Abstract
Purpose
Admission infarct core lesion size is an important determinant of management and outcome in acute (<9 hrs) stroke. Our purpose was to: (1) determine the optimal CT perfusion (CTP) parameter to define infarct core using various post-processing platforms, and (2) establish the degree of variability in threshold values between these different platforms.
Methods
We evaluated 48 consecutive cases with vessel occlusion and admission CTP and DWI within 3 hours of each other. CTP was acquired with a “second-generation” 66-second biphasic cine protocol, and post-processed using “standard” (from two vendors, “A-std” and “B-std”) and “delay-corrected” (from one vendor, “A-dc”) commercial software. ROC curve analysis was performed comparing each CTP parameter - both absolute and normalized to the contralateral uninvolved hemisphere - between infarcted and non-infarcted regions, as defined by co-registered DWI.
Results
Cerebral blood flow (CBF) had the highest accuracy (ROC “area under curve”, AUC), for all three platforms (p<0.01). The maximal AUC's for each parameter were: absolute CBF 0.88, CBV 0.81, and MTT 0.82, and relative CBF 0.88, CBV 0.83, and MTT 0.82. Optimal ROC operating point thresholds varied significantly between different platforms (Friedman test, p<0.01).
Conclusion
Admission absolute and normalized “second-generation” cine acquired CT-CBF lesion volumes correlate more closely with DWI defined infarct core than do those of CT-CBV or MTT. Although limited availability of DWI for some patients creates impetus to develop alternative methods of estimating core, the marked variability in quantification amongst different post-processing software limits generalizability of parameter map thresholds between platforms.
Keywords: Acute Stroke, CT Perfusion, Cerebral Blood Flow, Cerebral Blood Volume
Introduction
There is interest in studying core and penumbra to investigate whether they improve clinical outcomes from the use of thrombolytic therapy.1, 2 DWI is widely accepted as the de-facto clinical reference standard for core, but widespread implementation of MRI is hindered by availability as well as technical and safety limitations.3 Non-contrast CT (NCCT) is currently the standard of care to exclude hemorrhage before thrombolytic therapy, yet sensitivity for early infarction is poor.4 A reliable “DWI-like” CT measure of infarct core could therefore be of interest.
CT perfusion (CTP) is widely available and acquisition times are rapid.5 The most common and robust method to calculate CTP parameters is singular value decomposition (SVD) deconvolution.5 Potential sources of variability in this method include placement of the venous normalization region of interest (ROI), computational differences in algorithms, and the delay between the arterial and tissue time-density curves (common in patients with AIS).6-8 Hence, different post-processing software platforms have the potential to result in different values for CTP parameters.
Although multiple studies have been published to determine the CTP parameter maps and corresponding threshold values that optimally define infarct core, systematic technical differences in both acquisition and post-processing algorithms between different platforms limit their generalizability and reproducibility. Most were not only vendor dependent,9 but were performed using “first generation” CTP acquisition protocols and post-processing software10-12– specifically 45 second acquisitions and early versions of deconvolution algorithms– which might exaggerate the magnitude of the CT-CBV lesion size if there is truncation of the tissue time-density curves.13 Our purpose was to: (1) determine the optimal CTP parameter to define infarct core using various post-processing platforms (compared to a DWI reference standard, and using a more current “second generation” 66-second biphasic cine acquisition protocol), and (2) establish the degree of variability in the optimal threshold values between these different platforms.
Methods
Patient Selection
We reviewed the records of all consecutive patients admitted with the diagnosis of acute ischemic stroke (AIS) within 9 hours of symptom onset from December 2006 to April 2008. We identified 98 patients with AIS in the anterior circulation who had biphasic CTP and DWI obtained within 3 hours of one another. Cases were excluded for no visible vessel occlusion (n=28), punctate or no apparent DWI lesion (n=10), or poor quality DWI (n=5) or CTP (n=7) acquisition due to motion or truncated arterial or venous density curves, yielding 48 cases for analysis. The study received Institutional Review Board approval and was Health Insurance Portability and Accountability Act-compliant.
Imaging Acquisition
CTP was performed on a multidetector helical scanner (64 slice LightSpeed GE Medical Systems, Milwaukee, WI) as a 66-second biphasic cine series, beginning 5 seconds after power injection of 40 ml of contrast at 7 ml/s which contains 755mg/ml of iopamidol (Isovue Multipack - 370; Bracco Diagnostics Inc., Princeton, NJ). Image acquisition was every half second for the first 40 seconds, which was followed by a 2 second pause and 8 more acquisitions every 3 seconds. Imaging parameters were 80 kVp, 200 mAs, 1-second rotation time. Coverage consisted of 2 slabs positioned parallel and superior to the orbital roof. Each slab consisted of 8 slices of 5 mm thickness.
DWI was obtained on a 1.5 Tesla Signa scanner (GE Medical Systems, Milwaukee, WI) using single shot, spin-echo echo planar imaging. High-b-value images (b= 1000 s/mm2) were acquired in six different gradient directions, in addition to a single low-b-value (b= 0 s/mm2) image. Other parameters were: repetition time of 5000 ms, time to echo of 90 to 100 ms, field-of-view of 22 × 22 cm, image matrix of 128 × 128, slice thickness of 5 mm with a 1 mm gap, and five signal averages.
Image Analysis
CTP maps were post-processed using delay corrected software (CTP5 “A-dc”, GE Healthcare, WI) and two standard deconvolution software packages (CTP3 “A-std”, GE Healthcare, WI and Brain Perfusion “B-std”, Philips, The Netherlands). DWI images were co-registered to CTP data using a fully automated rigid method (CTI Molecular Imaging-Reveal-MVS 6.2, Mirada Solution Ltd). The images were manually adjusted in case of unsatisfactory co-registration.
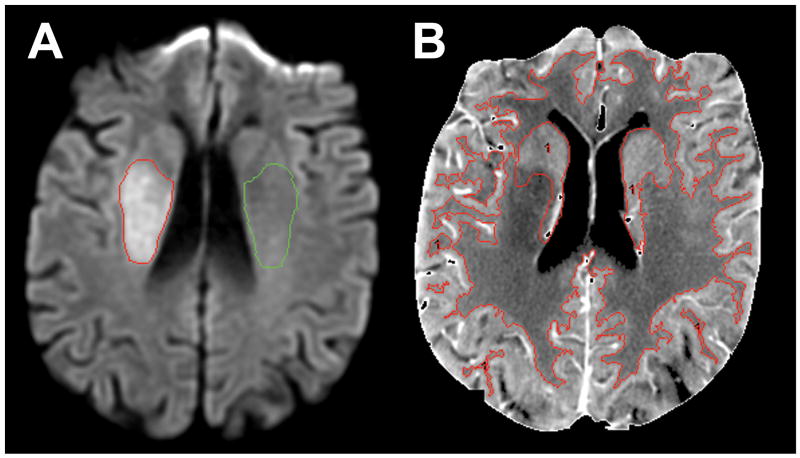
Visually detected DWI lesions were semi-automatically segmented, selecting only the slice with the largest area of the infarction, prior to the CTP analysis (Figure 1A). A mirrored region-of-interest (ROI) for normalization of the absolute voxel values was placed over the contralateral uninvolved hemisphere. Temporally averaged cine CTP images were served for segmentation of gray matter (GM), white matter (WM), and basal ganglia (BG) (Figure 1B). All ROI's were transposed onto the perfusion maps, and the voxel values were recorded using a commercial analysis program (Analyze 7.0, AnalyzeDirect, Mayo Clinic, Rochester, MN).
Figure 1.
Sample image segmentation methodology for right hemispheric infarct. A: Admission DWI lesion (red outline). A mirrored ROI (green outline) over the contralateral uninvolved hemisphere served for normalization of the absolute voxel values. B: Temporally averaged cine CTP image served as a template for the segmentation of GM, WM, and BG (red outline).
The normalized perfusion parameter values were calculated in 3 ways. First, by dividing each voxel value by the mean of the contralateral normal hemisphere voxel values; second, by dividing each voxel value by the mean contralateral reference ROI value; and third, by dividing each voxel value in GM, WM, and BG by the mean of the corresponding contralateral normal GM, WM, and BG values. Because there was no significant difference between these three normalization approaches, for simplicity we herein report only the results using the first method.
Statistics
For each CTP parameter map, both relative and absolute, ROC curves depicting the sensitivity/specificity for distinguishing core voxels from non-core voxels were generated from the pooled voxel values for all patients. Thresholds were then calculated as the optimum ROC operating point, with equally attributed weights to specificity and sensitivity; overall accuracy was estimated as the “area under curve” (AUC).
Because the millions of voxels contributing to the pooled ROC analysis could result in a statistically significant but not necessarily clinically meaningful comparison between the parameters being tested, a patient-based comparison of the individual ROC-AUC's was performed to compare relative CBF, CBV and CBV* CBF from each software package using a paired Wilcoxon analysis. Optimal ROC operating point thresholds for each parameter were also compared to assess for significant difference using a non-parametric paired comparison (Friedman Test) between different software package maps. STATA 10 (STATA; Stata, College Station, TX) software was used to perform the statistical analysis.
Results
Of the 48 patients included in the analysis, 22 (46 %) were male and mean age was 71.6 years (range: 26-97 years, SD: 14). Other important demographics were as follows [median (inter-quantile range)]: admission NIHSS 13 (8-20), symptom onset to CTP time 4.1 hours (2-5.3 hours), and CTP to DWI interval 34 minutes (28-43 minutes). Atrial fibrillation (AF) was present in 15 (31%) patients, all of whom had concomitant major intracranial vessel occlusions. Vessel occlusion was on the left in 29 (60%) patients. Location of occlusions were as follows: 4 (8%) internal carotid artery (ICA) and M1 segment of the middle cerebral artery (MCA); 4 (8%) ICA, M1 and M2; 3 (6%) ICA, M1 and M2 and anterior cerebral artery (ACA); 7 (15%) M1 only; 9 (19%) M1 and M2; 15 (31%) M2 only; 1 (2%) M2 and M3; 3 (6%) M3 only; 1 (2%) M1, M2 and ACA; and 1 (2%) M1 and ACA involved.
More than 2.5 million voxels were analyzed, approximately 250,000 of which corresponded to regions of restricted diffusion on DWI. Mean DWI lesion volume on the selected slices was 5.84 ml (range: 0.6-20.6, SD: 4.68).
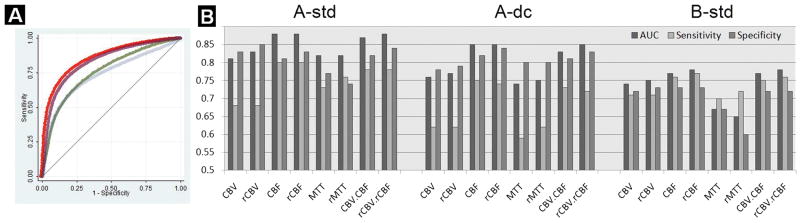
For each of the three software packages the relative and absolute CBF had higher AUCs for determination of core than CBV and MTT (Table 1, voxel-based analysis). The optimal thresholds for relative and absolute CBF and CBV varied substantially according across software packages. The optimal absolute CBF thresholds were 4.7, 5.4 and 10 ml/100g/min using A-std, A-dc and B-std software, respectively. The corresponding optimal normalized thresholds were 84%, 72%, and 68% reduction in CBF, respectively. AUC, thresholds, sensitivity and specificity are reported in Online Table 1 for the three software packages for absolute and relative CBF, CBV, MTT, and CBV*CBF for the whole brain and for segmented GM, WM and BG. All pairwise comparisons between the rCBF, and rCBV AUC's for each software were statistically significant (p<0.01, Table 2, patient-based analysis). Comparisons between the rCBF and rCBV*CBF AUC's were not statistically significant, except for software “B-std” (P<0.01). rCBF using software “A-std” had the highest AUC (P<0.01). Optimal ROC operating point thresholds varied significantly across the different platforms (p<0.01, Figure 2).
Table 1. Voxel-based ROC-AUC values (pooled data) for whole brain CTP map segmentation of DWI infarct core.
| Software | CBV | rCBV | CBF | rCBF | MTT | rMTT | CBV*CBF | rCBV*rCBF |
|---|---|---|---|---|---|---|---|---|
| A-std | 0.81 | 0.83 | 0.88 | 0.88 | 0.82 | 0.82 | 0.87 | 0.88 |
| A-dc | 0.76 | 0.77 | 0.85 | 0.85 | 0.74 | 0.75 | 0.83 | 0.85 |
| B-std | 0.74 | 0.75 | 0.77 | 0.78 | 0.67 | 0.65 | 0.77 | 0.78 |
AUC = Area Under the ROC Curve
Table 2. Patient-based comparison of the relative CTP parameter ROC-AUC's and post-processing software platforms.
| Software | Parameter | Median (95% CI*) | Comparison Software | Comparison Parameter | Median (95% CI*) | Wilcoxon P-value |
|---|---|---|---|---|---|---|
| a-std | rCBV | 0.81 (0.78-0.83) | a-std | rCBF | 0.86 (0.83-0.89) | <0.01 |
| a-dc | rCBV | 0.76 (0.72-0.78) | a-dc | rCBF | 0.83 (0.79-0.87) | <0.01 |
| b-std | rCBV | 0.73 (0.70-0.77) | b-std | rCBF | 0.80 (0.74-0.83) | <0.01 |
|
| ||||||
| a-std | rCBF | 0.86 (0.83-0.89) | a-std | rCBV* CBF | 0.86 (0.82-0.88) | 0.49 |
| a-dc | rCBF | 0.83 (0.79-0.87) | a-dc | rCBV* CBF | 0.83 (0.79-0.87) | 0.82 |
| b-std | rCBF | 0.80 (0.74-0.83) | b-std | rCBV* CBF | 0.80 (0.75-0.85) | <0.01 |
|
| ||||||
| a-std | rCBF | 0.86 (0.83-0.89) | a-dc | rCBF | 0.83 (0.79- 0.87) | <0.01 |
| a-std | rCBF | 0.86 (0.83-0.89) | b-std | rCBF | 0.80 (0.74-0.83) | <0.01 |
| a-dc | Rcbf | 0.83 (0.79-0.87) | b-std | rCBF | 0.80 (0.74-0.83) | <0.01 |
CI: Confidence interval for median
Figure 2.
A: Sample ROC curves for CTP delineation of DWI defined core using “A-dc” post-processing software (absolute parameter values only; Red: CBF; Purple: CBV*CBF; Green: CBV, and Blue: MTT). B: Bar graph of AUC (area under curve), sensitivity, and specificity (Y-axis) at the optimal ROC operating point for each CTP parameter and post-processing platform (“A-std”, “A-dc” and “B-std”; X-axis), for delineation of core (“r” = relative).
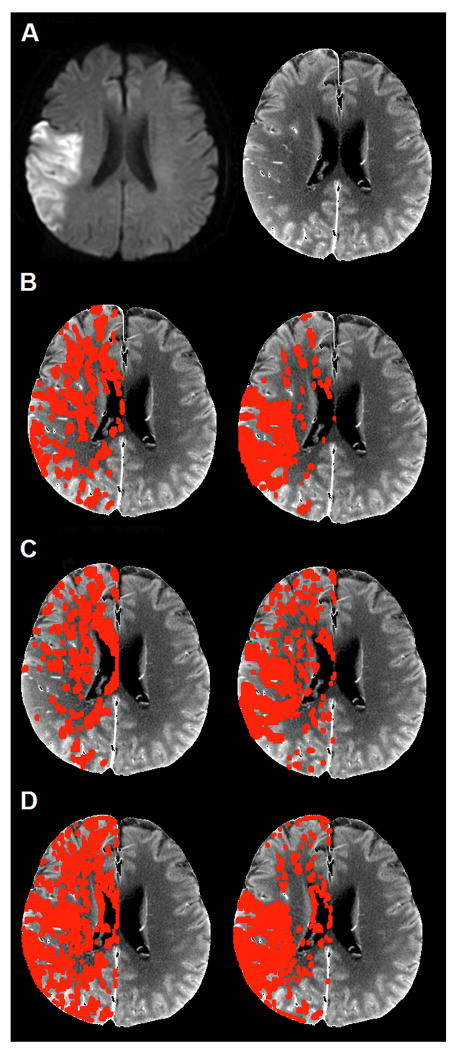
Figure 3 shows sample overlays for infarction core using the optimal operating point thresholds for absolute CBV and CBF, for all 3 software packages.
Figure 3.
Sample optimal absolute CBF and CBV pixel thresholds (red overlay applied to the temporally averaged cine CTP template) for right hemispheric stroke. A: Admission DWI (left) and temporally averaged CTP template (right); B: Software “A-std”: CBV (left), CBF (right); C: Software “A-dc”: CBV (left), CBF (right); D: Software “B-std”: CBV (left), CBF (right).
Discussion
We have shown that: (1) CBF is the optimal CTP parameter for estimating DWI-defined infarct core, exceeding CT-CBV in accuracy, and that (2) significant variation exists between the optimal parameter threshold values for different post-processing platforms. Strengths of our study include the use of advanced “second generation” CTP acquisition protocols that are sufficiently long to permit the complete transit of IV contrast through the brain (thus resulting in more physiologically correct perfusion maps), a co-registered CTP-DWI voxel based ROC analysis, comparison of multiple vendor software and post-processing platforms, and inclusion of heterogeneous patients with hemodynamic irregularities from both large vessel occlusion and atrial fibrillation. These methodological considerations may explain much of the difference between our results and those of earlier reports, and highlight the significant variability in optimal parameter thresholds between different platforms. Despite the variability in our reported thresholds, they remain within the range of prior meta-analyses,5, 10, 11, 14-16 and our conclusion that CBF is nominally more accurate than CBV in delineating core appears to be generalizable across platforms.
Our use of DWI as a reference standard, rather than follow-up infarct size in patients with early complete recanalization, may also have contributed to quantitative differences between our results and prior studies, including one that found higher CBF/CBV thresholds using the “A-std” software12. Technical differences in post-processing (such as our use of the “vessel exclusion off” clinical default mode) were likely also important. Although DWI lacks perfect specificity for infarct core, it is both highly accurate and widely accepted in research and clinical care.3, 17 With regard to our use of a more lengthy 66 second CTP acquisition time, rather than a “first generation” time of 45 seconds,10-12, 18 there is recent consensus that acquisition should be sufficiently long to permit the full wash-in and wash-out of contrast, so that complete, non-truncated time-density tissue curves can be obtained (crucial if concurrent permeability imaging is also performed).17 Short imaging times can lead to truncation of the tissue time-density curves (TDC) in regions with severe hemodynamic derangement due to severe vascular stenosis/occlusion and/or atrial fibrillation, which can distort the – typically vendor dependent - CTP parameter value calculations.9, 19 Indeed, our conclusion regarding the accuracy of CBF versus CBV in determining core is supported by the fact that calculation of CBV is typically more sensitive to TDC truncation than is CBF, and by previous work suggesting that CBV lesion size can be overestimated in the setting of marked hemodynamic derangement.13
That CBV has greater variability than CBF in delineating core is also consistent with the established hemodynamic alterations accompanying ischemia. Most relevant of these is luxury perfusion – CBV hyperemia of penumbra, or recanalized core – that occurs not infrequently in maximally vasodilated, critically ischemic tissue.20, 21
Our study highlights several technical limitations to the interpretation of CTP data. First, it is clear that the parameter thresholds obtained using one CTP post-processing platform may not be generalizable to other CTP methodologies. Currently, there is no standardization of CTP post-processing software across different vendors, different reconstruction algorithms, or even different versions of the same software package for a given vendor.9, 17, 19 Further variability in absolute quantification of flow values can be introduced by volume averaging effects in selecting the venous outflow ROI for normalization during CTP map construction.6 Indeed, variability in quantitation of perfusion parameter values has recently been identified in a simulated dataset comparing delay sensitive to delay insensitive deconvolution techniques.9
Unlike most prior investigations of CTP thresholds for infarct core, we minimized bias by using a more objective, voxel - rather than regional - based image analysis method. We determined the optimal core threshold values for each CTP parameter by transposing the segmented DWI core lesion directly onto the co-registered CTP maps, and performing ROC curve analyses to determine the optimal operating points for distinguishing infarcted from non-infarcted voxels. Hence, subjective differences in image display such as gray scale, window/level settings, and pixel conspicuity, which might introduce subjectivity in manual segmentation, were eliminated. A limitation inherent in all perfusion studies of acute ischemia is that they represent a “snapshot” in time, and that – specifically for very early times post ictus (<3 hours) – thresholds for irreversible ischemic damage may vary. Unfortunately, our study lacked sufficient patients to stratify our data by time-post-ictus. Moreover, although we could not control for the potential confounding effects of reperfusion just prior to scanning on our threshold analysis, there were no imaging findings (such as partially recanalized vessels on CTA) to suggest this.
Conclusion
Our study has demonstrated that appropriately thresholded CT-CBF maps, more so than CT-CBV, optimally delineate DWI-defined infarct core, but that the specific thresholds vary by post-processing software version and vendor (approximately 70-85% reduction in cerebral blood flow). Although CT perfusion imaging cannot replace DWI for the accurate delineation of infarct core, CTP is likely to be the best alternative modality – more accurate that unenhanced CT or CTA source images - for making this clinically important assessment in patients for whom MRI cannot be obtained.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) Network grant funded by the National Institute of Health (NIH/NINDS) (1 P50 NS051343-01A2), the Agency for Healthcare Research and Quality grant (AHRQ) R01 HS11392-01A1, and the Massachusetts General Hospital Clinical Research Center (No. 1 UL1 RR025758-01) Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
Karen L. Furie: NIH Research Grant (P50NS051343)
R. Gilberto González: NIH Research Grant (R01NS050041)
Michael H. Lev: NIH Research Grant (P50NS051343), Research Support from GE Healthcare, Consultancy for Co-Axia, GE Healthcare, and Millennium Pharmaceuticals.
Footnotes
Disclosures: Shahmir Kamalian, Shervin Kamalian, Matthew B. Maas, Gregory V. Goldmacher, Seyedmehdi Payabvash, Adnan Akbar and Pamela W. Schaefer: None
References
- 1.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 2.Ueda T, Sakaki S, Yuh WT, Nochide I, Ohta S. Outcome in acute stroke with successful intra-arterial thrombolysis and predictive value of initial single-photon emission-computed tomography. J Cereb Blood Flow Metab. 1999;19:99–108. doi: 10.1097/00004647-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Schellinger PD, Bryan RN, Caplan LR, Detre JA, Edelman RR, Jaigobin C, et al. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: Report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2010;75:177–185. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lev MH, Farkas J, Gemmete JJ, Hossain ST, Hunter GJ, Koroshetz WJ, et al. Acute stroke: Improved nonenhanced CT detection--benefits of soft-copy interpretation by using variable window width and center level settings. Radiology. 1999;213:150–155. doi: 10.1148/radiology.213.1.r99oc10150. [DOI] [PubMed] [Google Scholar]
- 5.Shetty SK, Lev MH. CT perfusion in acute stroke. Neuroimaging Clin N Am. 2005;15:481–501. ix. doi: 10.1016/j.nic.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Sanelli PC, Lev MH, Eastwood JD, Gonzalez RG, Lee TY. The effect of varying user-selected input parameters on quantitative values in CT perfusion maps. Acad Radiol. 2004;11:1085–1092. doi: 10.1016/j.acra.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki M, Kudo K, Ogasawara K, Fujiwara S. Tracer delay-insensitive algorithm can improve reliability of CT perfusion imaging for cerebrovascular steno-occlusive disease: Comparison with quantitative single-photon emission CT. AJNR Am J Neuroradiol. 2009;30:188–193. doi: 10.3174/ajnr.A1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu O, Ostergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med. 2003;50:164–174. doi: 10.1002/mrm.10522. [DOI] [PubMed] [Google Scholar]
- 9.Kudo K, Sasaki M, Yamada K, Momoshima S, Utsunomiya H, Shirato H, et al. Differences in CT perfusion maps generated by different commercial software: Quantitative analysis by using identical source data of acute stroke patients. Radiology. 2009;254:200–209. doi: 10.1148/radiol.254082000. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer PW, Roccatagliata L, Ledezma C, Hoh B, Schwamm LH, Koroshetz W, et al. First-pass quantitative CT perfusion identifies thresholds for salvageable penumbra in acute stroke patients treated with intra-arterial therapy. AJNR Am J Neuroradiol. 2006;27:20–25. [PMC free article] [PubMed] [Google Scholar]
- 11.Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, et al. Perfusion-CT assessment of infarct core and penumbra: Receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 12.Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer PW, Mui K, Kamalian S, Nogueira RG, Gonzalez RG, Lev MH. Avoiding “Pseudo-reversibility” Of CT-CBV infarct core lesions in acute stroke patients after thrombolytic therapy: The need for algorithmically “Delay-corrected” CT perfusion map postprocessing software. Stroke. 2009;40:2875–2878. doi: 10.1161/STROKEAHA.109.547679. [DOI] [PubMed] [Google Scholar]
- 14.Bandera E, Botteri M, Minelli C, Sutton A, Abrams KR, Latronico N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: A systematic review. Stroke. 2006;37:1334–1339. doi: 10.1161/01.STR.0000217418.29609.22. [DOI] [PubMed] [Google Scholar]
- 15.Lev MH, Gonzalez RG. Ct angiography and ct perfusion imaging. In: Toga AW, Mazziotta JC, editors. Brain mapping: The methods. San Diego: Academic Press; 2002. pp. 427–484. [Google Scholar]
- 16.Lev MH, Segal AZ, Farkas J, Hossain ST, Putman C, Hunter GJ, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: Prediction of final infarct volume and clinical outcome. Stroke. 2001;32:2021–2028. doi: 10.1161/hs0901.095680. [DOI] [PubMed] [Google Scholar]
- 17.Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, et al. Acute stroke imaging research roadmap. Stroke. 2008;39:1621–1628. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PubMed] [Google Scholar]
- 18.Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, et al. White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology. 2008;247:818–825. doi: 10.1148/radiol.2473070551. [DOI] [PubMed] [Google Scholar]
- 19.Konstas AA, Lev MH. CT perfusion imaging of acute stroke: The need for arrival time, delay insensitive, and standardized postprocessing algorithms? Radiology. 2010;254:22–25. doi: 10.1148/radiol.09091610. [DOI] [PubMed] [Google Scholar]
- 20.Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, et al. Variability of cerebral blood volume and oxygen extraction: Stages of cerebral haemodynamic impairment revisited. Brain. 2002;125:595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- 21.Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.