Waves of correlated activity sweeping across the early postnatal mouse retina promote the segregation and refinement of retinofugal projections. This process has been thought to be spontaneous and unaffected by visual experience. We found, however, that light prolongs spiking during the waves and enhances the segregation of retinogeniculate afferents, and that it did so by activating melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs).
Retinal waves are spontaneous depolarizing events that propagate across the developing inner retina, triggering bursts of spikes in retinal ganglion cells1. From birth through postnatal day 10 (P10), retinal waves are generated and propagated through the release of acetylcholine from starburst amacrine cells2. These events, termed Stage II waves, drive ganglion cells to fire synchronously with their neighbors while cells elsewhere in the same retina or in the other eye are largely silent. Perturbations in the frequency, intensity or duration of waves can disrupt segregation and refinement of retinofugal projections3, 4. Because waves occur well before rods and cones can drive the inner retina5, they have been viewed as occurring autonomously, without any instructive or permissive role for photoreceptors. The discovery of ganglion-cell photoreceptors, however, prompted us to reexamine this assumption.
Rare retinal ganglion cells express the photopigment melanopsin and function as autonomous photoreceptors6, 7. These ipRGCs fire tonically when illuminated and, like conventional ganglion cells, receive indirect rod and cone inputs8. Surprisingly, they also appear to exert centrifugal influences on other neurons of the mature retina, including dopaminergic amacrine cells9. ipRGCs differentiate concurrently with conventional ganglion cells and are photosensitive as early as P0 in mice, making them the first functional retinal photoreceptors10, 11. The precocious photosensitivity of ipRGCs and their capacity for intraretinal signaling provides a substrate for possible photic modulation of retinal waves.
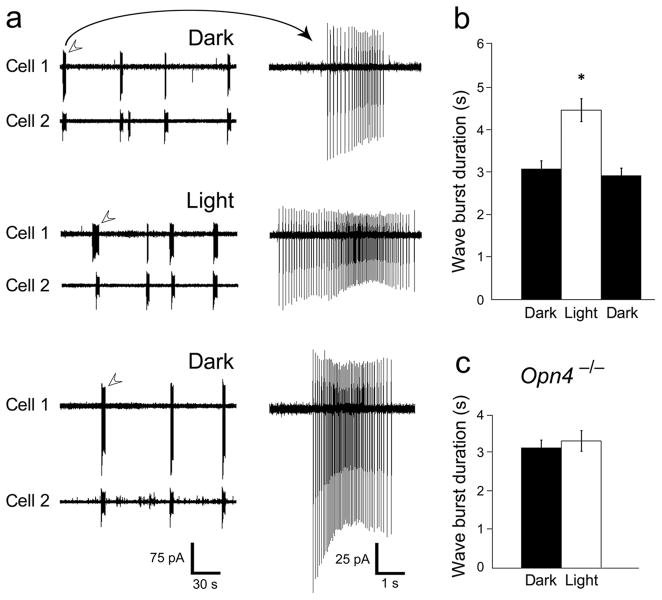
To test for such modulation, we recorded wave-associated spiking in P4-P7 wildtype mouse ganglion cells. (All animal procedures were approved by the Brown University Institutional Animal Care and Use Committee). Retinal illumination clearly increased the duration of the episodic bursts of spikes that are the hallmark of retinal waves. This was evident in multiunit recordings with a multi-electrode array (MEA; see Supplemental Fig. 1) and was confirmed at the single-cell level by recordings from conventional ganglion cells. An example of two such cells, recorded simultaneously by the loose-patch method, appears in Figure 1a. Bursts occurred synchronously in the two cells, confirming that they were triggered by retinal waves. Light appeared to increase the duration of bursts in Cell 1 whereas the photic effect was less obvious for Cell 2. Group data for such single and paired recordings (Fig. 1b) revealed that light increased burst duration by nearly 50% (dark: 3.08 ± 0.2 s; light: 4.47 ± 0.27 s; mean ± s.e.m.). Burst duration shrank again after a 10 minute period of dark recovery (2.93 ± 0.18 s; n=9). The effect of light was highly significant for the pooled data (p < 0.0001). It was also very consistent; every cell exhibited an increase in mean burst duration in the light, although these reached significance (p < 0.05) for only a minority of individual cells (3/9; 33%) due to variability and relatively small sample size. Mean firing rate during the burst was not altered by illumination and there did not appear to be a change in correlated firing (data not shown).
Figure 1.
Light increases wave duration in conventional ganglion cells. (a) Simultaneous loose-patch voltage-clamp recordings from two conventional ganglion cells located <150 μm apart, in the dark (top traces), in the light (middle traces) and after a 10 minute period of dark recovery (bottom traces). Representative wave-associated bursts of spikes (arrows) are shown on right at a faster time-base and increased gain. Both cells were conventional ganglion cells, not ipRGCs, because they lacked EGFP in this melanopsin reporter mouse and lacked any detectable direct photoexcitation. (b) Pooled data for burst duration in conventional ganglion cells recorded by this loose-patch method. Light increased wave burst duration by an average of 45% over the dark condition (* p < 0.0001, n=9). (c) In a melanopsin knockout retina (Opn4 −/−), wave-associated bursts were not significantly longer in the light than in the dark (p ≫ 0.05; n=13). Error bars represent s.e.m.
Because ipRGCs are the only functional photoreceptors at this stage of development, we suspected they would be essential for the observed effects of light on wave duration. To test this hypothesis, we turned to melanopsin knockout mice (Opn4 −/−), in which ipRGCs lack the capacity for phototransduction but remain otherwise normal in structure and function12. Light failed to alter wave burst duration in Opn4 −/− mice, confirming an essential role for ipRGCs in this process (Fig. 1C; mean burst durations in the dark: 3.14 ± 0.21 s; light: 3.31 ± 0.19 s; p>0.05; n=13). Wildtype littermate controls (Opn4 +/−) exhibited the same photic effect on wave duration we had seen earlier (see Supplemental Fig. 2 for MEA and wildtype littermate control data). Taken together, these data indicate that light acts through melanopsin to alter the dynamics of retinal waves in conventional ganglion cells.
Retinal waves contribute to the segregation and refinement of retinogeniculate projections. Perturbing waves can disrupt this developmental process. Because light affects the waves, we wondered whether exposing early postnatal mice to light might alter the segregation of retinogeniculate afferents in a melanopsin-dependent manner. We reared Opn4 −/− mice and their wildtype littermate controls from birth in continuous light. At P7, we injected contrasting fluorescent anterograde tracers into the two eyes, and at P8 harvested the brains and digitally imaged the distribution of the two tracers in the dorsal lateral geniculate nucleus (dLGN; Fig. 2a; Supplemental Fig. 3). In both genotypes, ipsilateral and contralateral retinal afferents terminated in largely distinct sectors of the nucleus, but the transition between crossed and uncrossed input zones appeared less sharp in the Opn4 −/− than in wildtype brains. To quantify this, we plotted the distribution among all dLGN pixels of the metric R, the log ratio of pixel intensities for the ipsilateral and contralateral eye tracers13 (Fig. 2b; see Supplemental Fig. 4 for details). Opn4 −/− mice (Fig. 2b, blue curve) differed from wildtype (orange curve) only in the right half of this distribution, corresponding to pixels in which the ipsilateral-eye signal was relatively strong. In Opn4 −/− mice, more of these pixels had substantial contralateral-eye signal, and fewer were overwhelmingly dominated by the ipsilateral-eye channel. To test the significance of this difference, we defined as ‘unsegregated’ those pixels in which the red and green pixel intensities were more nearly matched (i.e., R was closer to zero) than 99% of pixels within the core of the ipsilateral and contralateral sectors of the dLGN (see Supplemental Methods). Unsegregated pixels made up a significantly larger fraction of all dLGN pixels in Opn4 −/− mice than in their littermate controls (Fig. 2c; see also Supplemental Fig. 3; n = 5 wildtype mice and 3 Opn4 −/− mice with 8 images per mouse). This difference was apparent in both left and right dLGN (Fig. 2c). These data imply that melanopsin mediates light-driven enhancement of ocular segregation in the retinogeniculate pathway.
Figure 2.
Light enhances ocular segregation of retinogeniculate afferents by a melanopsin-dependent mechanism. (a) Segregation of retinogeniculate afferents in mice reared in constant light as revealed by injections of fluorescent tracer into the contralateral (green) and ipsilateral (red) eyes (horizontal plane; single optical sections; scale bar: 50 μm for main panels; 10 μm for the enlarged panel). Rightmost panel shows expanded view of region within the white rectangle, the transition zone between sectors dominated by inputs from one eye. Top row of images is from a wildtype (WT), melanopsin-expressing mouse (Opn4 +/−); bottom row is from a melanopsin-knockout littermate (Opn4 −/−). (b) The distribution of pixel intensity ratios (R) for all dLGN pixels in the right dLGN of all mice (for each pixel, R = log of the ipsilateral-eye to contralateral-eye signal intensity). Opn4 −/− mice (blue curve) have more pixels with nearly matched strength of input from the two eyes (‘unsegregated’ as shown in grey), and fewer strongly dominated by the ipsilateral eye, than wildtype mice (orange curve). (c) Pooled data across mice showing that the fraction of all dLGN pixels that were ‘unsegregated’ (relatively balanced input from the two eyes) was higher in Opn4 −/− mice than in their wildtype littermates (** p < 0.01; * p < 0.05). The effect was evident on both sides of the brain. Error bars represent s.e.m.
The mechanism by which light modulates retinal waves remains to be delineated beyond the first step, namely melanopsin-based phototransduction by ipRGCs. Both gap junctions and glutamate receptors have been said to support intraretinal signaling by ipRGCs in other contexts9, 14. Through these or other means, ipRGCs could prolong retinal waves by increasing the excitability of conventional ganglion cells, permitting nicotinic input from the starburst network to hold the cells above spike threshold longer than normal. Alternatively, ipRGCs could prolong the waves at their site of generation in the starburst network, perhaps by altering nicotinic transmission or the potassium conductance that terminates each wave2.
We estimate that enough sunlight can penetrate the closed eyelids of mouse pups to drive photic alterations in waves (see Supplemental Methods). In the wild, such light exposure would presumably be limited, with pups rarely leaving the nest during the day. However, early postnatal mice do exhibit melanopsin-dependent negative phototaxis15, a behavioral adaptation implying at least occasional light exposure at this age. Note also that ipRGCs at this stage are excited by the waves themselves (Supplemental Fig. 5), which may be as effective as light in inducing ipRGC-mediated modulation of the waves. Thus, ipRGCs may sculpt wave dynamics even in total darkness.
We have demonstrated that Stage II waves of the early postnatal retina are modulated by light through the actions of intrinsically photosensitive retinal ganglion cells. This is unexpected because retinal waves have been considered to arise and propagate without any influence from the visual environment. In addition, ipRGCs themselves exhibit wave-associated spiking. Thus, even in the absence of light, ipRGCs may exert control over key circuit elements to modulate wave generation and dynamics. Retinal waves are thought to play an important role in the normal development of retinofugal projections to the dLGN and we have found that light acts through melanopsin and ipRGCs to increase the ocular segregation of retinogeniculate afferents. Thus, our findings point to a previously unrecognized contribution of ipRGCs to the development of the central visual system.
Supplementary Material
Acknowledgments
We thank Dr. Samer Hattar for sharing his Opn4Cre mouse line, Jessica Gandy for assistance with data processing, and Dianne Boghossian for technical assistance. Additionally we would like to thank Dr. Michael Crair and Dr. Onkar Dhande for advice on ocular injection techniques.
Supported by NIH R01 grants EY012793 and EY017137 as well as F32 grant EY020108.
Footnotes
AUTHOR CONTRIBUTIONS
S.W. performed the multi-electrode array recordings. J.R. performed the loose-patch recordings and the anatomical tracing studies. J.R. carried out all data analysis for both electrophysiological and anatomical studies. J.R. and D.B. wrote the manuscript.
References
- 1.Huberman AD, Feller MB, Chapman B. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng J, Lee S, Zhou ZJ. Nat Neurosci. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. J Neurosci. 2005;25:6929–6938. doi: 10.1523/JNEUROSCI.1470-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butts DA, Kanold PO, Shatz CJ. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich KA, Zhan Y, Blanks JC. J Comp Neurol. 1997;388:47–63. [PubMed] [Google Scholar]
- 6.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berson DM, Dunn FA, Takao M. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 8.Do MT, Yau KW. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang DQ, et al. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu DC, et al. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Sekaran S, et al. Curr Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas RJ, et al. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 13.Torborg CL, Feller MB. J Neurosci Methods. 2004;135:17–26. doi: 10.1016/j.jneumeth.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J, et al. Proc Natl Acad Sci U S A. 2010;107:17374–17378. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.