Abstract
Long-term overweight and substantial weight gain over adulthood are known risk factors of endometrial cancer, but the timing of weight gain in relation to risk and the effect of weight change on age at diagnosis remain unclear. A population-based case–control study was conducted to evaluate the long-term effect of body weight on endometrial cancer risk. The study enrolled 668 incident cases and 674 population controls. Anthropometric features in each decade of adult life were ascertained through in-person interview and analyzed for their associations with endometrial cancer using unconditional logistic regression. As expected, high body mass index (BMI) was significantly associated with increased risk. Women who were overweight or obese at the time of interview had adjusted odds ratios of 1.54 (95%CI 1.13–2.10) and 4.76 (95%CI 3.50–6.49), respectively, compared to women of normal weight. Similar associations were observed for BMI assessed at each decade of adult life. More importantly, women who were overweight (BMI ≥ 25) in their 20s or 30s and maintained the overweight throughout life had significantly higher risk than those who became overweight at ages 40s or 50s. Women with substantial weight gain (≥35%) in early adulthood (age 20s) developed the disease 10 years earlier than those without such weight change in early life. These observations further confirm the critical link between body weight and development of endometrial cancer.
Keywords: endometrial cancer, body mass index, risk factors, epidemiology, case–control study, early adulthood
Endometrial cancer is the most commonly diagnosed gyneco-logical cancer in the US. The American Cancer Society estimates that 42,000 American women were diagnosed with it and 7,800 died from the disease in 2009.1 Despite the slight decline in annual incidence rates of endometrial cancer over the past 30 years,2 the number of women suffering from the disease has been steadily rising because of age-associated disease risks and the increasing numbers of older women in the population. Estrogens are believed to play a central role in endometrial carcinogenesis, as the sex hormones stimulate endometrial proliferation and glandular formation,3 and long-term exposure of endometrial tissue to unopposed estrogenic stimulation can cause abnormal proliferation and neo-plastic transformation.4 Epidemiologic studies support the involvement of estrogens in endometrial cancer: early menarche, late menopause, low parity and use of tamoxifen or exogenous estrogens without progestins are risk factors for the disease,5–9 whereas pregnancy and use of oral contraceptives or exogenous progesterone may protect the endometrium from estrogen exposure.10–12 and are associated with lowered risk of the disease.5,7
As a source of estrogen exposure, obesity and the lifestyles associated with obesity, such as high calorie intake and low physical activity, are associated with the risk of endometrial cancer.13–20 Recent studies further suggest that weight gain in early life may have a significant impact on the disease risk. Both case–control and cohort studies have shown that long-term overweight is a strong risk factor for endometrial cancer.21–24 The relationship between obesity and endometrial cancer appears to be similar across racial and ethnic groups.25 To understand better the long-term effect of obesity on endometrial cancer risk and the effect of weight gain in early life on age at diagnosis, we conducted a population-based case–control study with special focus on body weight in each decade of adulthood throughout life.
Material and Methods
Study design and population
A population-based case–control study was conducted in Connecticut between December 2004 and March 2009. The study was approved by the Institutional Review Boards of Yale University, the Connecticut State Department of Public Health and the 28 Connecticut hospitals involved. Connecticut residents newly diagnosed with primary endometrial cancer from October 2004 through September 2008 were identified for the study by the Yale Cancer Center Rapid Case Ascertainment (RCA) Shared Resource. RCA staff visited the 28 hospitals across the entire state of Connecticut on 1–4 week cycles to review medical records and pathology reports. During the study period, 1,663 potentially eligible patients diagnosed at ages 35–80 years were identified. Physician consent was sought sequentially for 1,270 patients and obtained from 1,242 (97.8%). Consent for the remaining 393 patients was not sought because of attainment of planned sample size. Of the consented patients, we attempted to contact 1,216 and 26 were not contacted due to completion of the study enrollment. Among the patients where contact was attempted, 317 (26.1%) refused to participate, 19 (1.6%) had died, 13 (1.1%) were too ill to participate, 44 (3.6%) were unable to be located, 68 (5.6%) were unable to be reached through available telephone numbers, 62 (5.1%) were found to be ineligible due to ineligible residence (n = 8), mental impairment (n = 17), facility resident (n = 10) barrier (n = 27) and 25 (2.1%) of potential participants had ineligible diagnoses on review of medical records. The total number of patients participating in our study was 668. The response rate for those whom we were able to approach was 59.2% (668 of 1,129) after excluding 87 ineligible patients.
Control women enrolled in the study were frequency matched to cases by age group (35–51, 52–59, 60–64, 65–69, 70–74 and 75–79 years), and were identified through preletter assisted random-digit dialing (RDD) methods. Each selected landline telephone number was first searched in reverse directories to find an address for the number, in order to mail a study letter prior to telephone contact. Potential control subjects were initially contacted by telephone for determination of study eligibility. Our RDD screen identified 8,168 residence numbers of which, 1,995 appeared to have female residents whose ages were within the desired range of our study. Of these, 1,447 (72.5%) agreed to be further contacted for possible participation in the study. From the list of potential candidates, we sequentially contacted 1,248 subjects before the end of the study, and found 111 subjects ineligible for the study due to ineligible residence, mental impairment, language barrier, diagnosis of cancer and ineligible medical conditions, as well as 92 disqualified for the study due to illnesses, death, relocation and no response. Of the remaining subjects, 674 completed in-person interview and 371 refused to participate. The response rate was 64.5% (674 of 1,045 subjects).
On agreement to participate, an appointment was scheduled for our interviewers to meet subjects at home for in-person interview and collection of biological specimens. Written informed consent was obtained before the interview and specimen collection. A structured questionnaire was used for interview, and it elicited information on birth weight, place and date of birth, years of education, self-identified ethnic group and ethnicity, menstrual and reproductive features at each decade from 20s through 40s, use of oral contraceptives and hormone replacement therapies, medical history of major chronic illnesses and gynecological disorders, family history of cancer, smoking and drinking habits, current and lifetime physical activities, current and lifetime body weight, including weight at age 20s, 30s, 40s, 50s, 60s and 5 years before the interview. At interview we also measured the subject's height and other physical dimensions.
Statistical analysis
Nine control subjects were excluded from data analysis due to a history of hysterectomy (n = 6) or age greater than 81 years (n = 3). Questionnaire data were first assessed for distributions, outliers and missing values. We computed descriptive statistics on each variable of interest in the cases and controls separately, including means, medians, ranges and standard deviations for numerical variables and frequency distributions for categorical or ordinal variables. After the initial assessment, we analyzed the associations between endometrial cancer and suspected risk factors, mainly body mass index. Unconditional logistic regression models were used to determine the strength and significance of associations through estimating odds ratios (OR) and their 95% confidence intervals. Multivariate logistic regression analyses were performed to adjust for covariates and potential confounding variables. In the multivariate models, age at interview or diagnosis, ethnic group (white vs. nonwhite), year of education (no college vs. college or higher), number of pregnancies, smoking habit (yes vs. no), drinking habit (yes vs. no), family history of cancer (yes vs. no), estrogen use (yes vs. no) and oral contraceptive (OC) use (yes vs. no) were included for adjustment. Regression models were also developed to assess interactions between risk factors. For variables with significant interactions, subgroup analyses were performed to assess the disease association with one risk factor while stratifying the data by another risk factor. All p-values calculated are two sided.
Results
Table 1 shows the demographic features of cases and controls in the study. Cases and controls were reasonably similar in age at interview, place of birth, mother's age at their birth, numbers of siblings, sisters and elder siblings (Table 1). The study enrolled somewhat more Caucasian women in the control group than in the case group (p = 0.033). Control women tended to have had more years of education than cases (p < 0.001); 34.2% and 25.6% of control subjects completed college or graduate education compared to 28.3% and 18.9% of cases, respectively. Reported birth weight and adult height were quite similar between cases and controls, but as expected reported current body weight and BMI [weight (kg)/height2 (m2)] were all significantly higher in cases than in controls (p < 0.001).
Table 1.
Demographic and anthropometric features of cases and controls in the study
| Variable | Cases | Controls | p value | ||
|---|---|---|---|---|---|
| Number | Mean (SD) | Number | Mean (SD) | ||
| Age at interview | 668 | 60.6 (9.5) | 665 | 61.5 (10.8) | 0.123 |
| Mother's age at birth | 618 | 28.6 (6.6) | 638 | 28.3 (6.1) | 0.444 |
| Number of siblings | 667 | 3.9 (2.3) | 663 | 3.7 (2.1) | 0.730 |
| Number of sisters | 665 | 2.4 (1.4) | 661 | 2.3 (1.3) | 0.324 |
| Number of elder siblings | 665 | 1.4 (1.8) | 660 | 1.3 (1.6) | 0.817 |
| Birth weight (lb) | 392 | 7.1 (1.4) | 409 | 7.0 (1.5) | 0.701 |
| Height (cm) | 668 | 162.7 (6.5) | 665 | 162.5 (6.5) | 0.553 |
| Weight (kg) | 665 | 86.5 (23.7) | 663 | 71.5 (16.4) | <0.001 |
| BMI | 665 | 32.7 (8.5) | 663 | 27.1 (6.1) | <0.001 |
| Percent | Percent | ||||
| Birth Place | 0.254 | ||||
| US | 602 | 90.3 | 612 | 92.0 | |
| Non-US | 65 | 9.7 | 53 | 8.0 | |
| Ethnic group | 0.033 | ||||
| White | 607 | 90.9 | 628 | 94.6 | |
| African American | 36 | 5.4 | 22 | 3.3 | |
| Other | 25 | 3.7 | 14 | 2.1 | |
| Education | <0.001 | ||||
| <12 yrs. | 237 | 35.5 | 175 | 26.4 | |
| 12 yrs. to 3 yrs. college | 116 | 17.4 | 92 | 13.9 | |
| College/university | 189 | 28.3 | 227 | 34.2 | |
| Graduate school | 126 | 18.9 | 170 | 25.6 | |
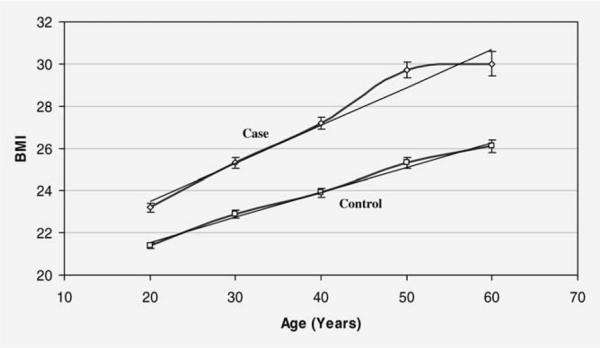
Both cases and controls were also asked to recall their height and weight at each decade of adulthood. Figure 1 shows the values of BMI from age 20s through 60s in cases and controls. As expected, BMI increased throughout adult life among all of the subjects regardless of their disease status. However, BMI values were consistently higher in cases than controls across the lifespan, and the increases in BMI values over time were not parallel, cases having greater increases than controls.
Figure 1.
BMI from ages 20s–60s in cases and controls.
The associations between endometrial cancer risk and BMI at each decade of age from 20s through 60s are shown in Table 2. Compared to women with BMI < 25, women overweight (BMI 25–30) at interview or at 5 years before interview had about 50% increased endometrial cancer risk after adjusting for confounding and other risk factors, including age at interview, ethnic group, years of education, number of pregnancies, estrogen use, oral contraceptive use, cigarette smoking, alcohol drinking and family history of cancer. The increase in risk among overweight women was even higher when BMI was assessed at earlier ages of adulthood (Table 2). For obese women (BMI ≥ 30), endometrial cancer risk was further elevated. A fourfold to fivefold increase in risk was observed when BMI was determined around the time of diagnosis or 5 years before the diagnosis. The odds ratios for obesity were lower but still statistically significant when BMI was assessed at ages 20s and 30s.
Table 2.
Associations of endometrial cancer risk with BMI at different ages of adulthood
| Variable | Cases | Controls | OR1 | 95% CI | p for trend | ||
|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | ||||
| BMI (current) | <0.001 | ||||||
| <25 | 132 | 20.4 | 294 | 45.0 | 1 | ||
| 25–30 | 161 | 24.9 | 217 | 33.2 | 1.54 | 1.13–2.10 | |
| ≥30 | 354 | 54.7 | 143 | 21.8 | 4.76 | 3.50–6.49 | |
| BMI (5 years in past) | <0.001 | ||||||
| <25 | 129 | 21.3 | 252 | 45.7 | 1 | ||
| 25–30 | 157 | 25.9 | 178 | 32.3 | 1.56 | 1.13–2.16 | |
| ≥30 | 321 | 52.9 | 122 | 22.1 | 4.22 | 3.05–5.84 | |
| BMI at age 20s | <0.001 | ||||||
| <25 | 519 | 79.1 | 598 | 90.7 | 1 | ||
| 25–30 | 77 | 11.7 | 37 | 5.6 | 2.21 | 1.42–3.44 | |
| ≥30 | 60 | 9.2 | 24 | 3.6 | 1.96 | 1.16–3.29 | |
| BMI at age 30s | <0.001 | ||||||
| <25 | 423 | 65.3 | 548 | 84 | 1 | ||
| 25–30 | 119 | 18.4 | 59 | 9.1 | 2.15 | 1.50–3.08 | |
| ≥30 | 106 | 16.4 | 45 | 6.9 | 2.19 | 1.46–3.28 | |
| BMI at age 40s | <0.001 | ||||||
| <25 | 285 | 48.5 | 446 | 73.6 | 1 | ||
| 25–30 | 152 | 25.9 | 109 | 18.0 | 1.90 | 1.40–2.59 | |
| ≥30 | 150 | 25.6 | 51 | 8.4 | 3.84 | 2.62–5.61 | |
| BMI at age 50s | <0.001 | ||||||
| <25 | 127 | ≥30.9 | 290 | 61.8 | 1 | ||
| 25–30 | 128 | 31.1 | 121 | 25.8 | 2.17 | 1.53–3.06 | |
| ≥30 | 156 | 38.0 | 58 | 12.4 | 5.44 | 3.62–8.17 | |
| BMI at age 60s | <0.001 | ||||||
| <25 | 45 | 26.8 | 147 | 52.1 | 1 | ||
| 25–30 | 56 | 33.3 | 81 | 28.7 | 2.24 | 1.36–3.72 | |
| ≥30 | 67 | 39.9 | 54 | 19.2 | 4.09 | 2.32–7.21 | |
Adjusted for age, ethnic group, education, pregnancy, family history of cancer, estrogen use, OC use, smoking and drinking.
To examine the long-term effect of BMI on endometrial cancer, we combined the BMI assessment across each decade of adulthood from 20s through 50s. The results of this longitudinal analysis are shown in Table 3. Compared to women whose BMIs were always under 25 between ages 20s and 50s, women whose BMIs were consistently 25 or greater throughout adult life had a nearly fivefold increase in risk for endometrial cancer (OR = 4.76; 95%CI: 2.75–8.25). Furthermore, a significant trend was observed between the time-span of being overweight and risk of endometrial cancer. For women whose BMI increased after 50s, 40s or 30s, the ORs were 2.17 (95%CI: 1.46–3.21), 3.24 (95%CI: 2.04–5.14) and 4.41 (95%CI: 2.52–7.70), respectively. If we changed the reference group to those who became overweight after age 50s, the disease risk was still significantly higher for those who were overweight since 30s or 20s, ORs 1.93 (95%CI: 1.05–3.56) and 2.02 (95%CI: 1.11–3.68). The data clearly indicate that the longer time being overweight, the higher the risk of endometrial cancer. These dose-response relationships were obtained after we had adjusted for other risk factors and confounding variables including age, ethnic group, education, number of pregnancies, family history of cancer, estrogen use, OC use, smoking and drinking habits.
Table 3.
Changes of BMI through each decade of adulthood in association with endometrial cancer risk
| BMI change overtime | Number (case/control) | OR1 (95% CI) | OR1 (95% CI) | OR1 (95% CI) | OR1 (95% CI) |
|---|---|---|---|---|---|
| BMI always <25 | 124/271 | 1.00 | |||
| BMI increase after 50s | 84/80 | 2.17 (1.46–3.21) | 1.00 | ||
| BMI increase after 40s | 75/44 | 3.24 (2.04–5.14) | 1.40 (0.84–2.34) | 1.00 | |
| BMI increase after 30s | 58/23 | 4.41 (2.52–7.70) | 1.93 (1.05–3.56) | 1.44 (0.75–2.74) | 1.00 |
| BMI always ≥25 | 62/27 | 4.762 (2.75–8.25) | 2.023 (1.11–3.68) | 1.37 (0.73–2.57) | 1.10 (0.54–2.25) |
Adjusted for age, ethnic group, education, pregnancy, family history of cancer, estrogen use, OC use, smoking and drinking.
p for trend < 0.001.
p for trend = 0.008.
Our data also show that endometrial cancer patients who had substantial increases in BMI during early adult life tended to be diagnosed at much younger age (Table 4). For women with 35% or greater change in BMI (mainly increase) between age 20s and 30s, the average age at diagnosis was 52.2 years. However, for women with less than 5% change in BMI during the same period of time, the average age at diagnosis was 62.5 years, a 10-year delay in diagnosis of the disease (p < 0.001). This age gap gradually narrowed when the substantial increases in BMI occurred later in life. Patients with 35% or more change in BMI in their 30s to 40s or 40s to 50s had average ages of 57.7 and 61.6 years at diagnosis, and for those with less than 5% change during the same times, ages at diagnosis of 63.3 and 68.0 years, differences of 5.6 and 6.4 years, respectively (p < 0.001). During 50s to 60s, the age gap at diagnosis between high and low BMI changes (67.4 versus 71.4 years) was 4 years, and this difference was no longer statistically significant (p = 0.107).
Table 4.
Ages at endometrial cancer diagnosis in relation to changes of BMI in different decades of adulthood
| BMI change | Number of cases | Mean age onset (SD) | Median age onset (range) | p |
|---|---|---|---|---|
| Between ages 20–30 years | <0.001 | |||
| <5% | 296 | 62.5 (9.3) | 62 (36–80) | |
| 5–20% | 267 | 60.0 (8.5) | 60 (38–80) | |
| 20–35% | 58 | 56.8 (9.4) | 58 (35–75) | |
| ≥35% | 26 | 52.2 (10.0) | 50 (36–73) | |
| Between ages 30–40 years | <0.001 | |||
| <5% | 206 | 63.3 (8.5) | 63 (30–79) | |
| 5–20% | 275 | 62.1 (7.9) | 61 (41–80) | |
| 20–35% | 76 | 60.1 (6.7) | 60 (45–79) | |
| ≥35% | 28 | 57.7 (5.4) | 59 (43–68) | |
| Between ages 40–50 years | <0.001 | |||
| <5% | 145 | 68.0 (7.2) | 68 (50–80) | |
| 5–20% | 185 | 64.6 (6.1) | 64 (50–80) | |
| 20–35% | 55 | 62.7 (6.3) | 62 (50–79) | |
| ≥35% | 25 | 61.6 (4.4) | 62 (51–69) | |
| Between ages 50–60 years | 0.107 | |||
| <5% | 68 | 71.4 (5.6) | 72 (60–80) | |
| 5–20% | 77 | 70.6 (6.2) | 72 (59–90) | |
| 20–35% | 12 | 68.3 (5.2) | 70 (60–79) | |
| ≥35% | 10 | 67.4 (4.7) | 67 (61–74) |
Potential interactions between BMI and other risk factors were also evaluated in our data analysis. We chose two BMI variables (at age 40s and 5 years before interview) for this assessment, and other risk factors evaluated for interaction included menopausal status (yes vs. no), pregnancy (yes vs. no), OC use (yes vs. no), estrogen use (yes vs. no), smoking habit (ever vs. never) and drinking habit (ever vs. never). The results showed no apparent interactions between BMI at age 40s and other risk factors in the study (data not shown). A potential interaction, however, was indicated for estrogen use and cigarette smoking. These variables seemed to have protective effects against the disease in overweight or obese women, but not in normal weight women when BMI was evaluated 5 years before interview (data not shown).
Discussion
This population-based case–control study confirms that BMI is an important risk factor for endometrial cancer. Obese women had fourfold to fivefold increased risk of endometrial cancer compared to women with normal weights. Overweight women also had nearly twofold increased risk. These associations between BMI and endometrial cancer risk were observed with adjustment for other risk and potential confounding factors, including age at diagnosis or interview, ethnic group, years of education, number of pregnancies, cigarette smoking, alcohol drinking, use of menopausal estrogens, use of oral contraceptives and family history of cancer. Our observations are consistent with the literature both in the direction and strength of the association. Several review articles have shown that among obesity-related female malignancies endometrial cancer has the strongest association with BMI.25–27 In general, risk of endometrial cancer is doubled (OR = 2) for overweight women, and redoubled for obese women (OR = 4), when compared to normal weight women.
We evaluated BMI not only at or before diagnosis of the disease, but also at ages 20s, 30s, 40s, 50s and 60s. BMI at each decade of adult life was significantly associated with endometrial cancer risk. In addition, we observed that the strength of association for obese women was low at age 20s (OR = 1.96), but gradually increased to 2.19 in the 30s, 3.84 in the 40s and 5.44 at age 50s, suggesting a strong cumulative effect of BMI on endometrial cancer risk over time. Similar associations have been observed in a case–control study of Chinese women.24 The accumulating effect was further confirmed in our longitudinal analysis when we grouped BMI assessments together from age 20s through 50s. Women who were continually overweight during their adulthood between 20s and 50s had nearly 5 times higher risk for the disease as compared to those whose BMI were always normal during the same adult time. Using the same reference group (BMI always normal), women becoming overweight after 50s had a twofold increase in risk, had threefold risk after 40s and fourfold risk after 30s. Thus, a longer time being overweight was clearly associated with higher risk of endometrial cancer.
These observations indicate that high BMI in early adult life may appreciably increase endometrial cancer risk later in life. This assertion is also supported by a number of previous studies. Chang et al. reported that American women who gained more than 20 kg body weight from age 18 years to postmenopause had nearly threefold increases in risk of endometrial cancer compared to those with less than 5 kg change in body weight during the same time interval.22 Schouten et al. found a similar association between weight gain and endometrial cancer risk in Dutch women.28 A study by Park et al. showed the same results in a multiethnic cohort, where women who gained weight more than 35% between ages 21 and 45–75 years had fourfold increased risk.21 However, none of these studies had evaluated the relationship between weight gain in early adulthood and age at disease diagnosis.
In our study, we found that considerable weight gain in early adult life could affect patient age at diagnosis; patients with 35% or more weight gain at their 20s were diagnosed 10 years younger than those with 5% or less weight change during the same period. The advance in age at diagnosis was quite substantial when weight gain developed earlier in life. The age gap at diagnosis, however, was gradually narrowed as the weight gain occurred later in life. This observation provides additional evidence in support of obesity or overweight playing a critical role in endometrial cancer. It suggests that as an important risk factor, early exposure to excessive body weight may shift the timeframe of carcinogenic process resulting in early development of the disease.
Adipose tissue is known to produce estrogens through peripheral aromatization of androstenedione,29 and unopposed estrogens are an established cause of endometrial cancer.8,30 However, the effects of estrogens from adipose tissue are suspected to occur later in life, mostly after menopause since premenopausal estrogens arise mainly from the ovaries. Our findings indicate that this effect may occur earlier in life and accumulate over time. It is also possible that other obesity-related factors are involved in endometrial carcinogenesis, such as proinflammatory cytokines and mitogenic growth factors.26,31 The results of our subgroup analyses further suggest that the effects of estrogens on the disease vary by BMI, indicating that adipocytes may have other biological effects on the uterine tissues and some of them may interfere or interact with estrogens.
Birth weight has been suspected to influence the risk of endometrial cancer.32 We collected the information on birth weight in the study. Although not all subjects were able to recall their birth weight, 59% of cases and 62% of controls provided the information. Among the subjects who supplied the data, no evidence of an association was observed. This result is consistent with the finding of the Nurses' Health Study which also found no association between birth weight and endometrial cancer risk.33 The observation of no association between birth weight and endometrial cancer suggests that in utero exposure of estrogens or growth factors may not be involved in endometrial cancer risk later in life.
There are some aspects of our study that should be considered when evaluating our findings. It is possible that our ascertainment of body weight at different decades of age throughout adult life is subject to recall bias, a standard consideration for all case–control studies. However, findings of cohort studies on weight gain in early adulthood and endometrial cancer risk have been similar to those for case–control studies. Compared to other variables, body weight for most individuals is relatively stable, and is relatively easy to recall because of its relevance to many aspects of a person's life. The reported weights and heights were all within reasonable ranges and no obvious outliers were identified in the data, i.e., weights between 34.0 and 197.5 kg and heights between 139.7 and 190.5 cm. In addition, there are no reasons to suspect that patients in our study are more likely to over report their body weight than controls or vise versa. We measured height at interview and found that using this variable instead of reported height in calculating BMI made no difference to the BMI estimates or to the observed associations with BMI. Also, our overall response fraction was lower than desired, but the consistency of our results with the vast literature on endometrial cancer, including both case–control studies and cohort studies, suggests that our findings are adequately representative of endometrial cancer cases and population controls in general.
In summary, this population-based epidemiologic study confirms that obesity is a strong risk factor for endometrial cancer, and that persistent overweight throughout adult life or substantial weight gain during adult life is also important to the disease risk. A strong positive correlation between the length of overweight in adulthood and risk of the disease is clearly demonstrated in the study. Moreover, BMI seems clearly related to the disease process as substantial weight gain in early adult life significantly advances the age at disease diagnosis. Women with 35% or more weight gain in their 20s are likely to have endometrial cancer diagnosed 10 years earlier than women with little change (<5%) in body weight during the same time. These observations suggest that obesity may play an important role in endometrial cancer.
Acknowledgements
The cooperation of 28 Connecticut hospitals, including Charlotte Hungerford Hospital, Bridgeport Hospital, Danbury Hospital, Hartford Hospital, Middlesex Hospital, New Britain General Hospital, Bradley Memorial Hospital, Yale/New Haven Hospital, St. Francis Hospital and Medical Center, St. Mary's Hospital, Hospital of St. Raphael, St. Vincent's Medical Center, Stamford Hospital, William W. Backus Hospital, Windham Hospital, Eastern Connecticut Health Network, Griffin Hospital, Bristol Hospital, Johnson Memorial Hospital, Day Kimball Hospital, Greenwich Hospital, Lawrence and Memorial Hospital, Milford Hospital, New Milford Hospital, Norwalk Hospital, MidState Medical Center, John Dempsey Hospital and Waterbury Hospital, in allowing patient access, is gratefully acknowledged. Our study was approved by the State of Connecticut Department of Public Health Human Investigation Committee. Certain data used in our study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data. The authors want to specially thank Rajni Mehta for her support in case identification through RCA, Helen Sayward for her effort in conducting the study, Ellen Andreson, Donna Bowers, Renee Capasso, Kristin DeFrancesco, Anna Florczak and Sherry Rowland for their assistance in recruiting and interviewing study participants and Na Ni for her help in SAS programming.
Grant sponsor: NCI-NIH; Grant number: 5R01CA098346
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrlich CE, Young PC, Cleary RE. Cytoplasmic progesterone and estradiol receptors in normal, hyperplastic, and carcinomatous endometria: therapeutic implications. Am J Obstet Gynecol. 1981;141:539–46. doi: 10.1016/s0002-9378(15)33275-0. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Elwood JM, Cole P, Rothman KJ, Kaplan SD. Epidemiology of endometrial cancer. J Natl Cancer Inst. 1977;59:1055–60. doi: 10.1093/jnci/59.4.1055. [DOI] [PubMed] [Google Scholar]
- 6.Parazzini F, La Vecchia C, Bocciolone L, Franceschi S. The epidemiology of endometrial cancer. Gynecol Oncol. 1991;41:1–16. doi: 10.1016/0090-8258(91)90246-2. [DOI] [PubMed] [Google Scholar]
- 7.Persson I, Weiderpass E, Bergkvist L, Bergstrom R, Schairer C. Risks of breast and endometrial cancer after estrogen and estrogen-progestin replacement. Cancer Causes Control. 1999;10:253–60. doi: 10.1023/a:1008909128110. [DOI] [PubMed] [Google Scholar]
- 8.Pike MC, Pearce CL, Wu AH. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23:6379–91. doi: 10.1038/sj.onc.1207899. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, Perlman JA, Ford L. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654–62. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- 10.Moyer DL, Felix JC. The effects of progesterone and progestins on endometrial proliferation. Contraception. 1998;57:399–403. doi: 10.1016/s0010-7824(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons WE, Thorneycroft IH. Protecting the endometrium. Opposing the hyperplasia/malignancy potential of ERT. J Reprod Med. 1999;44:203–8. [PubMed] [Google Scholar]
- 12.Satyaswaroop PG, Tabibzadeh S. Progestin regulation of human endometrial function. Hum Reprod. 2000;15(Suppl 1):74–80. doi: 10.1093/humrep/15.suppl_1.74. [DOI] [PubMed] [Google Scholar]
- 13.Carroll KK. Obesity as a risk factor for certain types of cancer. Lipids. 1998;33:1055–9. doi: 10.1007/s11745-998-0305-8. [DOI] [PubMed] [Google Scholar]
- 14.McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9:487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 15.Goodman MT, Hankin JH, Wilkens LR, Lyu LC, McDuffie K, Liu LQ, Kolonel LN. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. 1997;57:5077–85. [PubMed] [Google Scholar]
- 16.Willett WC. Diet and cancer. Oncologist. 2000;5:393–404. doi: 10.1634/theoncologist.5-5-393. [DOI] [PubMed] [Google Scholar]
- 17.Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev. 2001;10:287–301. [PubMed] [Google Scholar]
- 18.Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer. 2003;104:669–76. doi: 10.1002/ijc.10974. [DOI] [PubMed] [Google Scholar]
- 19.Xu WH, Matthews CE, Xiang YB, Zheng W, Ruan ZX, Cheng JR, Gao YT, Shu XO. Effect of adiposity and fat distribution on endometrial cancer risk in Shanghai women. Am J Epidemiol. 2005;161:939–47. doi: 10.1093/aje/kwi127. [DOI] [PubMed] [Google Scholar]
- 20.Matthews CE, Xu WH, Zheng W, Gao YT, Ruan ZX, Cheng JR, Xiang YB, Shu XO. Physical activity and risk of endometrial cancer: a report from the Shanghai endometrial cancer study. Cancer Epidemiol Biomarkers Prev. 2005;14:779–85. doi: 10.1158/1055-9965.EPI-04-0665. [DOI] [PubMed] [Google Scholar]
- 21.Park SL, Goodman MT, Zhang ZF, Kolonel LN, Henderson BE, Setiawan VW. Body size, adult BMI gain and endometrial cancer risk: the multiethnic cohort. Int J Cancer. 2010;126:490–9. doi: 10.1002/ijc.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SC, Lacey JV, Jr, Brinton LA, Hartge P, Adams K, Mouw T, Carroll L, Hollenbeck A, Schatzkin A, Leitzmann MF. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2007;16:723–30. doi: 10.1158/1055-9965.EPI-06-0675. [DOI] [PubMed] [Google Scholar]
- 23.Friedenreich C, Cust A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S, Linseisen J, Rohrmann S, Boeing H, Pischon T, Tjonneland A, et al. Anthropometric factors and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2007;18:399–413. doi: 10.1007/s10552-006-0113-8. [DOI] [PubMed] [Google Scholar]
- 24.Xu WH, Xiang YB, Zheng W, Zhang X, Ruan ZX, Cheng JR, Gao YT, Shu XO. Weight history and risk of endometrial cancer among Chinese women. Int J Epidemiol. 2006;35:159–66. doi: 10.1093/ije/dyi223. [DOI] [PubMed] [Google Scholar]
- 25.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 27.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–78. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 28.Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst. 2004;96:1635–8. doi: 10.1093/jnci/djh291. [DOI] [PubMed] [Google Scholar]
- 29.Enriori CL, Reforzo-Membrives J. Peripheral aromatization as a risk factor for breast and endometrial cancer in postmenopausal women: a review. Gynecol Oncol. 1984;17:1–21. doi: 10.1016/0090-8258(84)90055-6. [DOI] [PubMed] [Google Scholar]
- 30.Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15:341–54. doi: 10.1053/beog.2000.0180. [DOI] [PubMed] [Google Scholar]
- 31.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 32.McCormack VA, dos Santos Silva I, Koupil I, Leon DA, Lithell HO. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115:611–7. doi: 10.1002/ijc.20915. [DOI] [PubMed] [Google Scholar]
- 33.Xue F, Hilakivi-Clarke L, Maxwell G, Hankinson S, Michels K. Longitudinal study on birthweight and the incidence of endometrial cancer. Br J Cancer. 2008;98:1288–91. doi: 10.1038/sj.bjc.6604304. [DOI] [PMC free article] [PubMed] [Google Scholar]