Timmerman H, Leurs R, Chen Z. Histamine protects against NMDA- induced necrosis in cultured cortical neurons through H receptor/cyclic AMP/protein kinase A and H receptor/GABA release pathways. J Neurochem. 2006;96:1390–1400. doi: 10.1111/j.1471-4159.2005.03633.x. [DOI] [PubMed] [Google Scholar]
- Desiniotis A, Schafer G, Clocker H, Eder IE. Enhanced antiproliferative and proapoptotic effects on prostate cancer cells by simultaneously inhibiting androgen receptor and cAMP-dependent protein kinase A. Int J Cancer. 2010;126:775–89. doi: 10.1002/ijc.24806. [DOI] [PubMed] [Google Scholar]
- Ding B, Abe J, Wei H, Xu H, Che W, Aizawa T, Liu W, Molina CA, Sadoshima J, Blaxall BC, Berk BC, Yan C. A positive feedback loop of phosphodiesterase 3 (PDE3) and inducible cAMP early repressor (ICER) leads to cardiomyocyte apoptosis. Proc Nat Acad Sci USA. 2005;102:14771–6. doi: 10.1073/pnas.0506489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Zitt C, Auriga C, Hatzelmann A, Epstein PM. Inhibition of PDE3, PDE4 and PDE7 potentiates glucocorticoid-induced apoptosis and overcomes glucocorticoid resistance in CEM T leukemic cells. Biochem Pharmacol. 2010;79:321–9. doi: 10.1016/j.bcp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Dou AX, Wang X. Cyclic adenosine monophosphate signal pathway in targeted therapy of lymphoma. Chin Med J. 2010;123:95–9. [PubMed] [Google Scholar]
- Elberg G, Elberg D, Lewis TV, Guruswamy S, Chen L, Logan CJ, Chan MD, Turman MA. EP2 receptor mediates PGE2-induced cystogenesis of human renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1622–32. doi: 10.1152/ajprenal.00036.2007. [DOI] [PubMed] [Google Scholar]
- Faglia G, Spada A. Genesis of pituitary adenomas: state of the art. J Neurooncol. 2001;54:95–101. doi: 10.1023/a:1012988828164. [DOI] [PubMed] [Google Scholar]
- Ferdaoussi M, Abdelli S, Yang JY, Cornu M, Niederhauser G, Favre D, Widmann C, Regazzi R, Thorens B, Waeber G, Abderrahmani A. Exendin-4 protects beta-cells from interleukin-1 beta-induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway. Diabetes. 2008;57:1205–15. doi: 10.2337/db07-1214. [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N, Payet MD. Mechanism of action of ACTH: beyond cAMP. Microsc Res Rech. 2003;61:275–87. doi: 10.1002/jemt.10337. [DOI] [PubMed] [Google Scholar]
- Gao N, Hibi Y, Cueno M, Asamitsu K, Okamoto T. A-kinase-interacting protein 1 (AKIP2) acts as a molecular determinant of PKA in NF-kappaB signaling. J Biol Chem. 2010;285:28097–104. doi: 10.1074/jbc.M110.116566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates A, Hohenester S, Anwer MS, Webster CR. cAMP-GEF cytoprotection by Src tyrosine kinase activation of phosphoinositide-3-kinase p110 beta/alpha in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2009;296:G764–74. doi: 10.1152/ajpgi.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–75. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- Granata R, Settanni F, Gallo D, Trovato L, Biancone L, Cantaluppi V, Nano R, Annunziata M, Campiglia P, Arnoletti E, Ghe C, Volante M, Papotti M, Muccioli G, Ghigo E. Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function. Diabetes. 2008;57:967–79. doi: 10.2337/db07-1104. [DOI] [PubMed] [Google Scholar]
- Grandoch M, Bujok V, Fleckenstein D, Schmidt M, Fischer JW, Weber AA. Epac inhibits apoptosis of human leukocytes. J Leukoc Biol. 2009;86:847–9. doi: 10.1189/jlb.0109048. [DOI] [PubMed] [Google Scholar]
- Grandoch M, Lopez de Jesus M, Oude Weernink PA, Weber AA, Jakobs KH, Schmidt M. B cell receptor-induced growth arrest and apoptosis in WEHI-231 immature B lymphoma cells involve cyclic AMP and Epac proteins. Cell Signal. 2009;21:609–21. doi: 10.1016/j.cellsig.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol. 2010;159:265–84. doi: 10.1111/j.1476-5381.2009.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Growcott EJ, Spink KG, Ren X, Afzal S, Banner KH, Wharton J. Phosphodiesterase type 4 expression and anti-proliferative effects in human pulmonary artery smooth muscle cells. Resp Res. 2006;7:9. doi: 10.1186/1465-9921-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Iwai-Kanai E, Sasayama S. Neurohormonal regulation of myocardial cell apoptosis during the development of heart failure. J Cell Physiol. 2001;186:11–8. doi: 10.1002/1097-4652(200101)186:1<11::AID-JCP1013>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hawcroft G, Ko CW, Hull MA. Prostaglandin E2-EP4 receptor signaling promotes tumorigenic behaviour of HT-29 human colorectal cancer cells. Oncogene. 2007;26:3006–19. doi: 10.1038/sj.onc.1210113. [DOI] [PubMed] [Google Scholar]
- Holen I, Gordon PB, Stormhaug PE, Seglen PO. Role of cAMP in the regulation of hepatocytic autophagy. Eur J Biochem. 1996;236:163–70. doi: 10.1111/j.1432-1033.1996.00163.x. [DOI] [PubMed] [Google Scholar]
- Holla VR, Mann JR, Shi Q, DuBois RN. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem. 2006;281:2676–82. doi: 10.1074/jbc.M507752200. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Lindsten T, Pleasure D, Itoh T. Differing in vitro survival dependency of mouse and rat NG2+ oligoderoglial progenitor cells. J Neurosci Res. 2010;88:957–70. doi: 10.1002/jnr.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Christian F. p62(SQSTM1) forms part of a novel, reversible aggregate containing a specific conformer of the cAMP-degrading phosphodiesterase, PDE4A4. Autophagy. 2010;6:1198–200. doi: 10.4161/auto.6.8.13479. [DOI] [PubMed] [Google Scholar]
- Insel PA, Bourne HR, Coffino P, Tomkins GM. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science. 1975;190:896–8. doi: 10.1126/science.171770. [DOI] [PubMed] [Google Scholar]
- Iwai-Kanai E, Hasegawa K. Intracellular signaling pathways for norepinephrine- and endothelin-1-mediated regulation of myocardial cell apoptosis. Mol Cell Biochem. 2004;259:163–8. doi: 10.1023/b:mcbi.0000021368.80389.b9. [DOI] [PubMed] [Google Scholar]
- Jasinska A, Strakova Z, Szmidt M, Fazleabas AT. Human chorionic gonadotropin and decidualization in vitro inhibits cytochalasin-D-induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology. 2006;147:4112–21. doi: 10.1210/en.2005-1577. [DOI] [PubMed] [Google Scholar]
- Ji Z, Mei FC, Miller AL, Thompson EB, Cheng X. Protein kinase A (PKA) isoform RIIbeta mediates the synergistic killing effect of cAMP and glucocorticoid in acute lymphoblastic leukemia cells. J Biol Chem. 2008;283:21920–5. doi: 10.1074/jbc.M803193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JL, Peng YP, Qiu YH, Wang JJ. Adrenoreceptor-coupled signal-transduction mechanisms mediating lymphocyte apoptosis induced by endogenous catecholamines. J Neuroimmunol. 2009;213:100–11. doi: 10.1016/j.jneuroim.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Jiao S, Liu Z, Ren WH, Ding Y, Zhang YQ, Zhang ZH, Mei YA. cAMP/protein kinase A signaling pathway protects against neuronal apoptosis and is associated with modulation of Kv2/1 in cerebellar granule cells. J Neurochem. 2007;100:979–91. doi: 10.1111/j.1471-4159.2006.04261.x. [DOI] [PubMed] [Google Scholar]
- Joseph RR, Yazer E, Hanakawa Y, Stadnyk AW. Prostaglandins and activation of AC/cAMP prevents anoikis in IEC-18. Apoptosis. 2005;10:1221–33. doi: 10.1007/s10495-005-2049-y. [DOI] [PubMed] [Google Scholar]
- Journot L, Villalba M, Bockaert J. PACAP-38 protects cerebellar granule cells from apoptosis. Ann NY Acad Sci. 1998;865:100–10. doi: 10.1111/j.1749-6632.1998.tb11168.x. [DOI] [PubMed] [Google Scholar]
- Kamrava M, Simpkins F, Alejandro E, Michener C, Meltzer E, Kohn EC. Lysophosphatidic acid and endothelin-induced proliferation of ovarian cancer cell lines is mitigated by neurtralization of granulin-epithelin precursor (GEP), a prosurvival factor for ovarian cancer. Oncogene. 2005;24:7084–93. doi: 10.1038/sj.onc.1208857. [DOI] [PubMed] [Google Scholar]
- Kang KD, Andrade da Costa BL, Osborne NN. Stimulation of prostaglandin EP2 receptors on RGC-5 cells in culture blunts the negative effect of serum withdrawal. Neurochem Res. 2010;35:820–9. doi: 10.1007/s11064-010-0140-4. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wylie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Rai D, McKeller MR, Aguilar RC. Rational combined targeting of phosphodiesterase 4B and SYK in DLBCL. Blood. 2009;113:6153–60. doi: 10.1182/blood-2009-02-206128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Song SE, Kim YK, Kim JY, Park SC, Park YK, Baek SH, Lee IK, Park SY. Adiponectin inhibits palmitate-induced apoptosis through suppression of reactive oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase. J Endocrinol. 2010;207:35–44. doi: 10.1677/JOE-10-0093. [DOI] [PubMed] [Google Scholar]
- Kim YK, Park JH, Park SH, Lim B, Baek WK, Suh SI, Lim JG, Ryu GR, Song DK. Protective role of glucagon-like peptide-1 against glucosamine-induced cytotoxicity in pancreatic beta cells. Cell Physiol Biochem. 2010;25:211–20. doi: 10.1159/000276555. [DOI] [PubMed] [Google Scholar]
- Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML, Bonewald LF. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the β-catenin and PKA pathways. J Bone Miner Res. 2010;25:2381–92. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloster MM, Hafte TT, Moltzau LR, Naderi EH, Dahle MK, Skalhegg BS, Gaudernack G, Levy FO, Naderi S, Blomhoff HK. EBV infection renders B cell resistant to growth inhibition via adenylyl cyclase. Cell Signal. 2008;20:1169–78. doi: 10.1016/j.cellsig.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kostylina G, Simon D, Fey MF, Yousefi S, Simon HU. Neutrophil apoptosis mediated by nicotinic acid receptors (GPR109A) Cell Death Differ. 2008;15:134–42. doi: 10.1038/sj.cdd.4402238. [DOI] [PubMed] [Google Scholar]
- Kottyan LC, Collier AR, Cao KH, Niese KA, Hedgebeth M, Radu CG, Witte ON, Khurana Hershey GK, Rothenberg ME, Zimmermann N. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood. 2009;114:2774–82. doi: 10.1182/blood-2009-05-220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Bornfeldt KE, Fukumoto S, Nishizawa Y. Molecular pathways of cyclic nucleotide- induced inhibition of arterial smooth muscle cell proliferation. J Cell Physiol. 2001;186:1–10. doi: 10.1002/1097-4652(200101)186:1<1::AID-JCP1012>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Krakstad C, Christensen AE, Doskeland SO. cAMP protects neutrophils agains TNF-alpha-induced apoptosis by activation of cAMP-dependent protein kinase, independently of exchange protein directly activated by cAMP (Epac) J Leukoc Biol. 2004;76:641–7. doi: 10.1189/jlb.0104005. [DOI] [PubMed] [Google Scholar]
- Kumar B, Hanson AJ, Prasad KN. Sensitivity to proteasome to its inhibitors increases during cAMP-induced differentiation of neuroblastoma cells in culture and causes decreased viability. Cancer Lett. 2004;204:53–9. doi: 10.1016/j.canlet.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kostin S, Flacke JP, Reusch HP, Ladilov Y. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J Biol Chem. 2009;284:14760–8. doi: 10.1074/jbc.M900925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HJ, Park KM, Choi HE, Chung KS, Lim HU, Park HY. PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell Signal. 2008;20:803–14. doi: 10.1016/j.cellsig.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim JH, Chae G, Lee BK, Ha KS, Kwon YG, Kim YM. Cyclic AMP prolongs graft survival by suppressing apoptosis and inflammatory gene expression in acute cardiac allograft rejection. Exp Mol Med. 2010;42:69–79. doi: 10.3858/emm.2010.42.1.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone V, di Palma A, Ricchi P, Acquaviva F, Giannouli M, Di Prisco AM, Iuliano F, Acquaviva AM. PGE2 inhibits apoptosis in human adenocarcinoma Caco-2 cell line through Ras-PI3K association and cAMP-dependent kinase A activation. Am J Physiol Gastrointest Liver Physiol. 2007;293:G673–81. doi: 10.1152/ajpgi.00584.2006. [DOI] [PubMed] [Google Scholar]
- Lerner A, Epstein PM. Cyclic nucleotide phosphodiesterases as targets for treatment of haematological malignancies. Biochem J. 2006;393:21–41. doi: 10.1042/BJ20051368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Kim DH, Lee R. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk Lymphoma. 2000;37:39–51. doi: 10.3109/10428190009057627. [DOI] [PubMed] [Google Scholar]
- Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Cindrova-Davies T, Skepper JN, Sellers LA. Prostacyclin induces apoptosis of vascular smooth muscle cells by a cAMP-mediated inhibition of extracellular signal-regulated kinase activity and can counteract the mitogenic activity of endothelin-1 or basic fibroblast growth factor. Circ Res. 2004;94:759–67. doi: 10.1161/01.RES.0000121568.40692.97. [DOI] [PubMed] [Google Scholar]
- Li S, Allen KT, Bonanno JA. Soluble adenylyl cyclase mediates bicarbonate-dependent corneal endothelial cell protection. Am J Physiol-Cell Physiol. 2011;300:C368–74. doi: 10.1152/ajpcell.00314.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R, Martins RA, Silveira MS. Control of programmed cell death by neurotransmitters and neuropeptides in the developing mammalian retina. Prog Retin Eye Res. 2005;24:457–91. doi: 10.1016/j.preteyeres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Liu J, Li XD, Ora A, Heikkila P, Vaheri A, Voutilainen R. cAMP-dependent protein kinase activation inhibits proliferation and enhances apoptotic effect of tumor necrosis factor-alpha in NCI-H295R adrenocortical cells. J Mol Endocrinol. 2004;33:511–22. doi: 10.1677/jme.1.01535. [DOI] [PubMed] [Google Scholar]
- Loffler I, Grun M, Bohmer FD, Rubio I. Role of cAMP in the promotion of colorectal cancer cell growth by prostaglandin E2. BMC Cancer. 2008;19:380. doi: 10.1186/1471-2407-8-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan DG, Brunton VG, Baillie GS, Leslie NR, Houslay MD, Frame MC. Chemoresistant KM12C colon cancer cells are addicted to low cyclic AMP levels in a phosphodiesterase 4-regulated compartment via effects on phosphoinositide 3-kinase. Cancer Res. 2007;67:5248–57. doi: 10.1158/0008-5472.CAN-07-0097. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Bondioni S, Lania AG, Rodolfo M, Peverelli E, Polentarutti N, Veliz Rodriguez T, Ferrero S, Bosari S, Beck-Peccoz P, Spada A. High expression of PKA regulatory subunit 1A protein is related to proliferation of human melanoma cells. Oncogene. 2008;27:1834–43. doi: 10.1038/sj.onc.1210831. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Lania AG, Bondioni S, Peverelli E, Pedroni C, Ferrero S, Pellegrini C, Vicentini L, Arnaldi G, Bosari S, Beck-Peccoz P, Spada A. Different expression of protein kinase A (PKA) regulatory subunits in cortisol-secreting adrenocortical tumors: relationship with cell proliferation. Exp Cell Res. 2008;314:123– 30. doi: 10.1016/j.yexcr.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Martorana PA. The role of cyclic AMP in isoprenaline-induced cardiac necroses in the rat. J Pharm Pharmacol. 1971;23:200–3. doi: 10.1111/j.2042-7158.1971.tb08642.x. [DOI] [PubMed] [Google Scholar]
- McMurray JJ. Clinical Practice. Systolic heart failure. N Engl J Med. 2010;362:228–38. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation. J Biol Chem. 2005;280:38276–89. doi: 10.1074/jbc.M507332200. [DOI] [PubMed] [Google Scholar]
- Moghadam KK, Kessler CA, Schroeder JK, Buckley AR, Brar AK, Handwerger S. Cannabinoid receptor 1 activatioin markedly inhibits human decidualization. Mol Cell Endocrinol. 2005;229:65–74. doi: 10.1016/j.mce.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Muhl H, Nitsch D, Sandau K, Brune B, Varga Z, Pfeilschifter J. Apoptosis is triggered by the cyclic AMP signaling pathway in renal mesangial cells. FEBS Lett. 1996;382:271–5. doi: 10.1016/0014-5793(96)00179-2. [DOI] [PubMed] [Google Scholar]
- Naderi EH, Findley HW, Ruud E, Blomhoff HK, Naderi S. Activation of cAMP signaling inhibits DNA damage-induced apoptosis in BCP-ALL cells through abrogation of p53 accumulation. Blood. 2009;114:608–18. doi: 10.1182/blood-2009-02-204883. [DOI] [PubMed] [Google Scholar]
- Naviglio S, Di Gesto D, Romano M, Sorrention A, Illiano F, Sorvillo L, Abbruzzese A, Marra M, Caraglia M, Chiosi E, Spina A, Illiano G. Leptin enhances growth inhibition by cAMP elevating agents through apoptosis of MDA-MB-231 breast cancer cells. Cancer Biol Ther. 2009;8:1183–90. doi: 10.4161/cbt.8.12.8562. [DOI] [PubMed] [Google Scholar]
- Nishihara H, Hwang M, Kizaka-Kondoh S, Eckmann L, Insel PA. Cyclic AMP promotes cAMP-responsive element-binding protein-dependent induction of cellular inhibitor of apoptosis protein-2 and suppresses apoptosis of colon cancer cells through ERK and p38 MAPK. J Biol Chem. 2004;279:26176–83. doi: 10.1074/jbc.M313346200. [DOI] [PubMed] [Google Scholar]
- Nishihara H, Kizaka-Kondoh S, Insel PA, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Nat Acad Sci USA. 2003;100:8921–6. doi: 10.1073/pnas.1533221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H, Ichiki T, Miyazaki R, Inanaga K, Imayama I, Hashiguchi Y, Sadoshima J, Sunagawa K. Inducible cAMP early repressor inhibits growth of vascular smooth muscle cell. Arterioscler Thrmob Vasc Biol. 2007;27:1549–55. doi: 10.1161/ATVBAHA.107.145011. [DOI] [PubMed] [Google Scholar]
- Park MK, Kang YJ, Ha YM, Jeong JJ, Kim HJ, Seo HG, Lee JH, Chang KC. EP2 receptor activation by prostaglandin E2 leads to induction of HO-1 via PKA and PI3K pathways in C6 cells. Biochem Biophys Res Commun. 2009;379:1043–7. doi: 10.1016/j.bbrc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Parkkonnen J, Hasala H, Moilanen E, Giembycz MA, Kankaanranta H. Phosphodiesterase 4 inhibitors delay human eosinophil and neutrophil apoptosis in the absence and presence of salbutamol. Pulm Phamacol Ther. 2008;21:499–506. doi: 10.1016/j.pupt.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Passeron T, Namiki T, Passeron HJ, Le Pape E, Hearing VJ. Forskolin protects keratinocytes from UVB-induced apoptosis and increases DNA repair independent of its effects on melanogenesis. Invest Dermatol. 2009;129:162–6. doi: 10.1038/jid.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HH, Hamuro LL, Chun BJ, Kawaraguchi Y, Quick A, Rebolledo B, Pennypacker J, Thurston J, Rodriquez-Pinto N, Self C, Olson G, Insel PA, Giles WR, Taylor SS, Roth DM. Disruption of protein kinase A localization using a trans-activator of transcription (TAT)-conjugated A-kinase- anchoring peptide reduces cardiac function. J Biol Chem. 2010;285:27632–40. doi: 10.1074/jbc.M110.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino C, Felicello A, Schiattarella GG, Esposito G, Guerriero R, Zaccaro L, Del Gatto A, Saviano M, Garbi C, Carangi R, Di Lorenzo E, Donato G, Indolfi C, Avveedimento VE, Chiariello M. AKAP121 downregulation impairs protective cAMP signals, promotes mitochondrial dysfunction and increases oxidative stress. Cardiovasc Res. 2010;88:101–10. doi: 10.1093/cvr/cvq155. [DOI] [PubMed] [Google Scholar]
- Pon YL, Auersperg N, Wong AS. Gonadotropins regulate N-cadherin-mediated human ovarian surface epithelial cell survival at both post-translational and transcriptional levels through a cyclic AMP/protein kinase A pathway. J Biol Chem. 2005;280:15438–48. doi: 10.1074/jbc.M410766200. [DOI] [PubMed] [Google Scholar]
- Porcellini A, Messina S, De Gregorio G, Feliciello A, Carlucci A, Barone M, Picascia A, De Blasi A, Avvedimento EV. The expression of the thyroid-stimulating hormone (TSH) receptor and the cAMP-dependent protein kinase RII beta regulatory subunit confers TSH-cAMP-dependent growth to mouse fibroblasts. J Biol Chem. 2003;278:40621–30. doi: 10.1074/jbc.M307501200. [DOI] [PubMed] [Google Scholar]
- Pratt RM, Martin GR. Epithelial cell death and cyclic AMP increase during palatal development. Proc Nat Acad Sci USA. 1975;72:874–7. doi: 10.1073/pnas.72.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh PC, Margiotta JE. PACAP support of neuronal survival requires MAPK- and activity- generated signals. Mol Cell Neurosci. 2006;31:586–95. doi: 10.1016/j.mcn.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Qin Z, Sun Z, Huang J, Hu Y, Wu Z, Mei B. Mutated recombinant glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-beta peptide (1–42) Neurosci Lett. 2008;444:217–21. doi: 10.1016/j.neulet.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Quinteros Villarruel E, Borda E, Sterin-Borda L, Orman B. Lidocaine-induced apoptosis of gingival fibroblasts. Participation of cAMP and PKC activity. Cell Biol Int. 2010 doi: 10.1042/CBI20100200. in press. [DOI] [PubMed] [Google Scholar]
- Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme (PKA I) Immunol Res. 2006;36:91–9. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- Safa M, Kazemi A, Zand H, Azarkeivan A, Zaker F, Hayat P. Inhibitory role of cAMP on doxorubicin-induced apoptosis in pre-B ALL cells through dephosphorylation of p53 serine residues. Apoptosis. 2010;15:196–203. doi: 10.1007/s10495-009-0417-8. [DOI] [PubMed] [Google Scholar]
- Sastry KS, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A, Carson JP, Weber MJ, Register TC, Chen YQ, Penn RB, Kulik G. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem. 2007;282:14094–100. doi: 10.1074/jbc.M611370200. [DOI] [PubMed] [Google Scholar]
- Savai R, Pullamsetti SS, Banat GA, Weissmann N, Ghofrani HA, Grimminger F, Schermuly RT. Targeting cancer with phosphodiesterase inhbitors. Expert Opin Investig Drugs. 2010;19:117–31. doi: 10.1517/13543780903485642. [DOI] [PubMed] [Google Scholar]
- Shafer SH, Phelps SH, Williams CL. Reduced DNA synthesis and cell viability in small cell lung carcinoma by treatment with cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol. 1998;56:1229–36. doi: 10.1016/s0006-2952(98)00260-3. [DOI] [PubMed] [Google Scholar]
- Shao JL, Wan XH, Chen Y, Bi C, Chen HM, Zhong Y, Heng XH, Qian JQ. H(2)S protects hippocampal neurons from anoxia-reoxygenation through cAMP-mediated PI3K/Akt/p70S6K cell-survival signaling pathways. J Mol Neurosci. 2011 doi: 10.1007/s12031-010-9464-4. in press. [DOI] [PubMed] [Google Scholar]
- Shao W, Yu Z, Fantus IG, Jin T. Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic beta-cells. Cell Signal. 2010;22:1240–6. doi: 10.1016/j.cellsig.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Sinclair EM, Yusta B, Streutker C, Baggio LL, Koehler J, Charron MJ, Drucker DJ. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology. 2008;135:2096–106. doi: 10.1053/j.gastro.2008.07.075. [DOI] [PubMed] [Google Scholar]
- Singh K, Xiao L, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189:257–65. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- Slot KA, de Boer-Brouwer M, Houweling M, Vaandrager AB, Dorrington JH, Teerds KJ. Luteinizing hormone inhibits Fas-induced apoptosis in ovarian surface epithelial cell lines. J Endocrinol. 2006;188:227–39. doi: 10.1677/joe.1.06087. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Pisarchik A, Slominski RM, Smijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206:780–91. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PG, Wang F, Wilkinson KN, Savage KJ, Klein U, Neuberg DS, Bollag G, Shipp MA, Aquilar RC. The phosphodiesterase PDE4B limits cAMP-associated PI3K/AKT-dependent apoptosis in diffuse large B-cell lymphoma. Blood. 2005;105:308–16. doi: 10.1182/blood-2004-01-0240. [DOI] [PubMed] [Google Scholar]
- Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De Leon DD, Naji A, Kushner JA, Stoffers DA. Calcineurin signaling regulated human islet β-cell survival. J Biol Chem. 2010;285:40050–9. doi: 10.1074/jbc.M110.154955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa LP, Carmo AF, Rezende BM, Lopes F, Silva DM, Alessandri AL, Bonjardim CA, Rossi AG, Teixeira MM, Pinho V. Cyclic AMP enhances resolution of allergic pleurisy by promoting inflammatory cell apoptosis via inhibition of PI3K/Akt and NF-kappaB. Biochem Pharmacol. 2009;78:396–405. doi: 10.1016/j.bcp.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Sousa LP, Lopes F, Silva DM, Tavares LP, Vieira AT, Rezende BM, Carmo AF, Russo RC, Garcia C, Bonjardim CA, Alessandri AL, Rossi AG, Pinho V, Teixeira MM. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and NF-kappaB-independent manner. J Leukoc Biol. 2010;87:895–904. doi: 10.1189/jlb.0809540. [DOI] [PubMed] [Google Scholar]
- Steinert D, Kuper C, Bartels H, Beck FX, Neuhofer W. PGE2 potentiates tonicity-induced COX- 2 expression in renal medullary cells in a positive feedback look involving EP2-cAMP-PKA signaling. Am J Physiol Cell Physiol. 2009;296:75–87. doi: 10.1152/ajpcell.00024.2008. [DOI] [PubMed] [Google Scholar]
- Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Nat Acad Sci USA. 2009;10:17049–54. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Zukin RS, Vosler PS, Zhang L, Zhang F, Cao G, Bennett MV, Chen J. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proc Nat Acad Sci USA. 2010;107:3204–9. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Yokoyama U, Abe T, Kiyonari H, Yamashita N, Kato Y, Kurotani R, Sato M, Okumura S, Ishikawa Y. Differential roles of Epac in regulating cell death in neuronal and myocardial cells. J Biol Chem. 2010;285:24248–59. doi: 10.1074/jbc.M109.094581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda N, Zierler S, Kerschbaum HH. cAMP mediates ammonia-induced programmed cell death in the microglial cell line BV-2. Eur J Neurosci. 2007;25:2285–95. doi: 10.1111/j.1460-9568.2007.05452.x. [DOI] [PubMed] [Google Scholar]
- Szatmari E, Kalita KB, Kharebava G, Hetman M. Role of kinase suppressor of Ras-1 in neuronal survival signaling by extracellular signal-regulated kinase. J Neurosci. 2007;17:11389–400. doi: 10.1523/JNEUROSCI.3473-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Kobayashi H, Moriuchi T. Molecular basis of pituitary oncogenesis. J Neuroncol. 1999;45:83–96. doi: 10.1023/a:1006390306336. [DOI] [PubMed] [Google Scholar]
- Takadera T, Ohyashiki T. Prostaglandin E2 deteriorates N-methyl-D-aspartate receptor-mediated cytotoxicity possibly by activating EP2 receptors in cultured cortical neurons. Life Sci. 2006a;78:1878–83. doi: 10.1016/j.lfs.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Takedera T, Ohyashiki T. Prevention of rat cortical neurons from prostaglandin E2-induced apoptosis by glycogen synthase kinase-3 inhibitors. Neurosci Lett. 2006b;400:105–9. doi: 10.1016/j.neulet.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Thompson EB, Medh RD, Zhou F, Ayala-Torres S, Ansari N, Zhang W, Johnson BH. Glucocorticoids, oxysterols, and cAMP with glucocorticoids each cause apoptosis of CEM cells and suppress c-myc. J Steroid Biochem Mol Biol. 1999;69:453–61. doi: 10.1016/s0960-0760(99)00063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Felekkis K, Moon EY, Flies A, Sherr DH, Lerner A. Among circulating hematopoietic cells, B-CLL uniquely expressed functional EPAC1, but EPAC1-mediated Rap1 activation does not account for PDE4 inhibitor-induced apoptosis. Blood. 2004;103:2661–7. doi: 10.1182/blood-2003-06-2154. [DOI] [PubMed] [Google Scholar]
- Torella D, Gasparri C, Ellison GM, Curcio A, Leone A, Vicinanza C, Galuppo V, Mendicino I, Sacco W, Aquila I, Durace FC, Luposella M, Stillo G, Agosti V, Cosentino C, Avyedimento EV, et al. Differential regulation of vascular smooth muscle and endothelial cell proliferation in vitro and in vivo by cAMP/PKA-activated p85alphPI3K. Am J Physiol Heart Circ Physiol. 2009;297:H2015–25. doi: 10.1152/ajpheart.00738.2009. [DOI] [PubMed] [Google Scholar]
- Torgersen KM, Vagge JT, Levy FO, Hansson V, Rolstad B, Tasken K. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem. 1997;272:5495–500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- Ugland H, Boquest AC, Naderi S, Collas P, Blomhoff HK. cAMP-mediated induction of cyclin E sensitizes growth arrested adipose stem cells to DNA damage-induced apoptosis. Mol Biol Cell. 2008;19:5082–92. doi: 10.1091/mbc.E08-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan B, Ea HK, Launay JM, Garel JM, Champy R, Cressent M, Liote F. A critical rrole for adrenomedullin-calcitonin receptor-like receptor in regulating rheumatoid fibroblast-like synoviocyte apoptosis. J Immunol. 2006;176:5548–58. doi: 10.4049/jimmunol.176.9.5548. [DOI] [PubMed] [Google Scholar]
- Valente EG, Vernet D, Ferrini MG, Qian A, Raifer J, Gonzalez-Cadavid NF. L-arginine and phosphodiesterase inhibitors counteract fibrosis in the Peyronie’s fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–44. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MK. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179:8544–53. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NI, Harmon BV, Gobe GC, Kerr JF. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- Wang SM, Yang WL. Circulating hormone adrenomedullin and its binding protein protect neural cells from hypoxia-induced apoptosis. Biochim Biophys Acta. 2009;1790:361–7. doi: 10.1016/j.bbagen.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Werstiuk ES, Lee RMKW. Vascular β-adrenoceptor function in hypertension and in ageing. Can J Physiol Pharmacol. 2000;78:433–452. [PubMed] [Google Scholar]
- Whitaker CM, Beaumont E, Wells MJ, Magnuson DS, Hetman M, Onifer SM. Rolipram attenuates acute oligodendrocyte death in the adult rat ventrolateral funiculus following contusive cervical spinal cord injury. Neurosci Lett. 2008;438:200–4. doi: 10.1016/j.neulet.2008.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP. Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci STKE. 2001;104:re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- Yan L, Hermann V, Hofer JI, Insel PA. Beta-adrenergic receptor/cAMP-mediated signaling an apoptosis of S49 lymphoma cells. Am J Physiol Cell Physiol. 2000;279:1665–74. doi: 10.1152/ajpcell.2000.279.5.C1665. [DOI] [PubMed] [Google Scholar]
- Yan C, Miller CL, Abe J. Regulation of phosphodiesterase 3 and inducible cAMP early repressor in the heart. Circ Res. 2007;100:489–501. doi: 10.1161/01.RES.0000258451.44949.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Itoh Y, Kubota T, Sendo T, Koyama T, Fujita T, Saeki K, You A, Oishi R. A prostacyclin analog prevents radiocontrast nephropathy via phosphorylation of cyclic AMP response element binding protein. Am J Pathol. 2005;166:1333–42. doi: 10.1016/S0002-9440(10)62352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Melmed S. Oncogene activation in pituitary tumors. Brain Pathol. 2001;11:328–41. doi: 10.1111/j.1750-3639.2001.tb00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Jin T. New insights into the role of cAMP in the production and function of the incretin hormone glucagon-like peptide-1 (GLP-1) Cell Signal. 2010;22:1–8. doi: 10.1016/j.cellsig.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Yusta B, Boushev RP, Drucker DJ. The glucagon-like peptide receptor mediates inhibition of cellular apoptosis via a cAMP-dependent protein kinase-independent pathway. J Biol Chem. 2000;275:35345–52. doi: 10.1074/jbc.M005510200. [DOI] [PubMed] [Google Scholar]
- Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR, Insel PA. Gene expression patterns define key transcriptional events in cell cycle regulation by cAMP and protein kinase A. Proc Nat Acad Sci USA. 2005;102:8561–6. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang Q, Zhu N, Yu M, Shen B, Xiang J, Lin A. Cyclic AMP inhibits JNK activation by CREB-mediated induction of c-FLIP(L) and MKP-1, thereby antagonizing UV-induced apoptosis. Cell Death Differ. 2008;15:1654–62. doi: 10.1038/cdd.2008.87. [DOI] [PubMed] [Google Scholar]
- Zhang L, Insel PA. Bcl-2 protects lymphoma cells from apoptosis but not growth arrest promoted by cAMP and dexamethosone. Am J Physiol Cell Physiol. 2001;281:1642–7. doi: 10.1152/ajpcell.2001.281.5.C1642. [DOI] [PubMed] [Google Scholar]
- Zhang L, Insel PA. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem. 2004;279:20858–65. doi: 10.1074/jbc.M310643200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Murray F, Zahno A, Kanter JR, Chou D, Suda R, Fenlon M, Rassenti L, Cottam H, Kipps TJ, Insel PA. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc Nat Acad Sci USA. 2008a;105:19532–7. doi: 10.1073/pnas.0806152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zambon AC, Vranizan K, Pothula K, Conklin BR, Insel PA. Gene expression signatures of cAMP/protein kinase A (PKA)-promoted, mitochondrial-dependent apoptosis. Comparative analysis of wild-type and cAMP-deathless S49 lymphoma cells. J Biol Chem. 2008b;283:4304–13. doi: 10.1074/jbc.M708673200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Qian ZM, Zhang C, Wing HY, Du F, Ya K. Amyloid beta-peptide 31–35-induced neuronal apoptosis is mediated by caspase-dependent pathways via cAMP-dependent protein kinase A activation. Aging Cell. 2007;7:47–57. doi: 10.1111/j.1474-9726.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Zheng M, Zhu W, Han Q, Xiao RP. Emerging concepts and therapeutic implications of beta- adrenergic receptor subtype signaling. Pharmacol Ther. 2005;108:257–68. doi: 10.1016/j.pharmthera.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Zhou B, Li F, Chen H, Song J. The modulation of apoptosis involves Akt and epidermal factor receptor. Intl J Biochem Cell Biol. 2005;37:1483–95. doi: 10.1016/j.biocel.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yao J, Meng Y, Kasai A, Hiamatsu N, Hayakawa K, Miida T, Takeda M, Okada M, Kitamura M. Profiling of functional phosphodiesterase in mesangial cells using a CRE-SEAP-based reporting system. Br J Pharmacol. 2006;148:833–44. doi: 10.1038/sj.bjp.0706785. [DOI] [PMC free article] [PubMed] [Google Scholar]