Abstract
Background
Calorie restriction induces long-term changes in motivation to eat highly palatable food, and in body weight regulation, through an unknown mechanism.
Methods
Following a period of calorie restriction and re-feeding, mice were assessed by behavioral and metabolic studies and for levels of the transcription factor ΔFosB. ΔFosB levels were then increased specifically in nucleus accumbens (NAc) using viral-mediated gene transfer, and behavioral and metabolic studies were conducted.
Results
We show that accumulation of ΔFosB in the NAc shell after calorie restriction in mice corresponds to a period of increased motivation for high fat reward and reduced energy expenditure. Furthermore, ΔFosB over-expression in this region increases instrumental responding for a high fat reward via an orexin-dependent mechanism, while also decreasing energy expenditure and promoting adiposity.
Conclusions
These results suggest that ΔFosB signaling in NAc mediates adaptive responses to calorie restriction.
Keywords: Feeding, Metabolism, Nucleus Accumbens, Appetite, Orexin
Introduction
Obesity is a major health concern in the developed world. Even though many obese individuals are able to lose weight through short-term changes in diet, several studies show modest long-term results because of poor compliance and a tendency for individuals to regain lost weight (1, 2). While rebound weight gain after dieting is a significant clinical problem, the neural mechanisms involved are unknown. Previous studies have identified a role for the NAc, a key brain reward region, in the regulation of food intake (3, 4), but the underlying molecular regulation of this region is poorly understood. We investigated a possible role for the transcription factor ΔFosB in NAc for the following reasons: 1. ΔFosB accumulates in NAc following numerous stressors including exposure to drugs of abuse, food restriction, and restraint stress (5, 6); 2. ΔFosB over-expressing mice have altered sensitivity to highly rewarding diets (7); and 3. Over-expression of ΔFosB increases instrumental responding for sucrose (8). Together, these findings support the hypothesis that ΔFosB accumulation in NAc alters long-term responses to calorie restriction by increasing motivation for intake of highly palatable food.
Materials and Methods
Animals and housing
Animals were housed in the UT Southwestern vivarium in a temperature-controlled environment and maintained on regular chow (4% fat diet #7001, Harlan-Teklad, Madison, WI). All animal procedures were carried out in accordance with the UT Southwestern Institutional Animal Care and Use Committee (IACUC) guidelines.
Immunohistochemistry
Cell counts for ΔFosB+ neurons in NAc were performed as described (9). Complete description of methods can be found in the Supplement.
Operant Responding
Operant responding was performed as described recently (10). Complete description of methods can be found in the Supplement.
Stereotatic surgery
Adeno-associated viruses (AAV) expressing ΔFosB and GFP or GFP alone were performed as reported, see Supplement (9).
Metabolic Studies
Experiments were conducted in the UT Southwestern Metabolic Phenotyping Core using metabolic cages (TSE Systems, Chesterfield, MO). Oxygen consumption and carbon dioxide production measurements were corrected for lean body composition using the formula: (ml/hr/kgˆlean body mass). Body composition was determined by an mq10 series Bruker Minispec (Bruker Optics, The Woodlands, TX).
Statistical analyses
Data are reported as the mean ± SEM. GraphPad Prism 5 software (v 5.0, GraphPad Software Inc., San Diego, CA) was used to perform all statistical analyses.
Results
We first sought to determine the behavioral and metabolic consequences of exposure to calorie restriction (CR). Mice were exposed to a CR protocol in which they received 60% of ad lib calories daily for 10 days. During this time, mice lost ∼15-20% of their original body weight (Figure S1 in the Supplement). The mice were then given free access to regular chow. There was no significant difference in body weight between mice exposed to CR and ad lib fed mice within two days of re-feeding. Both groups were then allowed additional recovery, with behavioral and metabolic testing conducted the following week.
To test for motivation to obtain calorically dense food, mice were trained to nosepoke for higher in fat (HFD) pellets (22.7% fat) prior to exposure to CR. After the recovery period, the mice were moved to a progressive ratio schedule in which each successive reward required a greater number of nosepokes. The last reward earned within 30 min was used as our measure of instrumental responding for HFD. Mice with a history of CR earned a significantly greater number of rewards on the progressive ratio schedule compared to ad lib fed mice (Fig 1A) in the week after regaining their lost weight. No difference was detected between the two groups after 2 weeks recovery (data not shown).
Figure 1. ΔFosB expression in NAc enhances motivation for HFD.
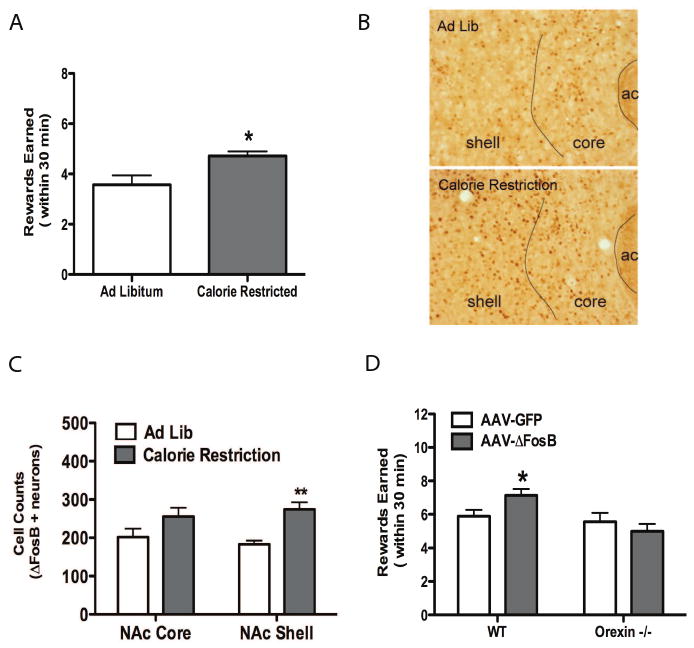
Eight week old c57BL/6 male mice were subjected to 10 days of 60% CR. (A) Number of rewards earned by operant responding prior to reaching breakpoint in mice one week after recovering body weight (Student's t-test. *p<0.05, n=7/group). (B) Representative image of ΔFosB+ neurons in NAc. (C) ΔFosB+ neurons in NAc (Student's t-test, **P<0.01, n=5/group). (D) Number of rewards earned by operant responding prior to reaching breakpoint in mice over-expressing ΔFosB in NAc by viral injection (significant effect of orexin [F1,28 = 7.04] by two-way ANOVA, Bonferroni post-test revealed a significant difference *P<0.05 between wild-type groups, n=12 for wild-type/GFP, n=10 for wild-type/ΔFosB and n=5 for both orexin-/- groups,). Data are presented as mean ± SEM.
Next we wanted to determine the effect of a history of CR on metabolic rate. A separate group of CR mice were analyzed for metabolic parameters using indirect calorimetry. One week after achieving stable weight, hourly measurements were collected for three consecutive days. Mice with a history of CR demonstrated reduced consumption of oxygen and production of carbon dioxide, suggesting a persistent decrease in energy expenditure (Fig 2A and 2B). Importantly, body weight and food intake did not differ between the two groups during this time (Fig 2C and 2D). Interestingly, mice with a history of CR displayed locomotor hyperactivity (Fig 2E), despite the reduced metabolic rate. Finally, we measured body composition at the end of the experiment. Animals with a history of CR displayed significantly increased levels of body fat (Fig 2F) compared to ad lib fed mice, which indicates that a history of CR promotes a repartitioning of energy stores into adipose tissue. These findings demonstrate that the increased energy expenditure and reduced adiposity seen in transgenic mice that over-express ΔFosB are mediated via non-NAc mechanisms (11).
Figure 2. Metabolic parameters after calorie restriction or ΔFosB over-expression.
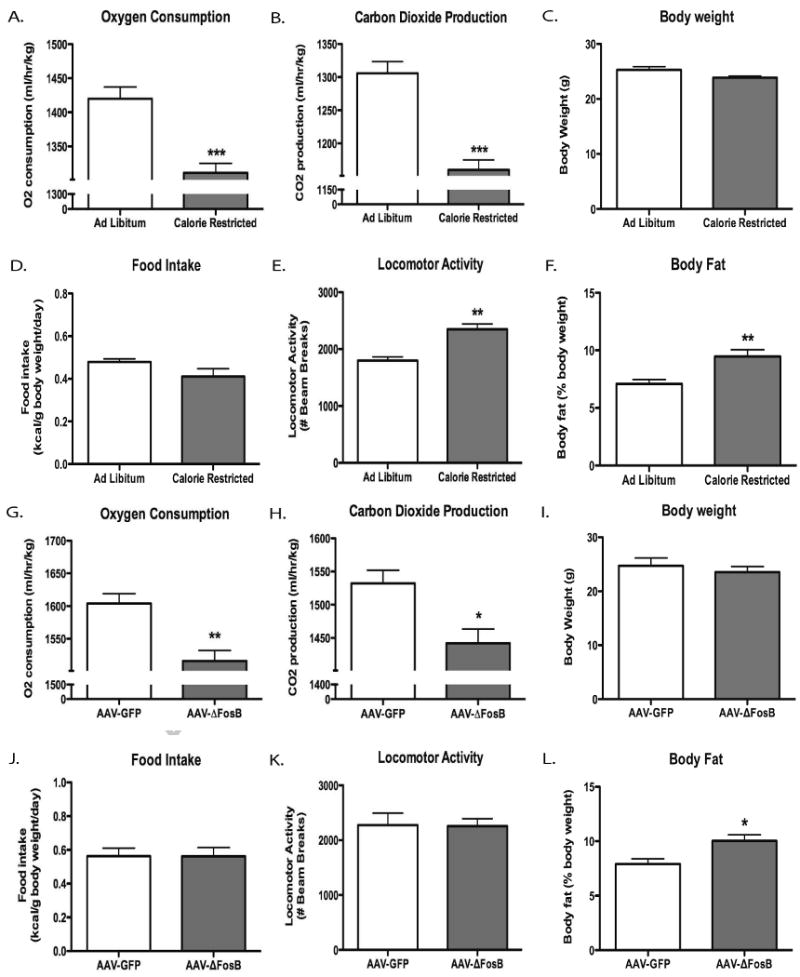
Eight week old c57BL/6 male mice were subjected to 10 days of 60% CR and then allowed to regain body weight. Mice (n=6/group) were then allowed to recover for one week and monitored for three days in metabolic cages. (A) Oxygen consumption, (B) carbon dioxide production, (C) body weight, (D) food intake, (E) locomotor activity, and (F) body composition were determined. Eight week old c57BL/6 mice received viral injections of either AAV-GFP or AAV-ΔFosB into the NAc. Four weeks later the mice were tested for (G) Oxygen consumption, (H) carbon dioxide production, (I) body weight, (J) food intake, (K) locomotor activity, and (L) body composition (*P<0.05, **P<0.01). Data are presented as mean ± SEM.
To test our hypothesis that accumulation of ΔFosB in NAc may be an important regulator of food intake and metabolism after CR, we first determined the effect of CR on ΔFosB levels. ΔFosB–positive neurons were quantified by immunohistochemistry (Fig 1B). Similar to published results (6), CR significantly increased the number of neurons in NAc shell, but not NAc core, expressing ΔFosB (Fig 1C). No significant differences in ΔFosB levels were detected two weeks after re-feeding; this time frame is consistent with the observation, noted above, that operant responding does not differ between either group two weeks after re-feeding.
Pharmacologic inhibition of NAc neurons has previously been demonstrated to increase the intake of high fat food via an orexin (also known as hypocretin)-dependent mechanism (4). Since CR increases motivation to obtain energy dense food (Fig 1A), the observed accumulation of ΔFosB in the NAc shell after CR may mediate the increased motivation to obtain highly palatable food observed after periods of CR. To directly test this hypothesis, we chose viral-mediated gene transfer (AAV-ΔFosB) to increase levels of ΔFosB in NAc, because this system allows for exact temporal and spatial control of ΔFosB expression in adult mice (Figure S2 in the Supplement). Four weeks after viral injection, mice were trained to nosepoke for HFD pellets. Wild-type mice receiving the control AAV-GFP vector earned fewer rewards than wild-type mice receiving AAV-ΔFosB into the NAc (Fig 1D), indicating that over-expression ΔFosB in NAc was sufficient to increase instrumental responding for HFD. We next determined if this effect was dependent on the presence of orexin, a peptide previously implicated in food intake regulated by the reward circuitry (4). Orexin-null mice received injection of AAV-GFP or AAV-ΔFosB into the NAc and the number of rewards earned on operant responding was determined. Unlike their wild-type littermates, mice expressing ΔFosB but lacking orexin failed to increase instrumental responding for HFD (Fig 1D).
Next we analyzed several metabolic parameters four weeks after viral injection using indirect calorimetry. Over-expression of ΔFosB decreased oxygen consumption and carbon dioxide production, indicating lower energy expenditure (Fig 2G and 2H). Similar to CR mice, there was no difference in body weight or food intake between the two groups during testing (Fig 2I and 2J). Interestingly, ΔFosB over-expression in NAc did not reproduce the locomotor hyperactivity phenotype (Fig 2K) observed in CR mice. Finally, mice receiving AAV-ΔFosB into the NAc also demonstrated significantly elevated body fat compared to control mice (Fig 2L).
Discussion
Identifying neural adaptations that mediate long-term regulation of appetite and body weight will be critical to the treatment of obesity. Our findings identify accumulation of ΔFosB in NAc as a regulator of motivation for food and of energy expenditure. The ability of ΔFosB to increase effortful responding to obtain HFD pellets requires the presence of orexin, consistent with previous observations that orexin receptor 1 signaling mediates motivation for highly palatable food (4, 10, 12-14). These data suggest that ΔFosB is an important physiological regulator of the influence of the NAc on brain circuits controlling food intake.
The mechanism by which ΔFosB over-expression in NAc lowers metabolic rate is unclear. It is possible that orexin neurons may mediate the effect on energy expenditure as well. Verty and colleagues recently demonstrated that the i.c.v. injection of an orexin receptor-1 antagonist increased thermogenesis in brown adipose tissue, a major metabolic pathway for the dissipation of excess calories (16, 17). Alternatively, genetic ablation of orexin neurons results in a late-onset obesity phenotype despite an overall reduction in food intake, suggesting a reduction in energy expenditure (18). Because of the disrupted sleep cycle, changes in body composition, and altered energy homeostasis in orexin null mice, we chose not to analyze the orexin-null mice that received AAV-ΔFosB by indirect calorimetry. Future experiments will need to determine the role of orexin in metabolic signaling through the use of pharmacologic agents.
ΔFosB accumulation may serve as an important neuronal adaptation that mediates the long-term effects of environmental stress on body weight regulation. A ten-day exposure to CR results in changes in operant responding and energy expenditure one week after return to normal body weight. Our findings are therefore consistent with a model in which repeated exposure to stressors, such as repeated low calorie diets, may promote obesity through ΔFosB signaling in NAc. Understanding this pathway may yield valuable new targets in treating obesity.
Supplementary Material
Acknowledgments
This work was supported by the following grants: K08 MH084058-1A1, UL1RR024923, RL1 DK081182, RL1 DK081185, R01 MH51399, R37 DK53301, R01 DK071320, 8--UL1-DE019584-02, K08 DK068069-01A2, P50 MH066172, R01 DA024680, NARSAD Young Investigator Award, Astra-Zeneca, The Physician Scientist Training Program, The Disease Oriented Clinical Scholars Program, and The Medical Scientist Training Program.
Abbreviations
- AAV
adeno-associated virus
- BMI
body mass index
- CR
calorie restriction
- HFD
higher in fat diet
- NAc
nucleus accumbens
Footnotes
Financial disclosure: Dr. Nestler consults for Merck Research Laboratories and PsychoGenics, and had a research alliance with AstraZeneca. Dr. Elmquist has received research support from Sanofi-Aventis. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 4.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, et al. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Teegarden SL, Nestler EJ, Bale TL. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiatry. 2008;64:941–950. doi: 10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe GC, Choi CS, Neff L, Horne WC, Shulman GI, Baron R. Increased energy expenditure and insulin sensitivity in the high bone mass ΔFosB transgenic mass. Endocrinology. 2009;150:135–143. doi: 10.1210/en.2008-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Davis JF, Krause EG, Melhorn SJ, Sakai RR, Benoit SC. Dominant rats are natural risk takers and display increased motivation for food reward. Neuroscience. 2009;162:23–30. doi: 10.1016/j.neuroscience.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 15.Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretinor melanin-concentrating hormone-containing neurons. J Neurosci. 2007;27:6948–6955. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, et al. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept. 2002;104:153–159. doi: 10.1016/s0167-0115(01)00358-5. [DOI] [PubMed] [Google Scholar]
- 17.Verty AN, Allen AM, Oldfield BJ. The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinology. 2010;151:4236–4246. doi: 10.1210/en.2009-1235. [DOI] [PubMed] [Google Scholar]
- 18.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


