Abstract
Interleukin 22 (IL-22), which acts as either a pro-inflammatory or anti-inflammatory cytokine in various disease models, is markedly upregulated in chronic liver diseases, including hepatitis B and C. In this report, we demonstrate a strong correlation between IL-22 expression in the liver with active, inflammatory human liver disease. To clarify the role of IL-22 upregulation in the pathogenesis of liver diseases, liver specific IL-22 transgenic (IL-22TG) mice, under the control of albumin promoter, were developed. Despite elevated IL-22 serum levels ranging from 4000 to 7000 pg/ml, IL-22TG mice developed normally without obvious adverse phenotypes or evidence of chronic inflammation except slightly thicker epidermis and minor inflammation in the skin compared with wild-type mice. Most interestingly, IL-22TG mice were completely resistant to Concanavalin A-induced T cell hepatitis with minimal effect on liver inflammation and had accelerated liver regeneration after partial hepatectomy. Although they did not spontaneously develop liver tumors, IL-22TG mice were more susceptible to diethylnitrosamine-induced liver cancer. Microarray analyses revealed that a variety of anti-oxidant, mitogenic, acute phase genes were upregulated in the livers from IL-22TG mice compared with those from wild-type mice. These findings indicate that localized production of IL-22 in the liver promotes hepatocyte survival and proliferation but primes the liver to be more susceptible to tumor development without significantly affecting liver inflammation.
Keywords: IL-22, transgenic mice, liver, cancer
Introduction
Interleukin 22 (IL-22) was originally identified as an IL-10-related T cell-derived inducible factor (IL-TIF), belonging to IL-10 family. 1 It is now known that IL-22 is mainly produced by Th17, Th22, γδT, natural killer (NK), and NKT cells etc. 2–4 IL-22 mainly targets epithelial cells including hepatocytes, playing an important role in controlling bacterial infection, homeostasis, and tissue repair.2–8 IL-22 exerts its functions via binding to the heterodimer IL-10R2/IL-22R1 complex, followed by activation of signal transducer and activator of transcription 3 (STAT3) as well as other signaling pathways (albeit to a lesser extent) including STAT1 and STAT5 etc. 2–4 IL-10R2 is ubiquitously expressed on a variety of cell types, whereas IL-22R1 expression is restricted to epithelial cells in the skin, liver, pancreas, lung, and gut. 2–4
IL-22 has been found to be upregulated and implicated as a proinflammatory cytokine in the pathogenesis in various human diseases and animal models including psoriasis,9 rheumatoid arthritis,10 and Crohn’s disease.11 In contrast, IL-22 has also been shown to prevent mice from liver injury,12–15 inflammatory bowel disease,16 and ulcerative colitis.17 To further clarify the biological significance of IL-22, Wolk et al 18 generated two types of IL-22 transgenic mice under the control of the EμLCK promoter expressed in lymphoid cells and the rat insulin II promoter expressed in pancreatic islet, respectively. Most of the IL-22 transgenic mice died within the first few days after birth. In addition, Savan et al.19 developed transgenic mice where the IL-22R1 was expressed on lymphocytes. These mice died of inflammation at 6–8 weeks after birth and also showed high levels of circulating IL-22, thus demonstrating that aberrant expression of the IL-22R1 was sufficient to drive IL-22 production by lymphocytes. In this manuscript, we demonstrate that a high percentage of inflammatory cells in the patients with chronic hepatitis B (HBV) or HCV expressed IL-22, and the number of IL-22 positive cells correlates positively with the grade of liver inflammation and serum levels of aspartate aminotransferase, thus implicating IL-22 expressing lymphocytes as mediators of pathogenesis in these diseases. At present, there are no small animal models available with chronic viral hepatitis and liver inflammation that is associated with elevation of IL-22 in the liver. As mentioned above, transgenic mice with IL-22 overexpression in lymphoid cells died within the first few days after birth.18 Thus, in order to define the role of elevated IL-22 in the pathogenesis of liver disease; we developed transgenic mice with overexpression of IL-22 in the liver under the control of the albumin promoter to imitate the situation in viral hepatitis patients with high levels of IL-22 in the liver. In contrast to the EμLCK promoter- or the rat insulin II promoter-driven IL-22TG mice,18 liver-specific IL-22TG had no obvious adverse phenotypes and no overt inflammation, but were completely resistant to T cell hepatitis, had accelerated liver regeneration after partial hepatectomy and showed increased sensitivity to diethylnitrosamine (DEN)-induced liver cancer.
Materials and Methods
Human liver samples
Most of human cirrhotic liver samples were obtained from the recipient livers after transplantation. Some of liver samples from patients with chronic HBV or HCV were obtained by biopsy. The detailed information about these samples is described in supplemental Table S1. Evaluation of severity of disease was followed Scheuer criterion. The degree of inflammatory infiltration was defined as Grade (G) while the degree of fibrosis was defined as Stage (S). Normal healthy liver samples were obtained from the normal healthy donors for liver transplantation. The study protocol involved in human samples was approved by the local ethics committee and every patient provided written informed consent.
Generation of IL-22TG mice
Liver specific IL-22 transgenic mice on the C57BL/6 background were generated by microinjection of the recombinant IL-22 expression vector containing the full-length murine IL-22 cDNA driven under a liver specific albumin promoter. The detailed generation and genotyping are described in supplemental materials. All animal experiments were approved by the NIAAA animal care and use committee.
Diethylnitrosamine (DEN)-induced hepatocellular carcinoma model
Mice were injected with 10µg/g or 20µg/g body weight of DEN (Sigma, St Louis, MO) at age of 15 days and sacrificed at 9 months old to examine incidence, size, and number of the tumors in the liver.
Statistical analysis
Data are expressed as means ± SD. To compare values obtained from three or more groups, one-factor analysis of variance (ANOVA) was used, followed by Tukey’s post hoc test. To compare values obtained from two groups, the student t test was performed. The correlations between variables were assessed by the Spearman rank order test. Statistical significance was taken at the P<0.05 level.
The other methods are included in the supplemental materials
RESULTS
Upregulation of hepatic IL-22 expression in patients with chronic HBV or HCV
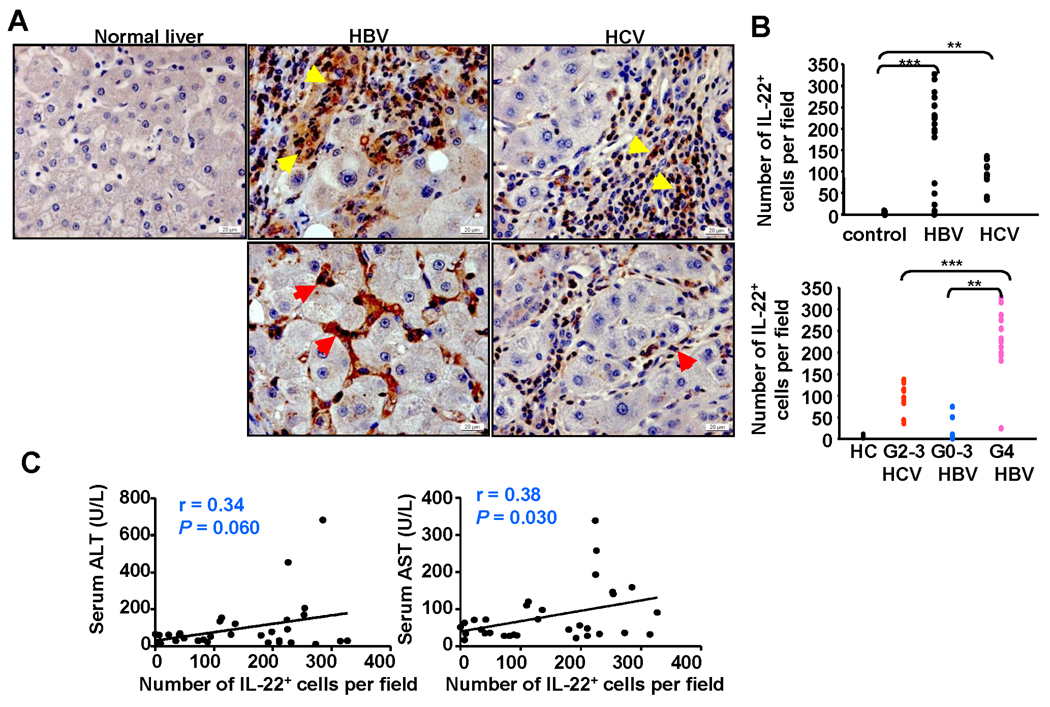
Immunohistochemistry analyses with anti-IL-22 antibody were performed on 37 human liver tissue samples from patients with hepatolithiasis (5 cases), HBV (22 cases), and HCV (10 cases). No obvious IL-22 positive staining was observed in the livers from healthy donors or from patients with hepatolithiasis. In contrast, the majority of inflammatory cells in HBV cirrhotic patients with active hepatitis (Inflammation grade 4: G4) stained positively for IL-22, while the number of IL-22+ lymphocytes was lower in those with lower inflammatory scores (G0-G3) (Figs. 1A–B and supplemental Table S1). A significant number of IL-22+ lymphocytes were also observed in HCV cirrhotic livers. All of these HCV cirrhotic liver samples had a grade of inflammation score G2 except one that had G3. The number of IL-22+ lymphocytes in these HCV patients with G2-3 was lower than that in HBV patients with a higher grade of inflammation (G4) (Fig. 1B). Furthermore, in both HBV and HCV cirrhotic livers, most of the IL-22+ lymphocytes were located in the inflamed regions, but also observed in liver sinusoids in some patients (Fig. 1A).
Fig. 1.
IL-22+ cells accumulate in the livers of patients with chronic HBV or HCV. (A) Immunohistochemical staining for IL-22 in normal healthy livers, HBV or HCV cirrhotic livers. Positive staining with IL-22 Ab is shown as a dark red color. IL-22+ immune cells are located mainly in inflammatory regions (upper panel: yellow arrows) but are also observed in sinusoids (lower panel: read arrows) from some patients. (B) The number of IL-22+ lymphocytes/per field (×100) correlates positively with the grade of inflammation. Each dot represents one patient. G: Grade of inflammation. **P<0.01; ***P<0.001 (C) Correlation between the number of IL-22+ cells/per field (×100) in the livers and serum ALT or AST from viral hepatitis patients.
IL-22 has been shown to protect against liver injury in various models,12–14 Thus, we examined the correlation between the number of IL-22+ cells and liver damage. As shown in Fig. 1C, the number of IL-22+ cells had a positive correlation with serum levels of aspartate aminotransferase (AST), and also had a trend to a positive correlation with serum levels of alanine transaminase (ALT).
Interestingly, a positive correlation between serum IL-22 and liver injury was also observed in a mouse model of T cell hepatitis. As shown in supplemental Fig. S1, serum or hepatic IL-22 levels had a positive correlation with serum ALT and AST in this T cell hepatitis model induced by different doses of Concanavalin A.
Generation of liver-specific IL-22 transgenic mice
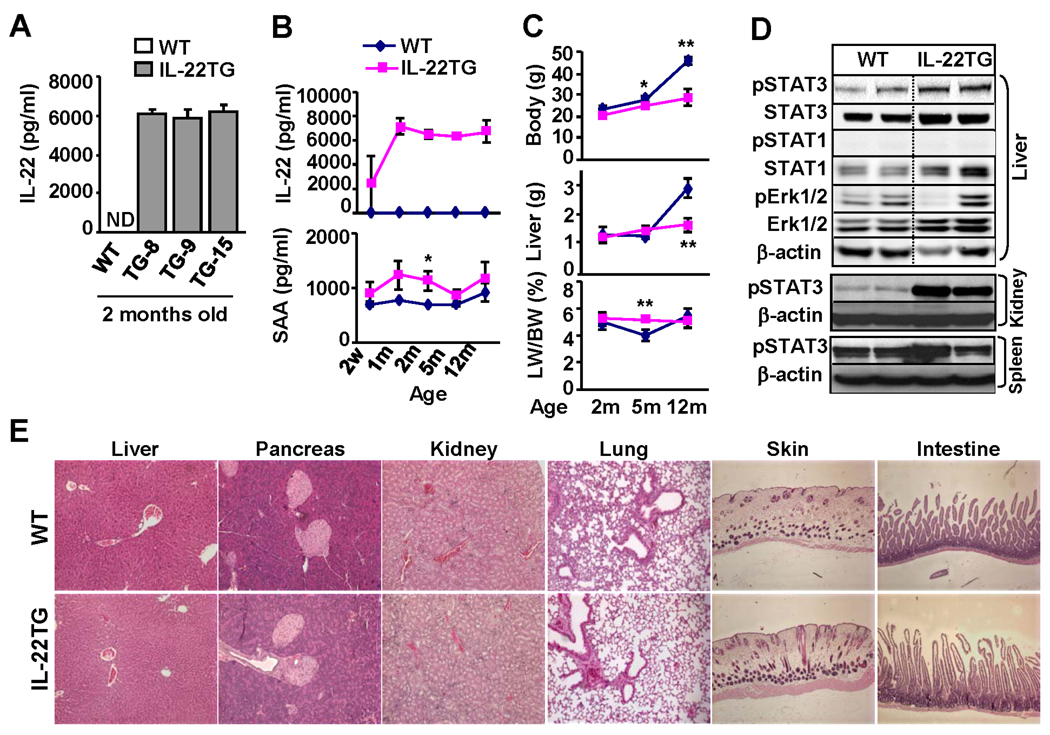
To understand the biologic significance of IL-22 upregulation in liver diseases, we generated liver specific IL-22 transgenic mice (C57BL/6 background) where IL-22 expression was under the control of the albumin promoter and enhancer. We obtained 3 founders (TG-8, TG-9, and TG-15) with serum levels of IL-22 reaching ~6000 pg/ml. All of experiments described below were obtained from the TG-8 (referred as IL-22TG). Many of these experiments were confirmed utilizing TG-9 or TG-15, thus demonstrating that our findings are due to the transgene, not the unique founder line of mice.
Fig. 2A shows that high levels of serum IL-22 were detected in the 3 founders of transgenic lines but not in WT mice. Serum levels of IL-22 were detected as early as 2 weeks in IL-22TG after birth and reached the peak level (~ 6000 pg/ml) at 1 month (Fig. 2B). Such levels of serum IL-22 were maintained for the lifetime of mice and did not change during the backcrossing with C57BL/6 mice.
Fig. 2.
Characterization of IL-22TG mice. (A) Serum IL-22 levels from 3 founders of IL-22TG mice. (B) Serum IL-22 and SAA levels at various ages of IL-22TG mice. (C) Body and liver weight. *P<0.05, **P<0.01 compared with the corresponding WT groups. (D) Western blot analyses of various tissues from 2-month-old mice. (E) H&E staining of various organs from 2-month-old mice.
IL-22 is known to induce expression of acute phase proteins (e.g. serum amyloid A: SAA) and multiple signaling pathways in hepatocytes.2, 20 Here we observed that IL-22TG mice had a trend to higher levels of serum SAA compared with WT mice, with a statistical difference being reached at age of 2 months (Fig. 2B). In addition, microarray data revealed that hepatic RNA expression of SAA, as well as several other acute phase proteins, were elevated in IL-22TG mice vs. WT mice (Table 1).
Table I.
Upregulation of genes in the livers from IL-22TG vs WT mice
| Gene title | UniGene ID | Gene symbol |
Fold change (IL-22TG vs WT) |
|---|---|---|---|
| Anti-oxidatant genes | |||
| Metallothionein 1 | Mm.192991 | Mt1 | 20.4 |
| Metallothionein 2 | Mm.147226 | Mt2 | 37.1 |
| Mitogenic and proliferative genes | |||
| Cyclin B1 | Mm.380027 | Ccnb1 | 1.5 |
| Cyclin D1 | Mm.273049 | Ccnd1 | 1.8 |
| Growth arrest specific gene 5 | Mm. 270065 | Gas5 | 1.5 |
| Growth arrest specific gene 6 | Mm. 3982 | Gas6 | 1.6 |
| Neuropilin 1 | Mm.271745 | Nrp1 | 2.4 |
| Acute phase genes | |||
| Serum amyloid A1 | Mm.148800 | Saa1 | 3.2 |
| Serum amyloid A2 | Mm.200941 | Saa2 | 5.3 |
| Serum amyloid A3 | Mm.14277 | Saa3 | 1.7 |
| Serum amyloid A4 | Mm.3489 | Saa4 | 2.1 |
| Serum amyloid P-component | Mm.330510 | Apcs | 2.5 |
| Fibrinogen-like protein 1 | Mm.277955 | Fgl1 | 3.0 |
| Orosomucoid 2 | Mm.14173 | Orm2 | 9.2 |
| Orosomucoid 3 | Mm.381399 | Orm3 | 2.8 |
| CD14 | Mm.3460 | Cd14 | 1.6 |
| LPS binding protein | Mm.218846 | LBP | 2.0 |
| Antimicrobial genes | |||
| Lipocalin 2 | Mm. 9537 | Lcn2 | 25.1 |
| Proteinase 3 | Mm. 2364 | Prtn3 | 7.4 |
| Cytokine signaling –related genes | |||
| Suppressor of cytokine signaling 3 | Mm.3468 | Socs3 | 4.0 |
| Suppressor of cytokine signaling 2 | Mm.474283 | Socs2 | 2.8 |
| Signal transducer and activator of transcription 3 | Mm.249934 | Stat3 | 1.6 |
| Janus kinase 3 | Mm. 249645 | Jak3 | 1.6 |
All IL-22TG mice grew normally without obvious adverse phenotypes except a lower body weight after 5 months of age compared with WT mice (Fig. 2C). Food intake was similar in both IL-22TG and WT mice (data not shown). In addition, at 2 months of age, both IL-22TG and WT mice had a similar liver weight and liver/body weight ratio; at 5 months of age, IL-22TG mice had similar liver weights but a higher liver/body weight ratio compared with WT mice. In contrast, at 12 months of age, IL-22TG mice had a lower liver weight but similar liver/body weight ratio compared with WT mice.
Western blot analyses in Fig. 2D show that pSTAT3 but not pSTAT1 and ERK1/2 activation was elevated in the livers of IL-22TG mice vs. WT mice. Activation of pSTAT3 was also detected in the kidney but not the spleen from IL-22TG mice (Fig. 2D), thus indicating that the circulating IL-22 had effects beyond the tissue in which it is being produced. The lack of effects in the spleen was not surprising, as normal mouse lymphocytes/leukocytes lack IL-22R1.4
Histology analyses showed that all of the organs from IL-22TG mice had a normal histology except for slightly thicker epidermis and minor inflammation in the skin compared with WT mouse skin (Fig. 2E and supplemental Fig. S2a). No obvious inflammation or necrosis was observed in the organs obtained from IL-22TG mice. Furthermore, flow cytometric analyses of leukocytes from the liver and other organs revealed no significant difference in the total number of leukocytes and the percentages of cell populations when comparing WT and IL-22TG mice (supplemental Table S2). Finally, expression of IL-22R1 and IL-10R2 in the liver was comparable in WT and IL-22TG mice as demonstrated by RT-PCR and real-time PCR (supplemental Fig. S2b).
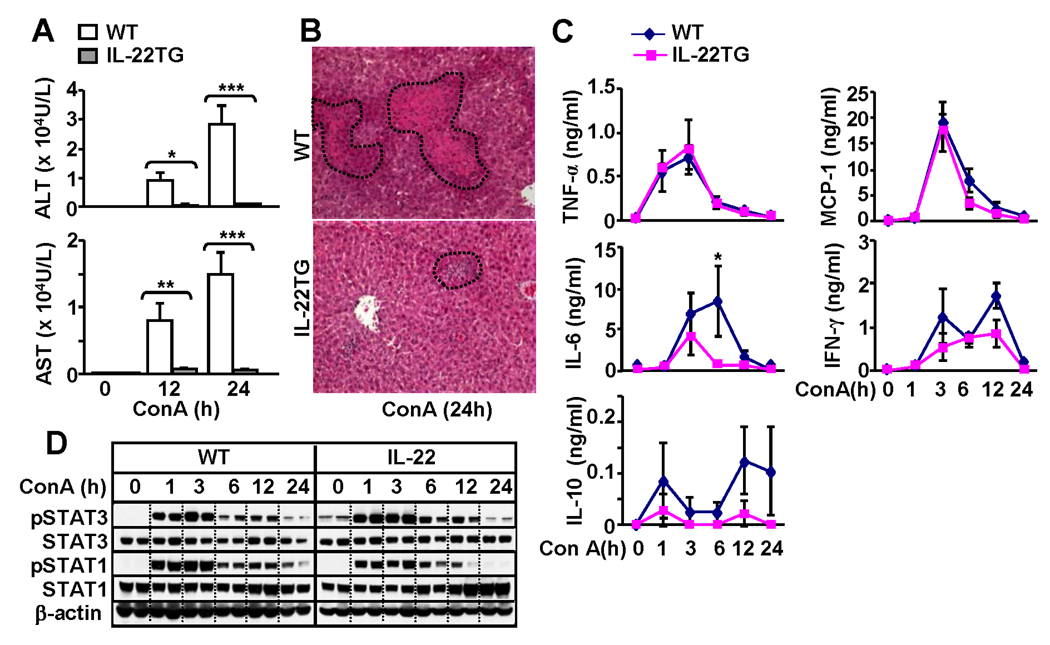
IL-22TG mice are protected from Con A-induced T cell hepatitis
Next we compared the liver injury in WT and IL-22TG mice in a model of Con A-induced T cell hepatitis. As illustrated in Figs. 3A–B, IL-22TG mice were completely protected from the liver injury induced by Con A injection. While WT and IL-22TG mice had comparable levels of multiple cytokines and chemokines including TNF-α, IL-10, MCP-1, and IFN-γ, serum levels of IL-6 were higher in WT than in IL-22TG mice (Fig. 2C). This increase in IL-6 may be due to massive liver necrosis observed in WT mice after Con A injection since necrotic hepatocytes are known to stimulate Kupffer cells to produce IL-6.21
Fig. 3.
IL-22TG mice are resistant to Con A-induced T cell hepatitis. Mice were treated with Con A (10µg/g) and then euthanized at different time points. (A) Serum ALT and AST levels. (B) H&E staining of the liver tissues from the mice treated with Con A for 24h. (C) Serum cytokines. (D) Western blot analyses. *P<0.05, **P<0.01, and ***P<0.001 compared with the corresponding WT groups.
To further understand the mechanisms underlying the resistance of IL-22TG mice to Con A-induced liver injury, we examined hepatic STAT3 and STAT1 activation, as these transcription factors play important roles in protecting against and promoting Con A-induced liver injury, respectively.22 As illustrated in Fig. 3D, Con A injection induced activation of both STAT3 and STAT1 in WT mice. In contrast, IL-22TG mice had higher basal levels of pSTAT3 and activation of STAT3 was slightly enhanced while STAT1 activation was lower in IL-22TG mice compared with WT mice.
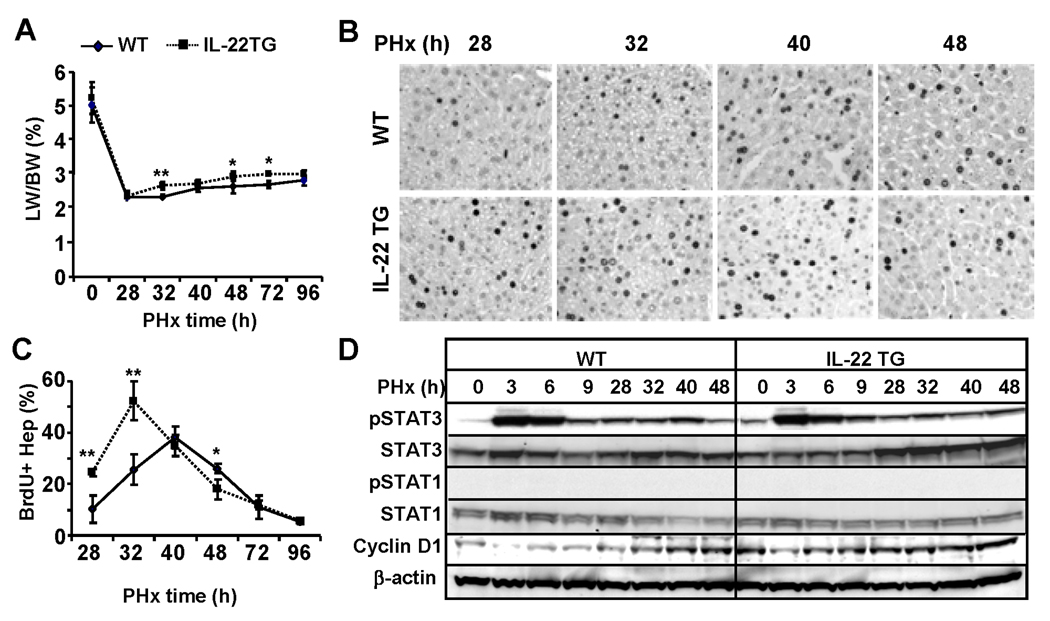
IL-22TG mice have accelerated liver regeneration after partial hepatectomy (PHx)
Liver regeneration induced by PHx was also examined in IL-22TG mice. As illustrated in Fig. 4A, the liver/body weight ratio was similar before PHX (0) but was significantly higher in IL-22TG mice than in WT mice 32h, 48h and 72h post PHx, and returned to the same levels 96h after PHx in both groups. BrdU+ incorporation experiments demonstrated that IL-22TG mice had an accelerated peak of hepatocyte proliferation at 32h post PHx, and this peak is significantly higher when compared with WT mice. In addition, the number of BrdU+ hepatocytes was higher at 28h but lower at 48h post PHx in IL-22TG mice than that in WT mice, respectively, and was comparable 72h and 96h post surgery in both groups (Figs. 4B–C). These findings indicate that IL-22TG mice have accelerated liver regeneration after PHx.
Fig. 4.
IL-22TG mice have accelerated liver regeneration after PHx. Mice were subject to PHx and injected with BrdU 2h before sacrifice. Liver tissues were collected for BrdU immunostaining. (A) Liver/body weight ratio. (B) Immunohistochemical staining with anti-BrdU antibody. BrdU+ cells are stained with as dark nuclei. (C) The number of BrdU+ hepatocytes is shown. (D) Western blot analyses of liver tissues from partially hepatectomized WT and IL-22TG mice. *P<0.05, **P<0.01, and ***P<0.001 compared with the corresponding WT mice.
In order to define the underlying mechanism for this enhanced liver regeneration in IL-22TG mice, western blot analyses for STAT3 and STAT1 activation were performed. Fig. 4D revealed that STAT3 but not STAT1 activation was detected after PHx and that such activation was comparable between WT and IL-22TG mice. However, IL-22TG mice had higher basal levels of pSTAT3 than WT mice. In addition, expression of cyclin D1 was higher in IL-22TG mice than in WT mice prior to treatment and at early time points after PHx (Fig. 4D). Thus the increased levels of cyclin D1 may contribute to the accelerated liver regeneration in IL-22TG mice after PHx.
IL-22TG mice do not spontaneously develop liver tumor but exhibit accelerated DEN-induced tumorigenesis
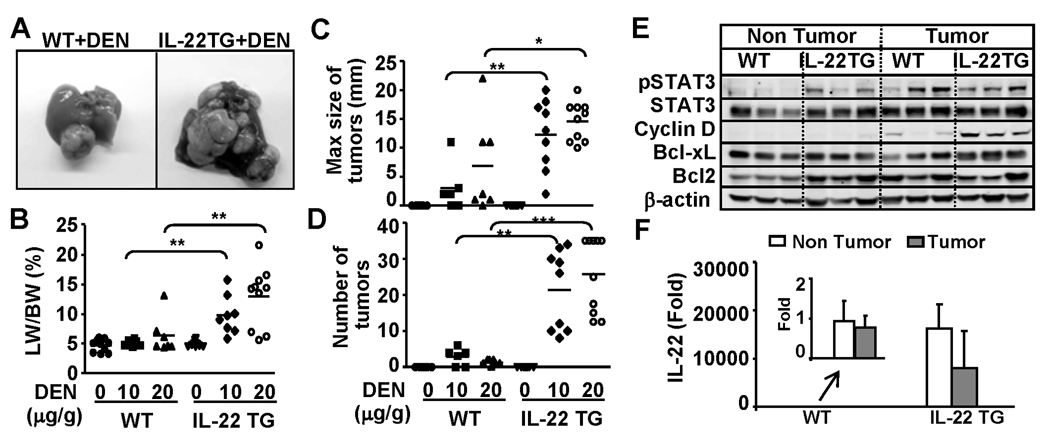
IL-22 has been shown to promote hepatoma cell proliferation and survival;12 however, no tumor was observed in all organs including the liver from IL-22TG mice up to 2 years of age (data not shown). Next, we examined whether IL-22TG mice were more susceptible to DEN-induced liver tumorigenesis. As illustrated in Figs. 5A–5B, IL-22TG mice had a bigger liver tumor size and a higher liver/body weight ratio than WT mice 9 months post DEN injection. Liver histology confirmed that the liver tumors from both WT and IL-22TG mice are hepatocarcinoma (supplemental Fig. S3a). The incidence of tumor development in IL-22TG mice was 100% whereas the WT littermate group only had 60% and 80% tumor incidence after injection of 10µg/g and 20µg/g, respectively (supplemental Fig. S3b). In addition, the maximum size of tumors and the number of tumors were greater in IL-22TG mice compared with WT mice post DEN injection (Figs. 5C–D)
Fig. 5.
IL-22TG mice do not spontaneously develop liver tumor but are more susceptible to DEN-induced liver tumorigenesis. Mice were injected with 10µg/g or 20µg/g body weight of DEN at 15 days of age and then sacrificed at 9 months old. Liver tissue and tumors were collected. (A) Representative photos of liver with tumors. (B) Liver/body weight ratio. (C) Size of tumors. (D) Number of tumors. (E) Western blot analysis. (F) Real-time PCR analyses of IL-22 expression in the 9-month-old DEN-treated WT and IL-22TG mice. The value of non-tumor tissues from DEN-treated WT mice was set as 1. *P<0.05, **P<0.01, and ***P<0.001.
Western blot analysis demonstrated that pSTAT3 activation was detected in both non-tumor and tumor regions in IL-22TG mice, but was detected only in the tumor region in WT mice. In addition, cyclin D1 and Bcl-xL expression were higher in the tumors from IL-22TG mice than those from WT mice (Fig. 5E). Real-time PCR analyses showed that IL-22 was detected at low levels in non-tumor and tumor tissues from DEN-treated WT mice, but was detected at very high levels in non-tumor and tumor tissues from DEN-treated IL-22TG mice (Fig. 5F). The levels of IL-22 in tumor tissues were slightly lower compared with those in nontumor tissues from DEN-treated IL-22 mice (Fig. 5F). Furthermore, the number of Ki67 positive cells was higher in the tumor tissues from IL-22TG mice compared with WT mice (supplemental Fig. S3c).
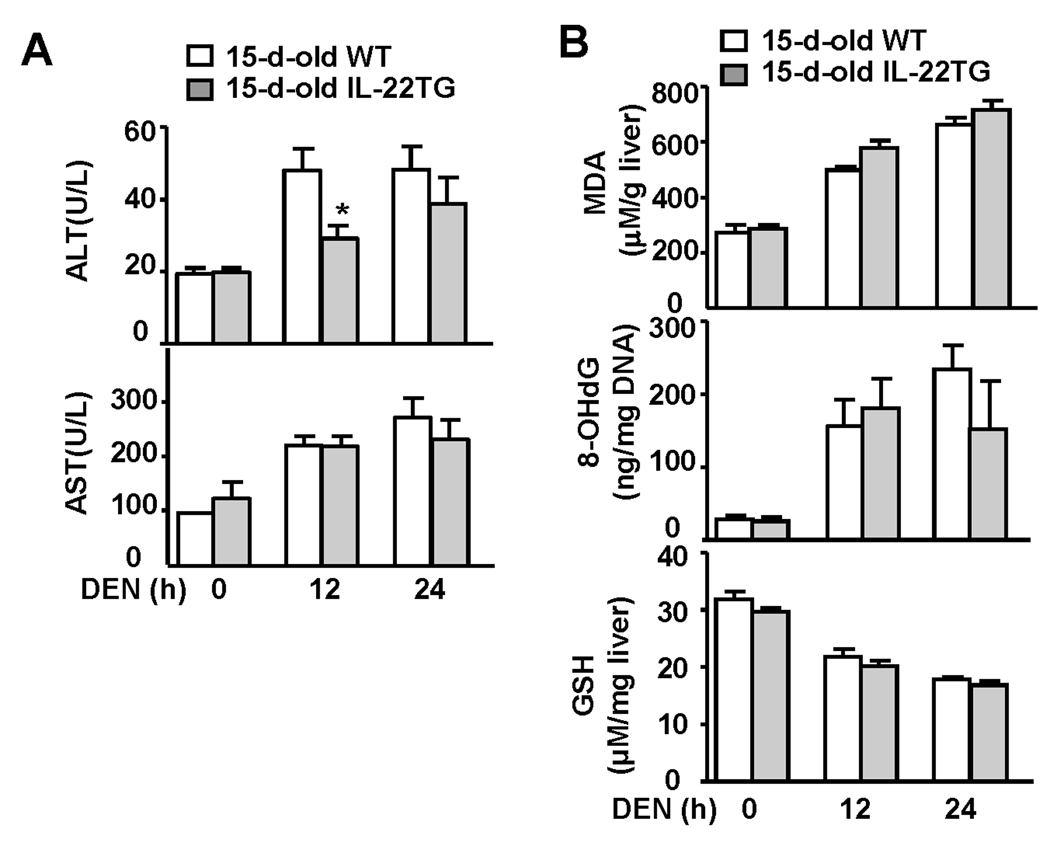
To rule out whether increased DEN-induced liver tumorigenesis in IL-22TG mice was due to an increase in DEN metabolism-associated liver injury and oxidative stress induced by a single dose of DEN injection at age 15 days, we measured serum ALT and AST, liver glutathione (GSH, an antioxidant), malondialdehyde (MDA: a marker of lipid peroxidation), and 8-Hydroxy-2'-deoxyguanosine (8-OHdG: a major product of DNA oxidation). As illustrated in Fig. 6A, DEN injection induced elevation of serum ALT and AST in both WT and IL-22TG mice with lower serum ALT in the latter group 12 h post injection. A single dose of DEN injection induced elevation of MDA and 8-OHdG and similar reduction of GSH in WT mice. Similar changes were also observed in IL-22TG mice. These findings indicated that a single injection of DEN induced similar levels of lipid peroxidation and DNA oxidation in the liver of WT and IL-22TG mice at age 15 days, suggesting the increased DEN-induced liver tumorigenesis in IL-22TG is not caused by an increase in DEN-metabolism.
Fig. 6.
Liver injury, oxidative stress, and DNA damage in 15-day-old WT and IL-22TG mice after a single dose of DEN injection. Fifteen-day-old WT and IL-22TG mice were injected (i.p) with DEN (10 µg/g) for 12h and 24h. (A) Sera were collected for measurement of ALT and AST. * P < 0.05. (B) Liver tissues were collected for measurement of MDA, 8-OHdG, and GSH.
Upregulation of acute phase protein, antioxidant, mitogenic, and antimicrobial genes in the lives from IL-22TG mice
Microarray analyses show that many genes are induced in the livers from IL-22TG mice compared with WT mice (Table 1). Among them, the expression of two antioxidant genes, metallothionein 1 and 2, was upregulated 20 and 37 fold in IL-22TG mice vs. WT mice, respectively. Induction of these antioxidant genes in hepatocytes may be responsible for the hepatoprotection of IL-22. Several mitogenic and proliferative genes were also upregulated from 1.5 to 2.4 fold in the livers from IL-22TG compared with WT mice, which likely attribute IL-22 promotion of liver regeneration and DEN-induced liver carcinongenesis.
IL-22 has been shown to stimulate hepatocytes to produce several acute phase proteins, including SAA, CD14, and LPS binding protein and these genes were upregulated in IL-22TG mice as compared to WT mice (Table 1). In addition, several other acute phase genes such as orosomucoid, fibrinogen-like protein and serum P-component were also upregulated in the livers from IL-22TG as compared to WT mice. It has been well documented that IL-22 plays an important role in protecting against bacterial infection via stimulating epithelial cells to produce anti-bacterial proteins.2, 3 Here we show that expression of two antimicrobial genes including lipocalin 2 and proteinase 3 was highly induced in the livers of IL-22TG vs WT mice. Collectively, these findings suggest that targeting hepatocytes by IL-22 may also play an important role in the host defense against bacterial infection via induction of acute phase proteins and antimicrobial proteins.
Discussion
Although the hepatoprotection of IL-22 has been well documented,12–14 the current study from IL-22TG mice provides several novel findings and implications of IL-22 in the pathogenesis of human liver diseases. (1) IL-22TG mice grew normally, suggesting that the therapeutic application of IL-22 in treating patients with liver injury may have minimal side effects; (2) The protective role of IL-22 in liver injury is due to its direct hepatoprotection and not due to modulation of the inflammatory response; (3) hepatic IL-22 is upregulated in patients with chronic HBV and HCV, and likely promotes hepatocyte survival and may accelerate liver cancer promotion in these patients.
The fact that liver-specific IL-22TG mice had no obvious adverse phenotypes suggests that the therapeutic application of IL-22 in treating patients with acute liver injury and alcoholic hepatitis may have few side effects. IL-22TG mice driven by the EμLCK or insulin II promoter had severe adverse phenotypes (most of mice died within a few days after birth).18 In contrast, the liver-specific IL-22TG mice described here develop normally and have no obvious adverse phenotypes. One possible explanation for the differences in the studies is that the IL-22TG driven by the EμLCK or insulin II promoter resulted in high levels of IL-22 expression before birth, while the IL-22TG driven by the albumin promoter only expressed IL-22 after birth (albumin expression by hepatocytes occurs after birth). Recently, Liang et al 20 reported that injection of 5×1010 particles of IL-22 adenovirus resulted in serum levels of 35,000–95,000 pg/ml IL-22 and causes hematological changes, loss of body weight, and thymic atrophy etc., while we have previously shown that injection of 1×108 particles of IL-22 adenovirus resulted in serum levels of 5,000 pg/ml IL-22 (similar to those in liver-specific IL-22TG mice) and did not induce obvious adverse phenotypes.14 These findings suggest that only very high doses of IL-22 may cause severe adverse phenotypes. However it is unlikely that therapeutic application of IL-22 will reach such high concentrations of IL-22 (35,000–95,000 pg/ml) in the serum reported in the study by Liang et al. 20 Regardless, monitoring IL-22 levels will be important in any therapeutic applications.
Although the hepatoprotection of IL-22 is well documented,12–14 the role of IL-22 in liver inflammation remains obscure. Liang et al 20 reported a single injection of IL-22 upregulated expression of CXCL1 in the liver, followed by a transient increase in circulating neutrophils, suggesting IL-22 may promote liver inflammation. In contrast, blockage of IL-22 via either using a neutralizing antibody 12 or genetic deletion 13 exacerbated inflammation while treatment with IL-22 12 or overexpression of IL-22 (this study) ameliorated Con A-induced liver inflammation, indicating IL-22 suppresses liver inflammation in this model. It is plausible that IL-22 plays dual roles in controlling liver inflammation: promoting liver inflammation via stimulating hepatocytes to produce acute phase proteins and chemokines, and inhibiting liver inflammation via preventing hepatocyte damage and subsequently reducing necrosis-associated liver inflammation. The final effect of IL-22 on liver inflammation is likely determined by the balance between the proinflammatory and anti-inflammatory effects of IL-22 and is dependent on the types of liver diseases and liver injury models.
A previous study reported that the expression of hepatic IL-22 mRNA was upregulated in the patients with viral hepatitis.23 Here we demonstrated that IL-22+ immune cells are accumulated in the livers of patients with viral hepatitis (Fig. 1); however, the types of inflammatory cells responsible for IL-22 production in viral hepatitis patients remain obscure. As Th17, Th22, NK, and NKT cells that are known to produce IL-22 2–4 and are elevated in the livers in patients with viral hepatitis,24–26 thus these cells likely contribute to hepatic IL-22 expression in the patients. Further studies are needed to confirm this.
The next obvious question is how IL-22 upregulation might affect the progression of viral hepatitis disease progression. First, it has been reported that IL-22 does not inhibit HCV replication in vitro,23 suggesting that elevated IL-22 may not affect HCV replication directly. However, we cannot rule out the possibility that IL-22 may affect indirectly HCV or HBV replication via modulation of the anti-viral effect of IFNs, since STAT3, a major IL-22 downstream signal, is known to modulate IFN-mediated signaling pathways.27 Second, although the number of IL-22+ cells correlated positively with the serum levels of AST in patients with viral hepatitis (Fig. 1), and serum and hepatic IL-22 levels correlated positively with serum ALT and AST in mice with T cell hepatitis (supplemental Fig. S1), the studies from animal models suggest that IL-22 protects against liver injury and promotes hepatocyte proliferation (this study).12–14 Thus, elevation of IL-22 in patients with viral hepatitis likely plays a compensatory role in protecting against hepatocellular damage. Third, while IL-22TG mice did not spontaneously develop liver tumor, these mice had accelerated DEN-induced liver tumor formation. This observation suggests that high levels of IL-22 alone may not induce liver tumor but that IL-22 may synergistically promote hepatic carcinogenesis in patients with chronic viral hepatitis. It is plausible that liver cancer in viral hepatitis patients is often associated with inflammation that produces IL-22, which may act as a local paracrine factor to stimulate liver cancer cell proliferation. Thus inhibition of IL-22 could be a potential therapeutic option for the treatment of liver cancer in those with high levels of inflammation and IL-22 in the liver.
Supplementary Material
Acknowledgments
This work is supported by the intramural program of NIAAA, NIH
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- CCl4
carbon tetrachloride
- ConA
concanavalin A
- DEN
diethylnitrosamine
- H&E
hematoxylin and eosin
- IL-22
interleukin 22
- TG
Transgenic
- PHx
partial hepatectomy
- pSTAT
phospholylated signal transducer and activator of transcription
- STAT
signal transducers and activators of transcription
- SAA
serum amyloid A
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- 1.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 2.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witte E WK, Warszawska K, Sabat R, Wolk K. Interleukin-22: A cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–379. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Lafdil F, Miller AM, Ki SH, Gao B. Th17 cells and their associated cytokines in liver diseases. Cell Mol Immunol. 2010;7:250–254. doi: 10.1038/cmi.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivella DB, Ferreira-Junior JR, Dumoutier L, Renauld JC, Polikarpov I. Structure and function of interleukin-22 and other members of the interleukin-10 family. Cell Mol Life Sci. 2010;67:2909–2935. doi: 10.1007/s00018-010-0380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med. 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 8.Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin' through a glass onion. Eur J Immunol. 2008;38:3265–3268. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- 9.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, Nojima Y. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–1046. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 11.Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- 12.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 13.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, Huang H, Wang Z, Huang Z, Huang Y, Yan X, Chen Y. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339–347. doi: 10.1016/j.jhep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, Johnson-Leger C, Volk HD, Sterry W, Sabat R. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med. 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 19.Savan R, McFarland AP, Reynolds DA, Feigenbaum L, Ramakrishnan K, Karwan M, Shirota H, Klinman DM, Dunleavy K, Pittaluga S, Anderson SK, Donnelly RP, Wilson WH, Young HA. A novel role for IL-22R1 as a driver of inflammation. Blood. 2011;117:575–584. doi: 10.1182/blood-2010-05-285908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang SC N-NC, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL, Collins M, Dunussi-Joannopoulos K, Fouser LA. IL-22 Induces an Acute-Phase Response. J Immunol. 2010;185:5531–5538. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- 21.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 22.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, Gao B. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dambacher J, Beigel F, Zitzmann K, Heeg MH, Goke B, Diepolder HM, Auernhammer CJ, Brand S. The role of interleukin-22 in hepatitis C virus infection. Cytokine. 2008;41:209–216. doi: 10.1016/j.cyto.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, Fu JL, Shi F, Shi M, Wang HF, Wang FS. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
- 25.Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, Rehermann B. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–335. e1–e2. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281:14111–14118. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.