Abstract
The t(14;18) chromosomal translocation is the most common cytogenetic abnormality in NHL, occurring in 70–90% of follicular lymphomas (FL) and 30–50% of diffuse large B-cell lymphomas (DLBCL). Previous t(14;18)-NHL studies have not evaluated risk factors for NHL defined by both t(14;18) status and histology. In this population-based case-control study, t(14;18) status was determined in DLBCL cases using fluorescence in situ hybridization on paraffin-embedded tumor sections. Polytomous logistic regression was used to evaluate the association between a wide variety of exposures and t(14;18)-positive (N=109) and −negative DLBCL (N=125) and FL (N=318), adjusting for sex, age, race and study center. Taller height, more lifetime surgeries, and PCB180 exposure were associated with t(14;18)-positivity. Taller individuals (3rd tertile vs. 1st tertile) had elevated risks of t(14;18)-positive DLBCL [odds ratio (OR)=1.8, 95% confidence interval (CI) 1.1–3.0] and FL (OR=1.4, 95%CI 1.0–1.9) but not t(14;18)-negative DLBCL. Similar patterns were seen for individuals with more lifetime surgeries [13+ versus 0–12 surgeries; t(14;18)-positive DLBCL OR=1.4, 95%CI 0.7–2.7; FL OR=1.6, 95%CI 1.1–2.5] and individuals exposed to PCB180 greater than 20.8 ng/g [t(14;18)-positive DLBCL OR=1.3, 95%CI 0.6–2.9; FL OR=1.7, 95%CI 1.0–2.8]. In contrast, termite treatment and high alpha-chlordane levels were associated with t(14;18)-negative DLBCL only, suggesting that these exposures do not act through t(14;18). Our findings suggest that putative associations between NHL and height, surgeries, and PCB180 may be t(14;18)-mediated and provide support for case-subtyping based on molecular and histologic subtypes. Future efforts should focus on pooling data to confirm and extend previous research on risk factors for t(14;18)-NHL subtypes.
Keywords: lymphoma, non-Hodgkin, case–control studies, translocation, follicular lymphoma, diffuse large B-cell lymphoma, etiology
INTRODUCTION
Non-Hodgkin lymphoma (NHL) comprises a group of closely related yet heterogeneous diseases1–2. Except for severe immunosuppression, the risk factors for most NHLs remain largely unknown 3–4. Findings from epidemiologic studies have increasingly suggested that risk factors for NHL may differ by histologic subtype3–5.
Two recent studies have suggested that characterizing NHLs by the presence of the t(14;18)(q32;q21) chromosomal translocations may also have etiologic relevance 6–11. For example, a number of pesticides have been associated with t(14;18)-positive NHL but not t(14;18)-negative NHL in both studies 6, 9. One of the most common cytogenetic abnormalities in NHL, t(14;18) occurs in approximately 70–90% of follicular lymphomas (FL) and 30–50% of diffuse large B-cell lymphomas (DLBCL), and is rarer in other NHL subtypes 12–13. This chromosomal translocation results in deregulation and overexpression of the anti-apoptotic gene BCL2 by joining it with the regulatory region of the immunoglobulin heavy chain gene (IgH) found on chromosome 14 12. It is thought to occur early in B-lymphocyte development during rearrangement of immunoglobulin genes (V(D)J recombination) to produce a functional surface antigen receptor. Thus, t(14;18) is considered an initiating event in lymphomagenesis but is not sufficient for lymphoma development, as evidenced by the presence of t(14;18)-positive lymphocytes in healthy individuals and the lack of lymphoma development in bcl2 transgenic mice 12, 14–15. Alternatively, due to the fact that t(14;18) has not been directly studied as a risk factor for NHL 16, t(14;18) in lymphoma may merely act as a bystander whose presence is enriched due to the relative growth advantage of induced deregulated apoptosis in translocation-positive cells.
Risk factors that are specific for t(14;18)-positive NHL may contribute to lymphomagenesis by several mechanisms, including increasing the occurrence of t(14;18) or increasing the likelihood that t(14;18)-positive cells will undergo neoplastic transformation 6, 8. Alternatively, it is possible that certain exposures may increase risk for FL independent of t(14;18), but appear related to t(14;18)-positive NHL because t(14;18) is so common in FL. However, the two previous studies that have evaluated risk factors for t(14;18)-defined NHL subtypes have not been able to simultaneously consider both t(14;18) status and histologic type due to small sample sizes (N~180 cases of various histologies). We therefore investigated the risk of t(14;18)-positive and t(14;18)-negative DLBCL in association with a broad range of NHL risk factors in a large, population-based case–control study, comparing results with FL, which have been published previously 5. We hypothesized that t(14;18) is more likely to play a role in NHL development for exposures associated with both t(14;18)-positive DLBCL and FL, but not t(14;18)-negative DLBCL, than exposures associated with just one of these 3 subtypes.
MATERIAL AND METHODS
Study population
The study population has been described in detail previously 17. Briefly, we included 1321 cases diagnosed with a first primary NHL during 1998–2000, aged 20 to 74 years, and identified among residents of four Surveillance Epidemiology and End Results (SEER) registries (Iowa, Detroit, Los Angeles, Seattle). Known HIV-positive cases were excluded. Population controls (N=1057) were selected from residents of the same four SEER areas using random digit dialing (<65 years) or Medicare eligibility files (≥65 years), and frequency-matched to the cases (regardless of NHL subtype) by age (within five year groups), sex, race, and SEER area. Controls with a history of NHL or known HIV infection were excluded. Overall participation rates (total participating/total contacted) were 76% in cases and 52% in controls; overall response rates (total participating/total presumed eligible) were 59% and 44%, respectively. In-person interviews were conducted by trained personnel, blinded to case-control status. The study protocol was approved by Institutional Review Boards at the National Cancer Institute and each SEER center. Participants provided written, informed consent prior to interview.
Histopathology
All cases were histologically confirmed and coded according to the International Classification of Diseases for Oncology, 2nd Edition (ICD-O-2) 18 by the local diagnosing pathologist. We grouped cases into NHL subtypes according to the World Health Organization classification 13 using the International Lymphoma Epidemiology Consortium (InterLymph) guidelines 19. Individuals diagnosed with DLBCL (ICD-O-2: 9680–84, 9688; N=417) or FL (ICD-O-2: 9690–91, 9695–98; N=318) were eligible for the present analysis.
Exposure Assessment
Exposure assessment methods and definitions are described in detail in Supplemental Table 1. To accommodate a large number of questions, we used a split-sample design, with core questions for all respondents and additional questions for either Group A (all African American and 50% of non-African American participants) or Group B (50% of non-African American participants). Prior to the in-person interview, participants were mailed residential and job history forms, and questionnaires regarding either family and medical history (Group A) or diet and lifestyle (Group B). During the home visit, the interviewer administered a computer-assisted personal interview (CAPI), which included core questions on demographics, pesticide use, occupational history, hair coloring product use, and medical history. Group B participants were also queried on sun exposure. Dust samples from participants’ vacuum cleaners were collected to measure residential exposure to pesticides.
In the present analyses, we included all risk factors that were associated with NHL and/or NHL subtypes in our study, or have been demonstrated consistently in the literature to be associated with NHL and/or NHL subtypes.
Ascertainment of the t(14;18)(q32;q21) by FISH analysis for DLBCL cases
Paraffin-embedded tumor biopsies were obtained for 236 (57%) DLBCL cases. For each tumor block, 5 µm thick sections were cut and mounted on slides. Diagnostic areas were marked on each slide after review of hematoxylin and eosin-stained sections by an expert hematopathologist (MAV).
To define t(14;18) status, FISH studies were performed using the commercially available LSI IGH/BCL2 dual color, dual fusion probes (Abbott-Vysis Inc., Downers Grove, IL), which are approved by the US Food and Drug Administration as analyte specific reagents. Pretreatment and deparaffinization of slides with tissue sections was performed using VP2000® (Abbott-Vysis Inc.) following the manufacturer’s protocol specific for paraffin-embedded tissue sections, with minor modifications. Subsequently, the probe mixture was placed on the marked diagnostic areas of the tissue sections, coverslipped, and sealed. Co-denaturation of probes and target DNA at 75°C for 5 minutes was followed by overnight hybridization at 37°C using an automated hybridization chamber (HYBrite®, Abbott-Vysis, Inc.). The slides were then washed with standard post hybridization washes in 2X standard saline citrate (SSC)/0.1% Nonidet P-40 (NP-40) for 2 minutes each at 73°C and room temperature, respectively. The tissue sections were then counterstained with 4,6-diamidino-2-phenylindole (DAPI II) (Abbott-Vysis Inc.). Analysis was performed on an Olympus BX51 microscope equipped with appropriate filters, and images were captured with CytoVision® image capture software (Applied Imaging, Santa Clara, CA).
For each case, a minimum of 100 interphase nuclei were evaluated independently by a cytotechnologist (SJ) and an expert cytogeneticist (BJD) for the presence of the t(14;18). Agreement between the readers was 100%. For the purposes of quality control, duplicate slides from 24 randomly-selected individuals were interspersed and blinded from the laboratory and readers. Agreement for the quality control duplicates was 100%.
Statistical analysis
For each risk factor, relative risks for t(14;18)-negative DLBCL, t(14;18)-positive DLBCL, and FL compared with controls were estimated using odds ratios (OR) and 95% confidence intervals (CI) derived from polytomous unconditional logistic regression models. P values for the linear trend were computed by including ordinal rather than categorical variables in the logistic regression models. To test homogeneity among the NHL subtypes defined by t(14;18) status, we compared t(14;18)-negative DLBCL cases versus t(14;18)-positive DLBCL cases alone and in combination with FL cases. To test homogeneity among the NHL subtypes defined by histologic type, we compared DLBCL cases (regardless of t(14;18) status) with FL cases. Controls were excluded from these analyses. For risk factors with more than two categories, we used the ordinal variable for the homogeneity tests. For risk factors with at least one significant subtype association, the magnitude of the ORs for all subtypes was compared and the p-values from the homogeneity tests were noted.
All models included sex, age (<45, 45–64, 65+ years), race (non-Hispanic white, black, other), study center (Detroit, Iowa, Los Angeles, Seattle), and education (<12, 12–15, 16+ years) as covariates. Statistical analyses were performed using the SAS system, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Using interphase FISH, the t(14;18) status was determined in 234 of 236 (99%) DLBCL cases with available tumor tissue. Of these, the t(14;18) was detected in 109 (47%) cases. The demographic characteristics for controls and case subtypes, including t(14;18)-positive and −negative DLBCL and FL, are presented in Table 1. Overall, the majority of participants was over the age of 45 years, white, and had completed at least a high school education. Compared with controls, t(14;18)-negative DLBCL and FL cases were more likely to be under age 45 at diagnosis, whereas t(14;18)-positive DLBCL cases were more likely to reside in Iowa and less likely to reside in Los Angeles.
Table 1.
Selected characteristics of population controls and cases by non-Hodgkin lymphoma subtypes defined by histology and t(14;18), from the NCI-SEER multicenter case–control study of non-Hodgkin lymphoma
| Diffuse large B-cell lymphoma | |||||
|---|---|---|---|---|---|
| Controls (N=1057) |
t(14;18)- negative (N=125) |
t(14;18)- positive (N=109) |
Follicular lymphoma (N=318) |
||
| N (%) | N(%) | N(%) | N(%) | P1 | |
| Age at diagnosis or selection (years) | |||||
| <45 | 162 (15.3) | 33(26.4) | 16(14.7) | 63(19.7) | 0.0003 |
| 45–64 | 449 (42.5) | 56(44.8) | 47(43.1) | 158(49.5) | |
| 65+ | 446 (42.2) | 36(28.8) | 46(42.2) | 97(30.7) | |
| Sex | |||||
| Male | 546 (51.7) | 68(54.4) | 67(61.5) | 157(49.5) | 0.16 |
| Female | 511 (48.3) | 57(45.6) | 42(38.5) | 161(50.5) | |
| Race | |||||
| White | 843 (79.8) | 110(88.0) | 100(91.7) | 279(87.8) | <0.0001 |
| Black | 151 (14.3) | 4(3.2) | 6(5.5) | 21(6.6) | |
| Other/unknown | 63 (6.0) | 11(8.8) | 3(2.8) | 18(5.6) | |
| SEER area | |||||
| Detroit | 214 (20.2) | 16(12.8) | 26(23.9) | 70(21.9) | <0.0001 |
| Iowa | 276 (26.1) | 42(33.6) | 48(44.0) | 103(32.6) | |
| Los Angeles | 273 (25.8) | 36(28.8) | 9(8.3) | 65(20.4) | |
| Seattle | 294 (27.8) | 31(24.8) | 26(23.9) | 80(25.1) | |
| Education (years) | |||||
| <12 | 111 (10.5) | 13(10.4) | 16(14.7) | 22(6.9) | 0.18 |
| 12–15 | 616 (58.3) | 78(62.4) | 61(56.0) | 206(64.9) | |
| 16+ | 330 (31.2) | 34(27.2) | 32(29.4) | 90(28.2) | |
P value for the Pearson chi-square statistic testing independence between the demographic characteristics and control/case subtype category.
The highest tertile for height (versus lowest tertile) was associated with t(14;18)-positive DLBCL (OR=1.8, 95% CI=1.1–3.0; Ptrend=0.03) and FL (OR=1.4, 95% CI=1.0–1.9; Ptrend=0.03) but not t(14;18)-negative DLBCL (Phomogeneity=0.15) (Table 2a). Among males only, height was not significantly associated with any subtype, whereas among females, tall height was associated with both t(14;18)-positive DLBCL and FL (OR=2.6, 95% CI=1.1–6.0 and OR=1.6, 95% CI=1.0–2.5, respectively), but not t(14;18)-negative DLBCL (Phomogeneity=0.08). Compared with individuals who had 12 or fewer lifetime surgeries, risks for individuals with 13 or more lifetime surgeries were elevated for t(14;18)-positive DLBCL (OR=1.4, 95% CI 0.7–2.7) and FL (OR=1.6, 95%CI 1.1–2.5) but not elevated for t(14;18)-negative DLBCL (Phomogeneity=0.13) (Table 2a). Risks for individuals exposed to higher levels of PCB180 (greater than 20.8 ng/g) were elevated for t(14;18)-positive DLBCL (OR= 1.3, 95% CI 0.7–2.5) and for follicular lymphoma (OR=1.5, 95% CI 1.0–2.2), but not elevated for t(14;18)-negative DLBCL (Phomogeneity=0.06) (Table 2c). BMI ≥35 kg/m2 (versus <25 kg/m2) was significantly associated with t(14;18)-positive DLBCL (OR=2.0, 95% CI=1.0–4.0), but, in contrast to height, surgeries, and PCB180, was not associated with follicular lymphoma (Table 2a).
Table 2.
| Table 2a. Associations1 between family history, medical history, and anthropometrics and non-Hodgkin lymphoma subtypes defined by histology and t(14;18), from the NCI-SEER multicenter case–control study of non-Hodgkin lymphoma | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | Controls N |
Odds ratios and 95% CI for DLBCL subtypes and FL | Test of homogeneity p-values | ||||||||||
| DLBCL | FL | t(14;18)- positive vs. negative |
t(14;18)-positive DLBCL/FL vs. t(14;18)-negative DLBCL |
DLBCL vs. FL |
|||||||||
| t(14;18)-negative | t(14;18)-postive | ||||||||||||
| N | OR | (95% CI)* | N | OR | (95% CI)* | N | OR | (95% CI)* | |||||
| Family and medical history | |||||||||||||
| Family history of NHL | |||||||||||||
| No | 969 | 115 | 1.0 | (reference) | 101 | 1.0 | (reference) | 284 | 1.0 | (reference) | |||
| Yes | 32 | 7 | 1.7 | (0.7, 4.0) | <5 | ~ | 16 | 1.5 | (0.8, 2.8) | 0.29 | 0.64 | 0.77 | |
| Autoimmune conditions | |||||||||||||
| No | 996 | 121 | 1.0 | (reference) | 103 | 1.0 | (reference) | 298 | 1.0 | (reference) | |||
| Yes | 61 | <5 | ~ | 6 | 1.0 | (0.4, 2.4) | 20 | 1.1 | 0.7, 2.0) | 0.55 | 0.34 | 0.28 | |
| Asthma | |||||||||||||
| No | 523 | 61 | 1.0 | (reference) | 53 | 1.0 | (reference) | 151 | 1.0 | (reference) | |||
| Yes | 66 | 8 | 1.0 | (0.4, 2.1) | <5 | ~ | 21 | 1.1 | (0.7, 1.9) | 0.53 | 0.85 | 0.28 | |
| Allergy | |||||||||||||
| No | 173 | 19 | 1.0 | (reference) | 22 | 1.0 | (reference) | 60 | 1.0 | (reference) | |||
| Yes | 289 | 36 | 1.2 | (0.6, 2.2) | 29 | 1.0 | (0.5, 1.8) | 84 | 0.9 | (0.6, 1.3) | 0.78 | 0.57 | 0.55 |
| Surgeries (total number) | |||||||||||||
| 0–12 | 235 | 26 | 1.0 | (reference) | 15 | 1.0 | (reference) | 45 | 1.0 | (reference) | |||
| 13+ | 354 | 43 | 1.1 | (0.6, 1.9) | 41 | 1.4 | (0.7, 2.7) | 127 | 1.6 | (1.1, 2.5) | 0.59 | 0.13 | 0.16 |
| Transfusion | |||||||||||||
| No | 876 | 111 | 1.0 | (reference) | 94 | 1.0 | (reference) | 265 | 1.0 | (reference) | |||
| Yes | 170 | 14 | 0.7 | (0.4, 1.3) | 15 | 0.8 | (0.4, 1.4) | 50 | 1.0 | (0.7, 1.5) | 0.73 | 0.53 | 0.22 |
| Birth order | |||||||||||||
| First/Middle | 327 | 34 | 1.0 | (reference) | 32 | 1.0 | (reference) | 100 | 1.0 | (reference) | |||
| Last | 105 | 23 | 2.0 | (1.1, 3.6) | 16 | 1.7 | (0.9, 3.2) | 36 | 1.1 | (0.7, 1.8) | 0.97 | 0.41 | 0.21 |
| Anthropometrics | |||||||||||||
| BMI (kg/m2) | |||||||||||||
| <25 | 305 | 46 | 1.0 | (reference) | 28 | 1.0 | (reference) | 104 | 1.0 | (reference) | |||
| 25-<35 | 578 | 58 | 0.7 | (0.5, 1.1) | 58 | 1.0 | (0.6, 1.7) | 161 | 0.9 | (0.6, 1.2) | |||
| 35+ | 94 | 15 | 1.1 | (0.6, 2.1) | 17 | 2.0 | (1.0, 4.0) | 23 | 0.7 | (0.4, 1.2) | |||
| p trend | 0.63 | 0.10 | 0.15 | 0.38 | 0.95 | 0.17 | |||||||
| Height (inches) | |||||||||||||
| 1st tertile | 396 | 44 | 1.0 | (reference) | 29 | 1.0 | (reference) | 106 | 1.0 | (reference) | |||
| 2nd tertile | 291 | 40 | 1.2 | (0.8, 1.9) | 37 | 1.7 | (1.0, 2.9) | 65 | 0.8 | (0.6, 1.2) | |||
| 3rd tertile | 301 | 35 | 1.1 | (0.6, 1.7) | 38 | 1.8 | (1.1, 3.0) | 118 | 1.4 | (1.0, 1.9) | 0.20 | 0.15 | 0.90 |
| p trend | 0.79 | 0.03 | 0.03 | ||||||||||
| Height (inches) males only | |||||||||||||
| 1st tertile | 205 | 23 | 1.0 | (reference) | 19 | 1.0 | (reference) | 55 | 1.0 | (reference) | |||
| 2nd tertile | 143 | 23 | 1.4 | (0.7, 2.6) | 25 | 1.7 | (0.9, 3.3) | 33 | 0.8 | (0.5, 1.4) | |||
| 3rd tertile | 156 | 18 | 1.1 | (0.6, 2.3) | 20 | 1.3 | (0.7, 2.6) | 53 | 1.2 | (0.8, 1.9) | 0.75 | 0.78 | 0.79 |
| p trend | 0.64 | 0.39 | 0.36 | ||||||||||
| Height (inches) females only | |||||||||||||
| 1st tertile | 191 | 21 | 1.0 | (reference) | 10 | 1.0 | (reference) | 51 | 1.0 | (reference) | |||
| 2nd tertile | 148 | 17 | 1.0 | (0.5, 2.0) | 12 | 1.7 | (0.7, 4.0) | 32 | 0.8 | (0.5, 1.3) | |||
| 3rd tertile | 145 | 17 | 0.9 | (0.5, 1.9) | 18 | 2.6 | (1.1, 6.0) | 65 | 1.6 | (1.0, 2.5) | 0.06 | 0.08 | 0.67 |
| p trend | 0.81 | 0.02 | 0.04 | ||||||||||
| Table 2b. Associations1 between lifestyle and diet and non-Hodgkin lymphoma subtypes defined by histology and t(14;18), from the NCI-SEER multicenter case–control study of non-Hodgkin lymphoma | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratios and 95% CI for DLBCL subtypes and FL | Test of homogeneity p-values | ||||||||||||
| DLBCL | FL | t(14;18)- positive vs. negative |
t(14;18)-positive DLBCL/FL vs. t(14;18)-negative DLBCL |
DLBCL vs. FL |
|||||||||
| Controls N |
t(14;18)-negative | t(14;18)-positive | |||||||||||
| Exposure | N | OR | (95% CI)* | N | OR | (95% CI)* | N | OR | (95% CI)* | ||||
| Smoking, alcohol & diet | |||||||||||||
| Smoking | |||||||||||||
| Never | 184 | 24 | 1.0 | (reference) | 27 | 1.0 | (reference) | 56 | 1.0 | (reference) | |||
| Former | 161 | 15 | 0.7 | (0.3, 1.4) | 12 | 0.5 | (0.2, 1.0) | 37 | 0.8 | (0.5, 1.4) | |||
| Current | 61 | 9 | 1.1 | (0.5, 2.5) | 9 | 0.9 | (0.4, 2.2) | 24 | 1.2 | (0.6, 2.1) | 0.52 | 0.93 | 0.58 |
| Smoking duration (years) | |||||||||||||
| Never | 186 | 25 | 1.0 | (reference) | 28 | 1.0 | (reference) | 57 | 1.0 | (reference) | |||
| <10 to 30 years | 128 | 11 | 0.6 | (0.3, 1.3) | 10 | 0.5 | (0.2, 1.1) | 37 | 0.9 | (0.6, 1.5) | |||
| 30 or more years | 96 | 13 | 1.1 | (0.5, 2.4) | 11 | 0.7 | (0.3, 1.6) | 24 | 1.0 | (0.5, 1.7) | 0.26 | 0.71 | 0.59 |
| p trend | 0.91 | 0.24 | 0.87 | ||||||||||
| Cigarettes per day | |||||||||||||
| Never | 186 | 25 | 1.0 | (reference) | 28 | 1.0 | (reference) | 57 | 1.0 | (reference) | |||
| 1 to 30 | 145 | 13 | 0.7 | (0.3, 1.4) | 9 | 0.4 | (0.2, 0.9) | 39 | 0.9 | (0.6, 1.5) | |||
| 30+ | 77 | 12 | 1.1 | (0.5, 2.4) | 13 | 1.0 | (0.5, 2.1) | 21 | 0.9 | (0.5, 1.7) | |||
| p trend | 0.92 | 0.59 | 0.79 | 0.72 | 0.85 | 0.97 | |||||||
| Smoking pack-years | |||||||||||||
| Never | 186 | 25 | 1.0 | (reference) | 28 | 1.0 | (reference) | 57 | 1.0 | (reference) | |||
| <7 to 35 | 142 | 12 | 0.6 | (0.3, 1.3) | 8 | 0.4 | (0.2, 0.9) | 40 | 0.9 | (0.6, 1.5) | |||
| 35+ | 79 | 12 | 1.1 | (0.5, 2.5) | 12 | 0.9 | (0.4, 2.1) | 20 | 0.9 | (0.5, 1.7) | |||
| p trend | 0.98 | 0.48 | 0.81 | 0.55 | 0.77 | 0.87 | |||||||
| Ethanol | |||||||||||||
| Non-drinkers | 156 | 23 | 1.0 | (reference) | 25 | 1.0 | (reference) | 65 | 1.0 | (reference) | |||
| Ever drinkers | 233 | 25 | 0.6 | (0.3, 1.2) | 23 | 0.7 | (0.3, 1.2) | 52 | 0.5 | (0.3, 0.7) | 0.63 | 0.50 | 0.19 |
| Dietary vitamin B6 (mg/1000kcal) | |||||||||||||
| <0.97 | 197 | 30 | 1.0 | (reference) | 29 | 1.0 | (reference) | 68 | 1.0 | (reference) | |||
| 0.97+ | 192 | 18 | 0.7 | (0.4, 1.3) | 19 | 0.7 | (0.4, 1.3) | 49 | 0.8 | (0.5, 1.3) | 0.87 | 0.61 | 0.31 |
| Dietary MeIQx (ng/day) | |||||||||||||
| <33.41 | 235 | 26 | 1.0 | (reference) | 21 | 1.0 | (reference) | 77 | 1.0 | (reference) | |||
| 33.41+ | 154 | 22 | 1.0 | (0.5, 1.9) | 27 | 1.5 | (0.8, 2.9) | 40 | 0.7 | (0.4, 1.1) | 0.35 | 0.57 | 0.03 |
| Dietary PhIP (ng/day) | |||||||||||||
| <105.64 | 234 | 29 | 1.0 | (reference) | 34 | 1.0 | (reference) | 74 | 1.0 | (reference) | |||
| 105.64+ | 155 | 19 | 0.7 | (0.4, 1.4) | 14 | 0.5 | (0.3, 1.0) | 43 | 0.7 | (0.4, 1.1) | 0.55 | 0.68 | 0.78 |
| Table 2c. Associations1 between environmental exposures and non-Hodgkin lymphoma subtypes defined by histology and t(14;18), from the NCI-SEER multicenter case–control study of non-Hodgkin lymphoma | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratios and 95% CI for DLBCL subtypes and FL | Test of homogeneity p-values | ||||||||||||
| DLBCL | FL | t(14;18)- positive vs. negative |
t(14;18)-positive DLBCL/FL vs. t(14;18)-negative DLBCL |
DLBCL vs. FL |
|||||||||
| Controls N |
t(14;18)-negative | t(14;18)-positive | |||||||||||
| Exposure | N | OR | (95% CI)* | N | OR | (95% CI)* | N | OR | (95% CI)* | ||||
| Sunlight | |||||||||||||
| Sun in teens (hrs/wk) | |||||||||||||
| <7 | 46 | 7 | 1.0 | (reference) | 10 | 1.0 | (reference) | 18 | 1.0 | (reference) | |||
| 7–<14 | 68 | 6 | 0.6 | (0.2, 2.1) | 7 | 0.4 | (0.1, 1.2) | 24 | 1.0 | (0.5, 2.1) | |||
| 14–<28 | 155 | 16 | 0.6 | (0.2, 1.6) | 21 | 0.5 | (0.2, 1.3) | 44 | 0.7 | (0.4, 1.4) | |||
| 28+ | 187 | 26 | 0.8 | (0.3, 2.0) | 13 | 0.2 | (0.1, 0.6) | 56 | 0.8 | (0.4, 1.5) | |||
| p trend | 0.86 | 0.00 | 0.34 | 0.06 | 0.29 | 0.40 | |||||||
| Sun in past ten years (hrs/wk) | |||||||||||||
| <7 | 159 | 23 | 1.0 | (reference) | 18 | 1.0 | (reference) | 58 | 1.0 | (reference) | |||
| 7–<14 | 126 | 10 | 0.5 | (0.2, 1.1) | 14 | 0.9 | (0.4, 2.0) | 43 | 0.9 | (0.6, 1.4) | |||
| 14–<28 | 123 | 11 | 0.5 | (0.2, 1.2) | 12 | 0.7 | (0.3, 1.6) | 29 | 0.6 | (0.4, 1.0) | |||
| 28+ | 51 | 11 | 1.1 | (0.4, 2.5) | 7 | 0.7 | (0.3, 2.0) | 14 | 0.6 | (0.3, 1.3) | |||
| p trend | 0.58 | 0.42 | 0.05 | 0.93 | 0.60 | 0.47 | |||||||
| Eye color | |||||||||||||
| Green/blue-green/blue | 244 | 21 | 1.0 | (reference) | 23 | 1.0 | (reference) | 72 | 1.0 | (reference) | |||
| Hazel | 84 | 7 | 1.0 | (0.4, 2.5) | 10 | 1.3 | (0.6, 2.9) | 29 | 1.2 | (0.7, 2.0) | |||
| Brown | 134 | 27 | 2.6 | (1.4, 5.0) | 18 | 1.7 | (0.8, 3.3) | 43 | 1.2 | (0.7, 1.9) | 0.15 | 0.03 | 0.06 |
| Environmental exposures | |||||||||||||
| Termite treatment <1988 | |||||||||||||
| None, DK, not treated <1988 | 893 | 94 | 1.0 | (reference) | 95 | 1.0 | (reference) | 277 | 1.0 | (reference) | |||
| 1+ homes treated <1988 | 162 | 30 | 2.1 | (1.3, 3.4) | 14 | 1.4 | (0.7, 2.5) | 41 | 1.0 | (0.7, 1.5) | 0.30 | 0.01 | 0.04 |
| Alpha-chlordane (dust, ng/g) | |||||||||||||
| ND | 317 | 35 | 1.0 | (reference) | 40 | 1.0 | (reference) | 99 | 1.0 | (reference) | |||
| 20.8–60.1 | 98 | 11 | 1.1 | (0.5, 2.3) | 7 | 0.7 | (0.3, 1.5) | 26 | 0.9 | (0.6, 1.5) | |||
| 60.3–5870 | 98 | 19 | 2.0 | (1.0, 3.9) | 10 | 1.1 | (0.5, 2.3) | 34 | 1.3 | (0.8, 2.1) | |||
| p trend | 0.06 | 0.88 | 0.45 | 0.26 | 0.24 | 0.75 | |||||||
| PCB180 (dust, ng/g) | |||||||||||||
| <20.8 | 395 | 55 | 1.0 | (reference) | 40 | 1.0 | (reference) | 115 | 1.0 | (reference) | |||
| 20.8+ | 118 | 10 | 0.7 | (0.4, 1.5) | 17 | 1.3 | (0.7, 2.5) | 44 | 1.5 | (1.0, 2.2) | 0.10 | 0.06 | 0.15 |
| Pre-1980 Hair dye use | |||||||||||||
| Never | 98 | 11 | 1.0 | (reference) | 10 | 1.0 | (reference) | 24 | 1.0 | (reference) | |||
| Permanent | 123 | 10 | 0.5 | (0.2, 1.5) | 6 | 0.6 | (0.2, 1.9) | 49 | 1.8 | (1.0, 3.4) | 0.75 | 0.09 | 0.03 |
| Had farm or pest-exposed job | |||||||||||||
| Never | 805 | 91 | 1.0 | (reference) | 71 | 1.0 | (reference) | 233 | 1.0 | (reference) | |||
| Had 1 or more farm jobs | 249 | 33 | 1.0 | (0.6, 1.5) | 37 | 1.4 | (0.9, 2.2) | 85 | 1.1 | (0.8, 1.5) | 0.51 | 0.43 | 0.82 |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma
Odds ratios (95% confidence intervals) estimated using polytomous logistic regression models, adjusted for age, sex, race, study center, and education.
~ Risk estimates are not shown when there were fewer than 5 cases.
Abbreviations: DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MeIQx, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
Odds ratios (95% confidence intervals) estimated using polytomous logistic regression models, adjusted for age, sex, race, study center, and education.
Abbreviations: DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; ND, not detected.
Odds ratios (95% confidence intervals) estimated using polytomous logistic regression models, adjusted for age, sex, race, study center, and education.
~ Risk estimates are not shown when there were fewer than 5 cases.
Having one or more homes treated for termites before 1988 was associated with t(14;18)-negative DLBCL (OR=2.1, 95% CI=1.3–3.4), but not associated with t(14;18)-positive DLBCL or FL (Phomogeneity=0.01) (Table 2c). High levels of alpha-chlordane in dust (60.3+ ng/g) was associated with t(14;18)-negative DLBCL (OR=2.0, 95% CI=1.0–3.9;), but not associated with the other subtypes (Phomogeneity=0.24) (Table 2c).
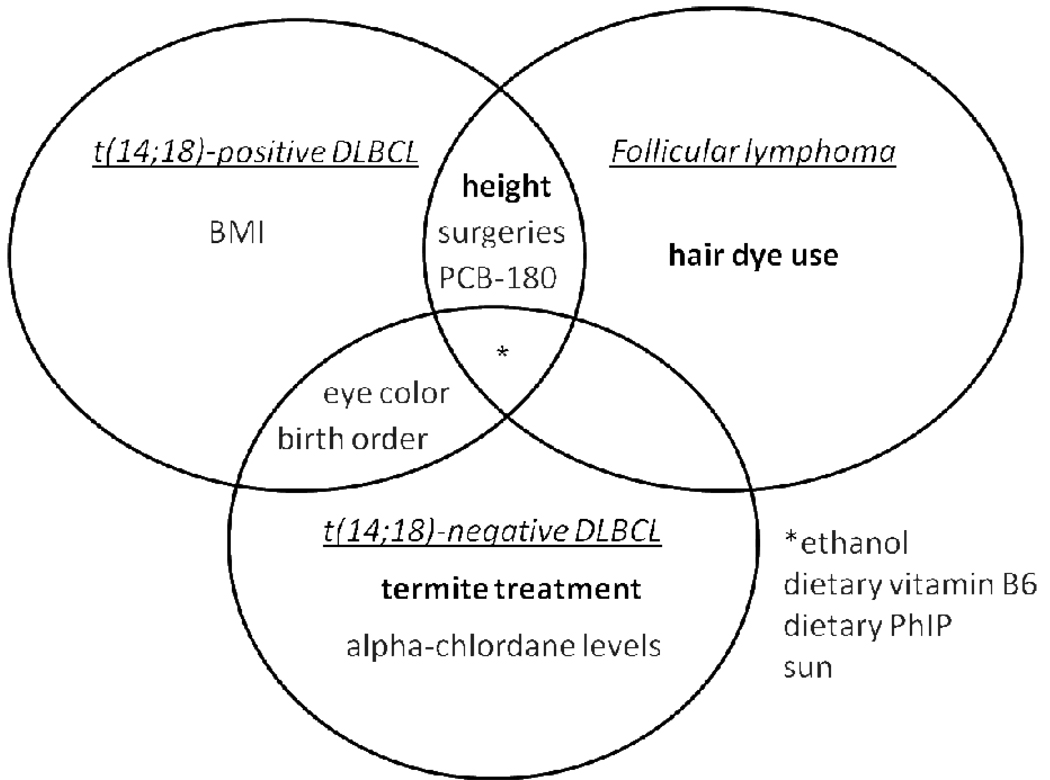
Several risk factors appeared to differ by histologic subtype, but not by t(14;18), whereas others did not differ by either histologic or t(14;18) subtype (Fig. 1). Pre-1980 hair dye use was associated with FL but not DLBCL (Table 2c). Late birth order and brown eye color were associated with DLBCL but not FL (Tables 2a and 2c). However, these exposures were relatively rare, therefore risk estimates for all were imprecise. No subtype-specific associations were found for a number of factors, including ethanol consumption, dietary vitamin B6 intake, dietary PhIP intake, and sun exposure, which were generally inversely associated with all case-subtypes (Tables 2b and 2c).
Figure 1.
Venn diagram of significant or suggestive t(14;18) and histologic-specific associations. Risk factors in bold had significant test of homogeneity p-values (<0.05). Overlap between t(14;18)-positive DLBCL and FL support a t(14;18)-pathway for height, surgeries, and PCB-180.
DISCUSSION
In this exploratory study, we found that NHL risk factors may vary by both t(14;18)- and histologically-defined subtypes of NHL (Fig. 1). In particular, we found positive associations with t(14;18)-positive DLBCL and FL, but not t(14;18)-negative DLBCL, for taller height among women only and, less strongly, for more lifetime surgeries and higher PCB180 exposure. In addition, we found an association with obesity (BMI ≥35 kg/m2) that was both t(14;18)-specific and histology-specific. We also found that termite treatment and the highest level of alpha-chlordane were positively associated with t(14;18)-negative DLBCL, but not t(14;18)-positive DLBCL or FL. Because of the small numbers in some strata, our findings require replication in future larger studies of NHL risk factors by t(14;18) status that simultaneously consider molecular and histologic subtypes of NHL.
Height, surgeries and PCB180 were associated with the presence of the t(14;18) due to the elevated risks of both t(14;18)-positive DLBCL and FL. Although height has not always been consistently linked with NHL, several studies have reported positive associations between height and NHL, particularly FL and/or DLBCL, which is consistent with our results 5, 20–25. The stronger association between height and NHL among women compared to men has also been observed in the EPIC cohort 23. Height is a marker of genetics as well as exposures early in life, including nutritional status which may more directly affect presence of t(14;18) 10. Surgeries were associated with both DLBCL and FL in the NCI-SEER study 5, 26 and have been speculated to increase NHL risk through low level antigenic stimulation or inflammation. PCB180 was more strongly associated with FL than with DLBCL in the NCI-SEER study5, but other studies reporting associations between different congeners of PCB and NHL lacked data on subtype-specific associations due to insufficient sample sizes 27. Not previously evaluated with respect to t(14;18) subtypes, the associations for height, surgeries, and PCB 180 need to be confirmed in additional or pooled studies based on t(14;18) subtypes.
We also observed an association between obesity and t(14;18)-positive DLBCL, but not t(14;18)-negative DLBCL or FL. Obesity, hypothesized to increase the risk of cancer through inflammation, has not been linked consistently with NHL, but pooled and meta-analyses have reported stronger associations for DLBCL than other NHL subtypes 25, 28. The reasons for our obesity findings are unclear, but may reflect a pathway that is both histologic and t(14;18)-specific.
Additional support for translocation-specific differences comes from positive associations between t(14;18)-negative DLBCL only and termite treatment and high alpha-chlordane levels. Both risk factors are associated with DLBCL and not with FL 5 and are hypothesized to affect NHL risk via immune dysfunction5. In the Factors Affecting Rural Men (FARM) study chlordane was positively associated with both t(14;18)-positive and −negative NHL 6. However, the FARM study considered chlordane used as a livestock or crop insecticide on farms whereas our study assessed alpha-chlordane used as a residential insecticide (i.e. termite treatment). Thus, the nature and intensity of chlordane use was likely to differ between the two studies and may account for the different results.
Among the factors with associations that differed by histology, but not by t(14;18) status, brown eye color and late birth order, both associated with DLBCL 5, have not been previously examined by t(14;18) status. In contrast, hair dye use, associated with FL 29, has not been consistently linked to t(14;18)-NHL subtypes 7–8, 11. Our histology-specific findings suggest that all three risk factors are not likely to be involved with t(14;18).
Smoking and follicular lymphoma were consistently associated in an InterLymph pooled study and other studies 30–32, but not in the current study population 5. The association between smoking and follicular lymphoma coupled with the observation that frequency of t(14;18) in the peripheral blood of heavy smokers was elevated compared with nonsmokers 33 suggested a possible role of t(14;18) in smoking and FL development. However, the previous t(14;18)-NHL studies 7–8, 11 and the current study have not found a consistent association between t(14;18)-positive NHL and smoking.
Among the strengths of this study, we defined case subtypes by both histology and t(14;18), whereas previous studies were based on t(14;18) status, grouping across all NHL histologic subtypes. The current study included men and women residing in 4 SEER areas, and is more representative of the U.S. general population, while the Nebraska and FARM studies were conducted in predominantly rural areas 6, 9, and the FARM study was restricted to men 6. In addition, the FARM study used PCR assays to detect t(14;18) status, which is less sensitive than FISH 34. A re-evaluation of a subset of factors in the FARM study based on FISH assays revealed associations inconsistent with those based on the previous PCR assays, further demonstrating the impact of different assays on associations 7.
The main limitation in the current study and the previous t(14;18)-NHL studies is the small sample size, which limited interpretation of the results. Another limitation in this study is our inability to retrieve tumor tissues for all cases (57%) due to difficulty in obtaining tumor blocks. However, clinical characteristics such as B symptoms and deaths did not differ between DLBCL cases with tissue biopsies and those without, and a comparison of putative risk factor associations showed no substantial differences between these two sets of cases. Other limitations include underpowered tests of homogeneity, chance associations due to multiple testing, and the potential for recall bias. We did not assess t(14;18) status in subtypes other than DLBCL. Thus, our findings may have been influenced by t(14;18)-negative FL, though 19% or less of FL were t(14;18)-negative in most US and European studies also using FISH assays34. The potential impact of t(14;18)-positive cases outside of DLBCL and FL (e.g. t(14;18)-positive SLL) is likely to be minimal because such cases accounted for only 3–14% of all t(14;18)-positive cases in the FARM and Nebraska study populations 8, 34.
In conclusion, this analysis revealed subtype-specific associations between t(14;18)-DLBCL and height, surgeries, PCB180, and termite treatment containing alpha-chlordane, not previously reported in similar studies. The findings provide support for case-subtyping based on both t(14;18) and histologic subtypes. Future efforts should focus on pooling data to confirm and extend previous research on the utility of t(14;18) status for defining etiologically-relevant subtypes of NHL.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of the staff and scientists at the SEER centers of Iowa, Los Angeles, Detroit, and Seattle for the conduct of the study’s field effort, and Mary McAdams, Pete Hui, Lonn Irish, and Michael Stagner (Information Management Services, Inc.) for programming support. This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, and Public Health Service (PHS) contracts N01-PC-65064, N01-PC-67008, N01-PC-67009, N01-PC-67010, N02-PC-71105.
Financial support: This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, and Public Health Service (PHS) contracts N01-PC-65064, N01-PC-67008, N01-PC-67009, N01-PC-67010, N02-PC-71105.
Reference List
- 1.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow S, Campo E, Harris N. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissuesed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 3.Alexander DD, Mink PJ, Adami HO, Chang ET, Cole P, Mandel JS, Trichopoulos D. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120 Suppl 12:1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 4.Hartge P, Bracci PM, Wang SS, Devesa SS, Holly EA, Schottenfeld D, Fraumeni JF., Jr . Non-Hodgkin lymphoma Cancer Epidemiology and Preventioned. vol. 3rd. Oxford: Oxford Press; 2006. [Google Scholar]
- 5.Morton LM, Wang SS, Cozen W, Linet MS, Chatterjee, Davis S, Severson RK, Colt J, Vasef MA, Rothman N, Blair A, Bernstein L, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes. Blood. 2008;112:5150–5160. doi: 10.1182/blood-2008-01-133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder JC, Olshan AF, Baric R, Dent GA, Weinberg CR, Yount B, Cerhan JR, Lynch CF, Schuman LM, Tolbert PE, Rothman N, Cantor KP, et al. Agricultural risk factors for t(14;18) subtypes of non-Hodgkin's lymphoma. Epidemiology. 2001;12:701–709. doi: 10.1097/00001648-200111000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Chang CM, Schroeder JC, Olshan AF, Dunphy CH, Huang WY, Baric RS, Dorsey KC, Cerhan JR, Lynch CF, Rothman N, Cantor KP, Blair A. A case-control study of tobacco use and other non-occupational risk factors for lymphoma subtypes defined by t(14;18) translocations and bcl-2 expression Cancer Causes & Control. 2010 doi: 10.1007/s10552-010-9531-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu BC, Dave BJ, Blair A, Gapstur SM, Chmiel JS, Fought AJ, Zahm SH, Weisenburger DD. Cigarette smoking, familial hematopoietic cancer, hair dye use, and risk of t(14;18)-defined subtypes of non-Hodgkin's lymphoma. Am J Epidemiol. 2007;165:652–659. doi: 10.1093/aje/kwk044. [DOI] [PubMed] [Google Scholar]
- 9.Chiu BC, Dave BJ, Blair A, Gapstur SM, Zahm SH, Weisenburger DD. Agricultural pesticide use and risk of t(14;18)-defined subtypes of non-Hodgkin lymphoma. Blood. 2006;108:1363–1369. doi: 10.1182/blood-2005-12-008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu BC, Dave BJ, Ward MH, Fought AJ, Hou L, Jain S, Gapstur S, Evens AM, Zahm SH, Blair A, Weisenburger DD. Dietary factors and risk of t(14;18)-defined subgroups of non-Hodgkin lymphoma. Cancer Causes Control. 2008;19:859–867. doi: 10.1007/s10552-008-9148-3. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder JC, Olshan AF, Baric R, Dent GA, Weinberg CR, Yount B, Cerhan JR, Lynch CF, Schuman LM, Tolbert PE, Rothman N, Cantor KP, et al. A case-control study of tobacco use and other non-occupational risk factors for t(14;18) subtypes of non-Hodgkin's lymphoma (United States) Cancer Causes & Control. 2002;13:159–168. doi: 10.1023/a:1014397920185. [DOI] [PubMed] [Google Scholar]
- 12.Bende RJ, Smit LA, van Noesel CJM. Molecular pathways in follicular lymphoma. Leukemia. 2006;21:18–29. doi: 10.1038/sj.leu.2404426. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 14.Schuler F, Dolken L, Hirt C, Kiefer T, Berg T, Fusch G, Weitmann K, Hoffmann W, Fusch C, Janz S, Rabkin CS, Dolken G. Prevalence and frequency of circulating t(14;18)-MBR translocation carrying cells in healthy individuals. Int J Cancer. 2009;124:958–963. doi: 10.1002/ijc.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janz S, Potter M, Rabkin CS. Lymphoma- and leukemia-associated chromosomal translocations in healthy individuals. Genes, Chromosomes & Cancer. 2003;36:211–223. doi: 10.1002/gcc.10178. [DOI] [PubMed] [Google Scholar]
- 16.Rabkin CS, Hirt C, Janz S, Dolken G. t(14;18) Translocations and risk of follicular lymphoma. J Natl Cancer Inst Monogr. 2008:48–51. doi: 10.1093/jncimonographs/lgn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee N, Hartge P, Cerhan JR, Cozen W, Davis S, Ishibe N, Colt J, Goldin L, Severson RK. Risk of non-Hodgkin's lymphoma and family history of lymphatic, hematologic, and other cancers. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:1415–1421. [PubMed] [Google Scholar]
- 18.Percy C, Van Holten V, Muir C, editors. Geneva: World Health Organization; International Classification of Diseases for Oncology. (2nd edition) 1990
- 19.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, Jack A, Cozen W, Maynadie M, Spinelli JJ, Seniori Costantini A, Rudiger T, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerhan JR, Janney CA, Vachon CM, Habermann TM, Kay NE, Potter JD, Sellers TA, Folsom AR. Anthropometric characteristics, physical activity, and risk of non-Hodgkin's lymphoma subtypes and B-cell chronic lymphocytic leukemia: a prospective study. American Journal of Epidemiology. 2002;156:527–535. doi: 10.1093/aje/kwf082. [DOI] [PubMed] [Google Scholar]
- 21.Cerhan JR, Bernstein L, Severson RK, Davis S, Colt J, Blair A, Hartge P. Anthropometrics, physical activity, related medical conditions, and the risk of non-Hodgkin lymphoma. Cancer Causes & Control. 2005;16:1203–1214. doi: 10.1007/s10552-005-0358-7. [DOI] [PubMed] [Google Scholar]
- 22.Troy JD, Hartge P, Weissfeld JL, Oken MM, Colditz GA, Mechanic LE, Morton LM. Associations between anthropometry, cigarette smoking, alcohol consumption, and non-Hodgkin lymphoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Epidemiol. 2010;171:1270–1281. doi: 10.1093/aje/kwq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Britton JA, Khan AE, Rohrmann S, Becker N, Linseisen J, Nieters A, Kaaks R, Tjonneland A, Halkjaer J, Severinsen MT, Overvad K, Pischon T, et al. Anthropometric characteristics and non-Hodgkin's lymphoma and multiple myeloma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Haematologica. 2008;93:1666–1677. doi: 10.3324/haematol.13078. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, Prescott J, Sullivan-Halley J, Henderson KD, Ma H, Chang ET, Clarke CA, Horn-Ross PL, Ursin G, Bernstein L. Body size, recreational physical activity, and B-cell non-Hodgkin lymphoma risk among women in the California teachers study. Am J Epidemiol. 2009;170:1231–1240. doi: 10.1093/aje/kwp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willett EV, Morton LM, Hartge P, Becker N, Bernstein L, Boffetta P, Bracci P, Cerhan J, Chiu BC, Cocco P, Dal Maso L, Davis S, et al. Non-Hodgkin lymphoma and obesity: a pooled analysis from the InterLymph Consortium. Int J Cancer. 2008;122:2062–2070. doi: 10.1002/ijc.23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerhan JR, Engels EA, Cozen W, Davis S, Severson RK, Morton LM, Gridley G, Hartge P, Linet M. Blood transfusion, anesthesia, surgery and risk of non-Hodgkin lymphoma in a population-based case-control study. Int J Cancer. 2008;123:888–894. doi: 10.1002/ijc.23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel LS, Laden F, Andersen A, Strickland PT, Blair A, Needham LL, Barr DB, Wolff MS, Helzlsouer K, Hunter DJ, Lan Q, Cantor KP, et al. Polychlorinated biphenyl levels in peripheral blood and non-Hodgkin's lymphoma: a report from three cohorts. Cancer Res. 2007;67:5545–5552. doi: 10.1158/0008-5472.CAN-06-3906. [DOI] [PubMed] [Google Scholar]
- 28.Larsson SC, Wolk A. Obesity and risk of non-Hodgkin's lymphoma: a meta-analysis. Int J Cancer. 2007;121:1564–1570. doi: 10.1002/ijc.22762. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Sanjose SD, Bracci PM, Morton LM, Wang R, Brennan P, Hartge P, Boffetta P, Becker N, Maynadie M, Foretova L, Cocco P, et al. Personal use of hair dye and the risk of certain subtypes of non-Hodgkin lymphoma. Am J Epidemiol. 2008;167:1321–1331. doi: 10.1093/aje/kwn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morton LM, Hartge P, Holford TR, Holly EA, Chiu BC, Vineis P, Stagnaro E, Willett EV, Franceschi S, La Vecchia C, Hughes AM, Cozen W, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph) Cancer Epidemiol Biomarkers Prev. 2005;14:925–933. doi: 10.1158/1055-9965.EPI-04-0693. [DOI] [PubMed] [Google Scholar]
- 31.Morton LM, Holford TR, Leaderer B, Boyle P, Zahm SH, Zhang Y, Flynn S, Tallini G, Zhang B, Owens PH, Zheng T. Cigarette smoking and risk of non-Hodgkin lymphoma subtypes among women. British Journal of Cancer. 2003;89:2087–2092. doi: 10.1038/sj.bjc.6601388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stagnaro E, Ramazzotti V, Crosignani P, Fontana A, Masala G, Miligi L, Nanni O, Neri M, Rodella S, Seniori Costantini A, Tumino R, Vigano C, et al. Smoking and hematolymphopoietic malignancies. Cancer Causes & Control. 2001;12:325–334. doi: 10.1023/a:1011216102871. [DOI] [PubMed] [Google Scholar]
- 33.Bell DA, Liu Y, Cortopassi GA. Occurrence of bcl-2 oncogene translocation with increased frequency in the peripheral blood of heavy smokers. Journal of the National Cancer Institute. 1995;87:223–224. doi: 10.1093/jnci/87.3.223. [DOI] [PubMed] [Google Scholar]
- 34.Chang CM, Schroeder JC, Huang WY, Dunphy CH, Baric RS, Olshan AF, Dorsey KC, Dent GA, Cerhan JR, Lynch CF, Rothman N, Cantor KP, et al. Non-Hodgkin lymphoma (NHL) subtypes defined by common translocations: Utility of fluorescence in situ hybridization (FISH) in a case-control study. Leuk Res. 2009 doi: 10.1016/j.leukres.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engels EA, Cerhan JR, Linet MS, Cozen W, Colt JS, Davis S, Gridley G, Severson RK, Hartge P. Immune-related conditions and immune-modulating medications as risk factors for non-Hodgkin's lymphoma: a case-control study. American Journal of Epidemiology. 2005;162:1153–1161. doi: 10.1093/aje/kwi341. [DOI] [PubMed] [Google Scholar]
- 36.Cozen W, Cerhan J, Martinez-Maza O, Ward M, Linet M, Colt J, Davis S, Severson R, Hartge P, Bernstein L. The effect of atopy, childhood crowding, and other immune-related factors on non-Hodgkin lymphoma risk. Cancer Causes & Control. 2007;18:821–831. doi: 10.1007/s10552-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 37.Morton LM, Zheng T, Holford TR, Holly EA, Chiu BC, Costantini AS, Stagnaro E, Willett EV, Dal Maso L, Serraino D, Chang ET, Cozen W, et al. Alcohol consumption and risk of non-Hodgkin lymphoma: a pooled analysis. Lancet Oncol. 2005;6:469–476. doi: 10.1016/S1470-2045(05)70214-X. [DOI] [PubMed] [Google Scholar]
- 38.Lim U, Schenk M, Kelemen LE, Davis S, Cozen W, Hartge P, Ward MH, Stolzenberg-Solomon R. Dietary determinants of one-carbon metabolism and the risk of non-Hodgkin's lymphoma: NCI-SEER case-control study, 1998–2000. American Journal of Epidemiology. 2005;162:953–964. doi: 10.1093/aje/kwi310. [DOI] [PubMed] [Google Scholar]
- 39.Cross AJ, Ward MH, Schenk M, Kulldorff M, Cozen W, Davis S, Colt JS, Hartge P, Cerhan JR, Sinha R. Meat and meat-mutagen intake and risk of non-Hodgkin lymphoma: results from a NCI-SEER case-control study. Carcinogenesis. 2006;27:293–297. doi: 10.1093/carcin/bgi212. [DOI] [PubMed] [Google Scholar]
- 40.Hartge P, Lim U, Freedman DM, Colt JS, Cerhan JR, Cozen W, Severson RK, Davis S. Ultraviolet radiation, dietary vitamin D, and risk of non-Hodgkin lymphoma (United States) Cancer Causes & Control. 2006;17:1045–1052. doi: 10.1007/s10552-006-0040-8. [DOI] [PubMed] [Google Scholar]
- 41.Colt JS, Davis S, Severson RK, Lynch CF, Cozen W, Camann D, Engels EA, Blair A, Hartge P. Residential insecticide use and risk of non-Hodgkin's lymphoma. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:251–257. doi: 10.1158/1055-9965.EPI-05-0556. [DOI] [PubMed] [Google Scholar]
- 42.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environmental Health Perspectives. 2004;112:1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colt JS, Lubin J, Camann D, Davis S, Cerhan J, Severson RK, Cozen W, Hartge P. Comparison of pesticide levels in carpet dust and self-reported pest treatment practices in four US sites. J Expo Anal Environ Epidemiol. 2004;14:74–83. doi: 10.1038/sj.jea.7500307. [DOI] [PubMed] [Google Scholar]
- 44.Colt JS, Severson RK, Lubin J, Rothman N, Camann D, Davis S, Cerhan JR, Cozen W, Hartge P. Organochlorines in carpet dust and non-Hodgkin lymphoma. Epidemiology. 2005;16:516–525. doi: 10.1097/01.ede.0000164811.25760.f1. [DOI] [PubMed] [Google Scholar]
- 45.Morton LM, Bernstein L, Wang SS, Hein DW, Rothman N, Colt JS, Davis S, Cerhan JR, Severson RK, Welch R, Hartge P, Zahm SH. Hair dye use, genetic variation in N-acetyl transferase 1 (NAT1) and 2 (NAT2), and risk of non-Hodgkin lymphoma. Carcinogenesis. 2007;28:1759–1764. doi: 10.1093/carcin/bgm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.