Abstract
Background and Purpose
White matter hyperintensities (WMHs) are associated with progressive age-related cognitive decline and cardiovascular risk factors, but their biological relevance as indicators of generalized white matter injury is unclear. DTI provides more sensitive indications of subtle white matter disruption and can therefore clarify whether WMHs represent foci of generalized white matter damage that extends over a broader neighbourhood.
Methods
208 participants from the University of California, Davis Alzheimer's Disease Center received a comprehensive clinical evaluation and brain MRI including FLAIR and DTI sequences. Voxel-wise maps of WMHs were produced from FLAIR using a standardized WMH detection protocol. Fractional anisotropy (FA) maps were calculated from DTI. All WMH and FA maps were coregistered to a standardized space. For each normal-appearing white matter voxel in each subject FLAIR scan, a neighbourhood WM injury (NWI) score was calculated that increased with increasing number and proximity of WMH in the vicinity of the voxel. FA was related to NWI using a nonlinear mixed effect model controlling for relevant confounding factors.
Results
FA was found to decrease as NWI increased (β=-0.0017/%, p<0.0001) with an accelerated rate (p<0.0001). An increase of 1% in NWI score was associated with a decrease in mean FA of 0.012 (p<0.001).
Conclusion
WMH may represent foci of more widespread and subtle white matter changes rather than distinct, sharply-delineated anatomical abnormalities. We use the term white matter hyperintensities penumbra to explain this phenomenon.
Keywords: Aging, Diffusion Tensor Imaging, Cerebrovascular Disease, White Matter Hyperintensity, Alzheimer Disease, Magnetic Resonance Imaging
Introduction
White matter hyperintensities (WMH) are a common finding in brain MRI images of older individuals, increasing with age and vascular risk factors1, 2. Although resulting from multiple etiologies, increased WMH are associated with cognitive impairment 3-6 and future risk for incident stroke and death7 emphasizing the importance of understanding the role of WMH in health and cognition. Pathological studies find no relationship between WMH and Alzheimer disease (AD) pathology8, but find expression of ischemic changes9. Furthermore, recent studies suggest that WMH are common to white matter watershed areas10 supporting the notion that at least some of these lesions are vascular in origin. Yet, the exact evolution of WMH, however remain unclear. For example, Schmidt et. al.11 identified two categories of WMH lesions: punctuate lesions that were considered relatively benign and confluent or early confluent lesions which progressed rapidly in size over time. They postulated that these more confluent areas represented white matter regions where the disease process is progressively affecting an at risk area in a fashion similar to the ischemic penumbra of acute infarction.
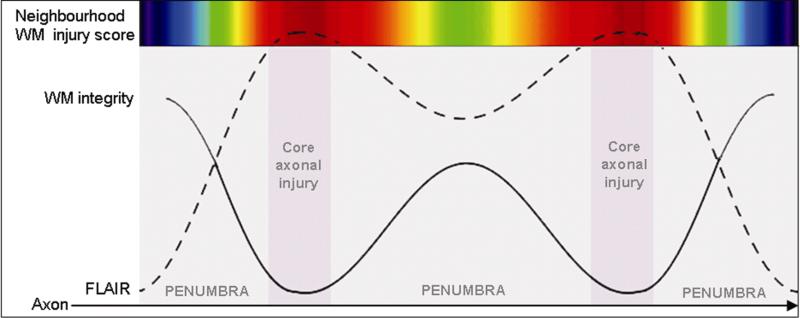
Newer studies using diffusion tensor imaging (DTI) and measures of fractional anisotropy (FA), support the notion of injury to white matter integrity beyond the area of WMH to include normal appearing white matter12. As such, FA analysis may reveal subtle changes in areas surrounding WMH. We hypothesize that WMH constitute evidence of a core injury surrounded by a penumbra1 reflecting more subtle injury (Figure 1). We tested this hypothesis by constructing a voxel based measure neighbourhood proximity to WMH, termed the white matter injury (NWI) score. The value of this measure increased with increasing number and proximity of WMH in the vicinity of each image voxel. With this information, we examined voxel based measures of FA surrounding WMH. We expected, that if our penumbra hypothesis was correct, FA would consistently decline some distance from each WMH voxel. This distance measure, therefore, would indicate the size of the penumbra, if one existed. In addition, this measure could be used to examine the overall effect of total WMH burden on mean FA for the entire white matter of each study subject.
Figure 1.
Schematic depiction of white matter (WM) integrity (measured by DTI-based FA) and FLAIR intensity along a row of white matter voxels. The colour scale indicates for the corresponding voxel the neighbourhood WM injury (NWI) score. For example, a voxel located in the injured core , i.e. in a white matter hyperintensity (WMH) as detected using FLAIR, will exhibit a high NWI score (red) while voxels in WMH penumbra (outside the lesion) will exhibit a mild NWI score (green) if surrounding by one or more WMH or a weaker NWI score (blue) if no WMH are in their proximity.
Materials and methods
Sample
Subjects included 45 patients with AD, 67 patients with mild cognitive impairment (MCI), and 96 cognitively normal (CN) individuals. The AD group consisted of 88.9% patients with probable AD, 8.9% patients with possible AD, and 2.2% patients with AD and sufficient cerebrovascular disease for the diagnosis of mixed dementia. This was defined as two or more strokes, at least 1 of which is outside the cerebellum on MRI, or else a single stroke with clearly documented temporal relationship to the onset or aggravation of cognitive impairment13. No subjects except those with mixed dementia had a clinical history of stroke. The diagnosis of AD was made according to the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria14. MCI was diagnosed according to current consensus criteria15. As part of the clinical evaluation, the presence or absence of stroke, diabetes, hyperlipidemia, transient ischemic attack, hypertension, and coronary artery disease was systematically assessed to create a composite score for vascular risks, which was the sum of the factors present, ranging from 0 to 6, and reported as a percentage12.
Subjects were recruited from the Alzheimer's Disease Center at the University of California, Davis. All participants received a comprehensive clinical evaluation and neuropsychological testing with a standardized test battery16. In addition, all subjects received a standardized MRI scan of the brain at the baseline evaluation. The Institutional Review Boards at all participating institutions approved this study, and subjects or their legal representatives gave written informed consent.
Image acquisition and processing
All brain imaging was performed at the University of California, Davis Imaging Research Center on a 1.5-T GE Signa Horizon LX Echospeed system. Three sequences were used: a 3-dimensional T1-weighted coronal spoiled gradient-recalled echo acquisition, a FLAIR sequence, and a DTI sequence. DTI was performed according to previously reported methods12.
The segmentation of WMH was determined from FLAIR images according to an in-house procedure that has been previously described10. For each subject, we calculated the total volume of WMHs and normalized this measurement for head size by dividing by total cranial volume. Since the distribution of total WMH volume across the population was skewed, it was log-transformed prior to normalization. Fractional anisotropy (FA) maps were calculated from DTI. Using a previously-described image registration method12, WMH and FA maps were linearly aligned to the corresponding T1-weighted scan, which was then linearly aligned and nonlinearly deformed to a minimal deformation template (MDT)17 with a 0.98 × 1.5 × 0.98 mm3 voxel size. These alignments allowed the transfer of all FA and WMH maps to the space of the MDT. Maps of GM and WM in the space of the MDT have been generated using previously-described tissue segmentation methods18.
An average young adult FA map previously described 12 was used for comparison with the individuals of the present study. This map was made by averaging 15 healthy young adult FA images (mean age = 24.1 ± 3.1 years, 60.0% male).
Calculation of neighbourhood white matter injury score and relation to FA
We first calculated, for each MDT WM voxel i, the number of WM voxels located at distance j (mij), for j ranging between 1 mm and its maximum, 214 mm. Then, for each subject and MDT WM voxel i, we used that individual's WMH map in MDT space to calculate the number of voxels located at distance j which were WMH (nij). The ratio of mij to nij can be interpreted as the probability (pij) that any randomly-selected voxel that is at distance j from i is WMH. The neighbourhood WM injury probability (NWI) for a voxel i adds together these WMH injury probabilities over a range of distances j to encapsulate the notion that an increased amount of WMH in the vicinity of i may be associated with an increased degree of WM injury at i. In addition, the NWI down-weights the influence that WMHs at larger distances from i have on this sum, to encapsulate the notion that the more proximal a WMH is to voxel i, the more severe its impact on the WM integrity at i. The influence of a WMH on WM integrity falls off by the square of its distance to voxel i; thus, the NWI score for voxel i is given by the weighted sum (Σ pij/j2)j normalized by (Σ 1/j2)j (see Figure 1).
For each individual, the NWI score was calculated at each WM voxel in the MDT space, resulting in an NWI score map across the brain. The NWI score was then discretized to one of ten integer values by breaking up the range of values from 0 to 1 into ten intervals that were .1 units in width; each subject WM voxel in MDT space was then labelled according to which of these intervals its NWI score fell into. We then calculated, for each subject and for each NWI score interval using individual's FA map, the mean FA value amongst WM voxels in that interval. Mean FA within NWI score intervals that included fewer than 10 voxels were omitted from the analysis2.
To summarize, NWI scores reflect the degree to which a given WM voxel is expected to be damaged, even if it appears normal on FLAIR, based on the presence of WMH in its vicinity.
Statistical analysis
The primary goals of the statistical analysis were to determine the strength and nature of the association between mean FA and NWI score interval within each individual, and to characterize commonalities and differences between individuals in the characteristics of this relationship. A secondary goal was to assess whether a variety of relevant factors served to systematically modify how mean FA relates to NWI score interval. Nonlinear mixed-effects regression was used to achieve these goals. Because the mean FA appeared to be quadratically related to NWI score interval on visual inspection (Figure 2, upper part), mean FA was modelled as a quadratic function of NWI score interval within each individual; that is, the within-individual model of mean FA included linear and quadratic terms for the fixed effect of NWI score interval. In addition, total WMH volume was added to the model as a fixed effect impacting mean FA as well as the linear and quadratic relationship between mean FA and NWI score interval. Inter-individual differences in the mean FA – NWI score interval relationship were modelled using random effects for the intercept as well as linear and quadratic NWI score interval terms. We tested whether the random intercept and slopes were necessary, omitting them one by one from the original model and using the likelihood-ratio test to contrast each refit model with the reference. Each refit model was found significantly different from the reference model (p values<0.001) justifying the inclusion of all random effects.
Figure 2.
Upper part: regression curves of mean fractional anisotropy (FA) as a quadratic function of the neighbourhood white matter injury (NWI) score across the 208 subjects. Lower part: Mean FA according to NWI scores and overall WMH load quartiles
We then used a stepwise model-building approach to adjust this model for potential confounders including gender, age, clinical diagnosis (CN, MCI, or AD), APOE genotype, number of years of education, and vascular risk, one at a time. Because APOE is a common stratification variable in AD studies, we also adjusted the reference model by the interaction between APOE and clinical diagnosis. In addition, since cognitive diagnosis may interact with overall WMH load, we conducted in a separate analysis the reference model across cognitively normal individuals only.
Continuous variables were mean centered in all analyses. Statistical analyses were performed using R version 2.10.0 (R Development Core Team, 2009, Vienna, Austria).
Results
Demographics
Table 1 summarizes participant characteristics. The three clinical groups did not differ significantly in terms of age, cardiovascular risks, education level, or gender distribution but, predictably, the AD group included a higher proportion of APOE ε4-carriers relative to CN. Individuals with AD also exhibited higher total WMH volume compared to CN subjects.
Table 1.
Summary of subjects characteristics, broken down by diagnostic category Data presented as means (SD).
| Variables | All | CN | MCI | AD | p |
|---|---|---|---|---|---|
| Number of subjects | 208 | 96 | 67 | 45 | |
| Vascular risk score | 0.29 (0.21) | 0.25 (0.22) | 0.24 (0.21) | 0.29 (0.20) | 0.51 |
| Age, y | 74.5 (7.9) | 74.3 (7.5) | 73.4 (7.2) | 76.5 (9.6) | 0.13 |
| Years of Education, y | 12.0 (5.1) | 11.9 (5.1) | 13.0 (5.3) | 10.9 (4.4) | 0.092 |
| Gender (# male; % male) | 84; 40.4 | 64; 66.7 | 35; 52.2 | 25; 55.5 | 0.15 |
| WMH load (% TCV) | 0.91 (1.09) | 0.70 (0.85) | 0.97 (0.91) | 1.26 (1.60) | 0.018* |
| Number of subjects with Apoe | 171 | 78 | 54 | 39 | |
| ApoE Genotype (# ε4(-,-); % ε4(-,-)) | 115; 67.2 | 63; 80.8 | 33; 61.1 | 19; 48.7 | 0.016* |
Chi-square tests were used for categorical variables and analysis of variance (ANOVA) for continuous variables. To normalize variance, WMH loads were log-transformed for ANOVA groups comparison.
Post hoc comparisons of significant group difference:
cognitively normal (CN) vs Alzheimer disease (AD)
MCI indicates mild cognitive impairments; WMH, White Matter Hyperintensities; TCV, Total Cranial Volume; WMH volumes were corrected for head size (% TCV).
Visualization of NWI score and FA
Figure 3 provides an example of WM alteration inside the core of a WMH and in its penumbra for one individual in this study. As expected, FA and FLAIR images showed respectively lower and higher values inside the lesion ((A) and (C)). Using the map of mean FA across a healthy young population sample as a reference (B), we observed that FA was relatively reduced in this subject within the WMH as well as in the surrounding peripheral WM (A). The map of NWI score for this individual (D) reflects this pattern of reduced WM integrity in the WMH periphery.
Figure 3.
Illustrations of FA in regions exhibiting high and low neighbourhood white matter injury (NWI) scores, along with a visual depiction of the NWI score calculation. Top row: Oblique 3D view of one elderly subject's fractional anisotropy (FA) (left) and FLAIR (right) maps, coregistered to the same space. Middle and bottom rows: FLAIR voxels from this elderly individual that passed threshold for categorization as WMHs are shown in a semi-translucent overlay on top of four anatomical closeup views, again rendered from an oblique angle. (A) The FA map of the elderly subject. (B) The mean FA map calculated over the sample of young individuals, again coregistered to the same space. Note that the young subjects had relatively higher (redder) FA values on average in the vicinity of the overlaid WMH than the elderly subject did. (C) The FLAIR of the elderly subject. (D) The NWI score map calculated from the overlaid WMH voxels. This WMH significantly increases the NWI score of voxels proximal to it (shown in red), and has very little impact on more distal voxels (black).
Figure 4 illustrates the average of the 208 NWI score maps across all participants in this study. This map suggests that NWI score is relatively increased at systematic locations in the brain, especially in the periventricular zones where WMHs tend to accrue6.
Figure 4.
Axial slices of the average (across subjects) neighbourhood white matter injury (NWI) score maps in the minimal deformation template space. Scores are given by the colour scale. Score maps are superimposed on the average T1-weighted images of the sample.
Effect of NWI on FA
An NWI score of 0 was associated with an FA of .37 (see Table 2). Each 10 percent of increasing NWI score was associated with a decrease in FA of 0.0168 (p<0.0001) from this baseline value, and this decrease significantly accelerated with increasing NWI score (see Table 2). Figure 2 (upper part) shows the regression curves relating NWI score interval to mean FA in each subject. Both linear and quadratic decreases in FA due to NWI were found to be larger in individuals exhibiting higher overall WMH burden (see Table 2).
Table 2.
Summary of the nonlinear mixed effects model of mean Fractional Anisotropy (FA) with NWI, NWI × NWI and the WMH load as main fixed factors, including NWI by WMH load and NWI × NWI by WMH load interactions. Entries show the regression coefficient for the listed fixed effect followed by the associated p value for an F test on the marginal sum of squares.
| All (n=208) | CN (n=96) | |||
|---|---|---|---|---|
| β | p | β | p | |
| Intercept (mean FA) | 0.370 | <0.001 | 0.372 | <0.001 |
| NWI | -0.168 | <0.001 | -0.164 | <0.001 |
| NWI × NWI | -0.406 | <0.001 | -0.394 | <0.001 |
| WMH load | -0.012 | <0.001 | -0.013 | <0.001 |
| NWI by WMH load | -0.039 | <0.001 | -0.056 | <0.001 |
| NWI × NWI by WMH load | -0.082 | <0.001 | -0.105 | <0.001 |
WMH: White Matter Hyperintensities
NWI: Neighbourhood White matter Injury
CN indicates cognitively normal
Overall WMH load effect
An increase of 1% in WMH burden was significantly associated with a decrease in mean FA of 0.012 (p<0.0001) and this rate accelerated with increasing WMI score interval (see Table 2). To illustrate such interaction, we calculated WMH loads quartiles (Q1= [0.029; 0.179[, Q2=[0.179; 0.465[, Q3=[0.465; 1.105[ and Q4=[1.105; 6.981[) and computed mean FA related to the NWI score interval across subjects pooled according to their overall WMH loads broken into quartiles (see Figure 2, lower part).
Confounders
Cognitive diagnosis was not significant (p=0.33) indicating that mean FA, with respect to overall WMH load and NWI score interval, was independent of cognitive status. In the separate analysis conducted in cognitively normal individuals group (N=96), all effects indicating a significant inverse relationship between mean FA and NWI interval and overall WMH load (see Table 2) remained significant.
None of the other potential confounders significantly modified the relationship between mean FA and NWI score interval (age: p=0.36, APOE: p=0.87, APOE by cognitive diagnosis: p=0.86, education: p=0.24, vascular risk: p=0.18, gender: p=0.41).
Discussion
The present study aimed to better understand the role of WMH on local white matter architecture. The first finding of this study is that WMH appear to be at the apex of the WM alteration since FA measures of WM integrity surrounding WMH decline in proximity to WMH. We use the term WMH penumbra to explain this phenomenon. The second result is that generalized WM integrity is function of the overall WMH load. The greater the total WMH load the more generalized the injury, including areas extremely distant from WMH. Importantly, both findings were found to be independent of cognitive diagnosis.
These results are significant for two reasons. First, the presence of a WMH penumbra strongly suggests that WMH indicate a process that extends beyond the region of tissue pathology determined by selecting any particular FLAIR intensity threshold to define WMH. This finding may have biological implications. For example, we have previously shown that periventricular WMH occur in anatomic areas consistent with vascular watershed with peak WMH prevalence approximately 3-4 mm from the ventricular edge3. Our current findings are surprisingly consistent in that decline in FA surrounding areas of high white matter injury probability was on the order of 1-2 voxels or about 3 mm (see Figures 2 and 3 (D)). In both instances, we see a gradient of effect surrounding potential vascular distributions, the previous report at a global level, the current findings, more locally. However, additional work is needed to determine exactly what biological mechanisms may be causing microstructural damage to surround WMHs.
Secondly, our findings strongly suggest that extensive WMH result in altered white matter integrity throughout the cerebral white matter, particularly when WMH burden is extensive. This second finding is important to interpreting any differences in FA measurements in the absence of adjusting for total WMH burden in studies of older individuals where WMH are more common. Few prior studies have examined the relationship between FA and total WMH loads in elderly19, 20. We further propose that WMH constitute only “the tip of the iceberg” and are only a crude estimate of WM injury and should not be dissociated from the somewhat more subtle white matter impairments in white matter integrity in the WMH penumbra.
To our knowledge, this is the first study that provides in-vivo evidence of an association between generalized white matter injury in one location and WMH in another. This work supports a previous study suggesting that, after WMH voxels are removed from FA maps, WMH volume is associated with regional loss of WM integrity in regions prone to WMHs 19. Our results are also consistent with a recent postmortem study that found decreased micro-vascular density using alkaline phosphatase staining in subjects with leukoaraiosis (LA) as compared with subjects without LA, both within WM lesions and the healthy appearing WM outside the lesions21. The weak associations between WMH and cognitive performance may be explained, as previously suggested22, by an inappropriate dichotomization of WM into WMH and normal appearing WM. Our results may indicate a method whereby we can better characterize the full impact of these more extensive losses of white matter integrity. Our results also support those of a previous study that reported negative and positive correlations between FLAIR within WMH and FA and mean diffusivity (MD) respectively20. We also performed analysis on MD (please see Supplementary Data at http://stroke.ahajournals.org) and as expected found a strong relationship between MD and NWI that was the reverse of that observed between FA and NWI. Our results extend their finding to all WM, even WM that is not immediately adjacent to WMH.
The key limitation of the study was that we assumed that the impact of a WMH on a normal WM location depended solely on the straight-line distance between the two, without taking into account the architecture of the underlying network of axonal tracts. In fact, it may be more biologically plausible to assume that WMH at one location on a WM tract more strongly influences WM integrity along the rest of the same tract than in others. Future work should explore the use of DTI tractography to model WMH penumbra effects more realistically in this fashion.
Summary/Conclusions
In conclusion, this study provides evidence of the existence of a penumbra of WM injury in the so-called normal WM surrounding WMH. WM integrity locally is associated with the overall WMH load globally; and it is more compromised at locations that are closer to WMH. Aging-associated mechanisms of WM degeneration are complex and this work suggests new considerations to better assess the broader spectrum of white matter injury.
Supplementary Material
Acknowledgments
Source of Funding
The study was supported by NIH grants K01 AG030514 and P30 AG010129
Footnotes
We use the most général définition of the term << penumbra >> : a surrounding région in which a property exists to a lesser degree. Unlike the more specific usage of the term in vascular neurology, the général usage does not ascribe any specific biological mechanism, such as ischemia, to the phenomenon.
Changing this threshold from 10 to 5 voxels did not significantly change the findings.
Conflict of Interest/ Disclosure
The authors report no actual or potential conflicts of interest in relation to this manuscript.
REFERENCES
- 1.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: The framingham study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 2.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 3.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the national heart, lung, and blood institute twin study. Arch Neurol. 2001;58:643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 4.Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Reed BR, Jagust WJ. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43:1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, mci, and ad. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, Ellis WG, Zarow C, Mungas D, Reed BR, Kramer JH, Schuff N, DeCarli C, Chui HC. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 10.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (wmh): Exploring the relationships between periventricular wmh, deep wmh, and total wmh burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke. 2007;38:2619–2625. doi: 10.1161/STROKEAHA.107.489112. [DOI] [PubMed] [Google Scholar]
- 12.Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Regional pattern of white matter microstructural changes in normal aging, mci, and ad. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the state of california alzheimer's disease diagnostic and treatment centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer's disease: Report of the nincds-adrda work group under the auspices of department of health and human services task force on alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de LM, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van DC, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. J.Intern.Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The uniform data set (uds): Clinical and cognitive variables and descriptive data from alzheimer disease centers. Alzheimer Dis.Assoc.Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 17.Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P. Regional spatial normalization: Toward an optimal target. J.Comput.Assist.Tomogr. 2001;25:805–816. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and alzheimer disease. Stroke. 2010;41:1791–1797. doi: 10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vernooij M, Degroot M, Vanderlugt A, Ikram M, Krestin G, Hofman A, Niessen W, Breteler M. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage. 2008;43:470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 20.Zhan W, Zhang Y, Mueller SG, Lorenzen P, Hadjidemetriou S, Schuff N, Weiner MW. Characterization of white matter degeneration in elderly subjects by magnetic resonance diffusion and flair imaging correlation. Neuroimage. 2009;47(Suppl 2):T58–T65. doi: 10.1016/j.neuroimage.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: Association with leukoaraiosis. Radiology. 1995;194:469–476. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 22.Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, Scheltens P, Geurts JJ. Heterogeneity of white matter hyperintensities in alzheimer's disease: Post-mortem quantitative mri and neuropathology. Brain. 2008;131:3286–3298. doi: 10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.