Abstract
Background and Purpose
Little is known about acute precipitants of ischemic stroke, although evidence suggests infections contribute to risk. We hypothesized that acute hospitalization for infection is associated with short-term risk of stroke.
Methods
The case-crossover design was used to compare hospitalization for infection during case periods (90, 30, or 14 days prior to incident ischemic stroke) and control periods (equivalent time periods exactly 1 or 2 years prior to stroke) in the Cardiovascular Health Study, a population-based cohort of 5888 elderly participants from 4 US sites. Odds ratios and 95% confidence intervals (OR, 95% CI) were calculated using conditional logistic regression. Confirmatory analyses assessed hazard ratios (HR) of stroke from Cox regression models with hospitalization for infection as a time-varying exposure.
Results
During a median follow-up of 12.2 years, 669 incident ischemic strokes were observed in participants without baseline history of stroke. Hospitalization for infection was more likely during case than control time periods; for 90 days prior to stroke, OR=3.4 (95% CI 1.8–6.5). The point estimates of risks were higher when examining shorter intervals: for 30 days, OR= 7.3 (95% CI 1.9–40.9), and 14 days, OR=8.0 (95% CI 1.7–77.3). In survival analyses, risk of stroke was associated with hospitalization for infection in the preceding 90 days, adjusted HR=2.4 (95% CI 1.6–3.4).
Conclusions
Hospitalization for infection is associated with a short-term increased risk of stroke, with higher risks observed for shorter intervals preceding stroke.
Keywords: Epidemiology, Cerebral Infarction, Infectious Diseases
Introduction
Knowledge of acute stroke precipitants is primitive. Identification of particular time periods during which stroke risk is elevated could prove a valuable strategy to reduce stroke incidence through the introduction of appropriate prevention strategies during a period of vulnerability. Studies of acute precipitants lend themselves to different methodologies than studies of chronic risk factors. In analysis of acute precipitants of stroke, for example, intra-individual differences may be more important than between-person differences. The factor of interest is not the characteristic making one person more likely to have a stroke than another, but rather what makes one individual more likely to have stroke at a particular point in time.
The case-crossover design is particularly suited to assessing potential precipitants.1 Case-crossover analyses are based on data about relatively short intervals of time leading to events, contrasted with data from comparable periods of time in the same individual. Events that occur more frequently just prior to stroke than at other time intervals are more likely to be precipitants. Each participant thus serves as his own control, and the analysis implicitly accounts for most inter-individual differences. The case-crossover design has been used only sparingly to identify triggers of stroke2 compared with myocardial infarction.3,4,5
We hypothesized that the risk of ischemic stroke would be higher during the 90 days after hospitalization for infection compared to the same period of time 1 and 2 years prior to stroke. The Cardiovascular Health Study (CHS) is a multi-center prospective study of vascular risk factors in an elderly population-based cohort. CHS afforded the opportunity to address our hypothesis with a case-crossover design and to seek confirmation with survival analyses.
Methods
Details of CHS have been described.6,7,8 Briefly, a random sample of men and women aged ≥65 years (n=5888) was recruited from Medicare eligibility lists in four U.S. communities. The institutional review board at each site approved the study methods, and all participants gave written informed consent.
The CHS Cerebrovascular Adjudication Committee adjudicated all suspected events according to standard criteria.9 Strokes were classified as ischemic or hemorrhagic using all available information from informant interviews, medical records, and brain imaging studies. These analyses are limited to incident ischemic strokes not related to a procedure.
We used a case-crossover design in which participants served as their own controls. Exposure was defined as hospitalization for infection within 90, 30, or 14 days prior to stroke (case period) or equivalent time periods exactly 1 or 2 years prior to stroke (control periods). In a few participants, control periods occurred prior to study enrollment and were coded as missing. Hospitalization for infection was based on hospital discharge ICD-9 codes (see eAppendix). Primary central nervous system infections and endocarditis were excluded because of their known potential to directly cause stroke. Up to 10 codes were abstracted per hospitalization. Codes in any position were counted. Hospitalizations that occurred ≤4 days before stroke were not treated as exposures, to avoid including hospitalizations for the stroke itself in which infections may have been diagnosed secondarily. When the relationship of infection to the originally recorded stroke date was unclear, medical charts were reviewed to determine the temporal relationship of stroke and infection. The first day of hospitalization was considered the first day of infection. For participants with more than one hospitalization for infection, each hospitalization within a case or control period was considered an exposure.
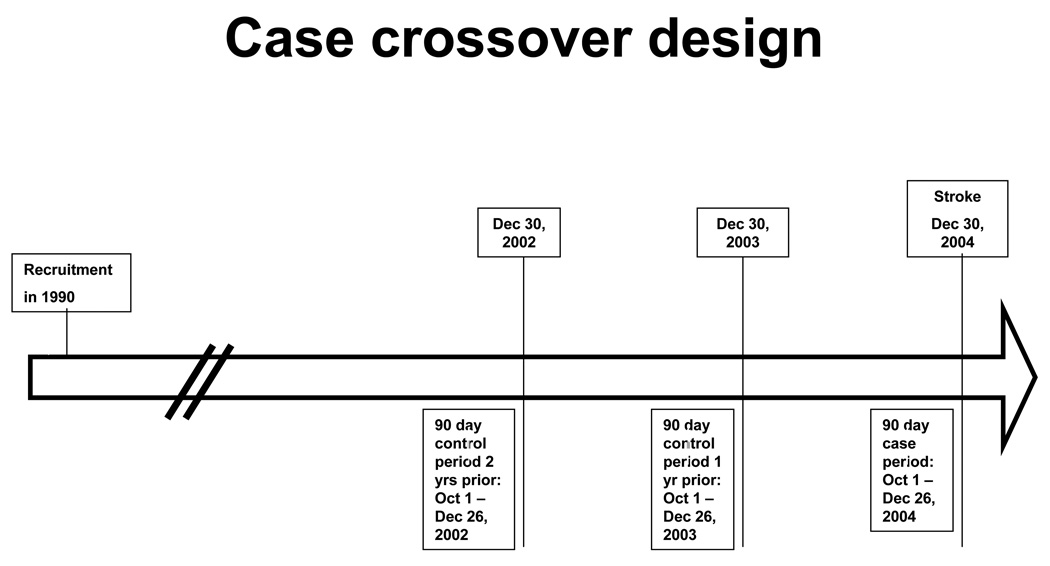
The prevalence of exposure during these time intervals was compared with the prevalence of exposure during the same calendar period one year and two years before the event (see Figure). Confounding by age is possible because as participants age their risk of stroke and hospitalization for infection increase. To reduce potential confounding, only time periods ≤2 years prior to stroke were included. Conditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). For analyses with <5 periods per cell we used exact conditional logistic regression. The null hypothesis was that prevalence of exposure would remain constant across all time intervals.
Figure 1. Illustration of the case crossover design.
The figure shows an example timeline illustrating case and control time periods for a hypothetical participant who enrolled in the study in January 1990 and had a stroke on December 30, 2004. The case period represents the 90 days prior to the stroke, excluding the 4 days immediately preceding the stroke, as infections during those dates were considered to possibly be secondary to stroke rather than preceding it. Control periods represent the same calendar periods 1 and 2 years prior to the stroke. More remote time periods were not included as their inclusion could add to bias due to participant aging in the case-crossover analyses, because participants serve as their own controls. Analyses then compared the prevalence of hospitalization for infection between case and both control time periods considered together. Analyses were repeated for 30 day and 14 day time windows.
To confirm our case-crossover findings, we also fit a Cox proportional hazards model among participants without baseline history of stroke, using the same ICD9 codes for hospitalization with infection, and incident ischemic stroke as the outcome. These analyses allowed adjustment for factors (such as age) that change over time and may confound the case-crossover findings. Hospitalization for infection was treated as a time-varying exposure in the Cox model; participants were non-exposed until hospitalization, remained exposed for 14, 30 or 90 day intervals, and then became non-exposed until their next eligible hospitalization. Hospitalizations for infection occurring after stroke were not included in analyses. Participants were followed for events from 1989 through June 2005. Censoring occurred on the date of death, loss to follow-up, study drop out, or non-ischemic stroke. Strokes occurring within 4 days after the hospital admission date were not counted; participants were censored at that date. Analyses were adjusted for baseline age, sex, race, diabetes mellitus (defined as self-reported use of insulin or oral hypoglycemics or having fasting glucose ≥126mg/dL), and current smoking, due to known associations with infections and stroke risk. We tested for interactions of hospitalized infection with diabetes and, as a measure of underlying atherosclerosis, carotid artery intima-media thickness (cIMT, as a continuous variable) to assess effect modification. Maximum common and internal cIMT were measured at baseline.10 Non-normally distributed measurements were log-transformed. Analyses were performed using Stata v.10.1 (StataCorp, College Station, Texas).
Results
Description of the cohort and hospitalizations for infections
During a median 12.2 years of follow-up, 5639 CHS participants without baseline history of stroke experienced 669 incident, non-procedure-related ischemic strokes. Baseline characteristics are provided in Table 1. Of these 669 participants, 29 had at least one hospitalization for infection during the preceding 90 days. Types and frequencies of infections are shown in the e-table.
Table 1.
Characteristics of Participants
| Baseline Characteristic | Case-Crossover Analysis | Survival Analysis |
|---|---|---|
| N (%)* | 669 (11.4) | 5639 (95.8) |
| Age in years, mean ± SD | 74.0 ± 5.7 | 72.8 ± 5.6 |
| Female sex, n (%) | 408 (61.0) | 3287 (58.3) |
| Self-reported race, n (%) | ||
| Black | 101 (15.1) | 860 (15.3) |
| White | 566 (84.6) | 4743 (84.1) |
| Other | 2 (0.3) | 36 (0.6) |
| Current Smoker, n (%) | 74 (11.1) | 671 (11.9) |
| Diabetes, n (%) | 132 (19.7) | 887 (15.9) |
| Hypertension, n (%) | 398 (59.5) | 2780 (57.3) |
| BMI in kg/m2, mean ± SD | 26.7 ± 4.7 | 26.9 ± 4.8 |
| Total Cholesterol in mg/dL, mean ± SD | 213.4 ± 45.2 | 208.6 ± 38.7 |
| LDL Cholesterol in mg/dL, mean ± SD | 130.5 ± 36.6 | 127.2 ± 33.9 |
| HDL Cholesterol in mg/dL, mean ± SD | 53.0 ± 15.3 | 53.3 ± 14.4 |
| Triglycerides in mg/dL, mean ± SD | 152.7 ± 104.3 | 143.5 ± 85.3 |
| Maximum common IMT in mm, median (IQR) | 1.03 (0.92–1.16) | 1.07 (0.96–1.21) |
| Maximum internal IMT in mm, median (IQR) | 1.30 (0.99–1.77) | 1.42 (1.07–1.90) |
Abbreviations: SD=standard deviation. HDL=high density lipoprotein. LDL=low density lipoprotein; BMI=body mass index; IMT= carotid artery intima-media thickness
As a percentage of the initially-recruited cohort, n=5888. The case-crossover analysis includes only participants who had incident stroke.
Case-crossover analyses
Among 669 stroke cases, 8 individuals were hospitalized for infection during the 4–14 days before stroke, whereas only 2 stroke cases had hospitalizations in the same 10 day calendar periods one and two years prior (Table 2). Hospitalization for infection within 14 days was associated with increased risk of stroke (OR 8.0, 95% CI 1.6–77.3; p=0.007). The elevated risk persisted for each pre-defined time window, with decreasing point estimate for magnitude of risk as the time interval lengthened (Table 2). The risk of stroke following hospitalization for infection within the previous 90 days remained elevated (OR 3.4, 95% CI 1.8–6.5).
Table 2.
Association of recent hospitalization for infection with risk of ischemic stroke based on case-crossover analyses
| Exposure | Case time intervals, n |
Control time intervals, n |
Odd ratio, 95% confidence interval |
|---|---|---|---|
| Hospitalization for infection within 14 days prior to stroke | |||
| No | 660 | 1194 | |
| Yes | 8 | 2 | 8.0, 1.6–77.3* |
| Missing/not eligible | 1 | 142 | |
| Hospitalization for infection within 30 days prior to stroke | |||
| No | 655 | 1193 | |
| Yes | 11 | 3 | 7.3, 1.9–40.9* |
| Missing/not eligible | 3 | 142 | |
| Hospitalization for infection within 90 days prior to stroke | |||
| No | 631 | 1179 | |
| Yes | 29 | 17 | 3.4, 1.8–6.5 |
| Missing/not eligible | 9 | 142 | |
Result is from exact conditional logistic regression.
Survival analyses
Incidence of first ischemic stroke was 11.0 per 1000 person-years in the 5639 CHS participants without a baseline history of stroke (Table 1). Of 2387 participants hospitalized for infection, 29 (1.2%) had stroke within 90 days of the hospitalization. Most infections were classified as miscellaneous (ICD9 codes 1–134), respiratory (acute respiratory infections, influenza, and pneumonia), and urinary tract infections.
Adjusting for age, sex, and race, hospitalization for infection was associated with an increased risk of ischemic stroke in the following 30 days (hazard ratio (HR)= 2.5, 95% CI: 1.4–4.6). The results remained essentially unchanged after additionally adjusting for diabetes and smoking (HR=2.5, 95% CI 1.4–4.5). Further adjusting for cIMT did not appreciably attenuate the findings (Table 3). Hospitalization for infection during the 14-day time window was associated with a higher point estimate for risk of stroke than hospitalization during the 30 and 90 day time windows. The association between hospitalization for infection and risk of stroke was modified by internal carotid IMT in the 90-day (p=0.04), 30-day (p=0.05) and 14-day windows (p=0.01) such that risk of stroke associated with hospitalization decreased with increasing IMT. We found similar interactions with common carotid IMT. We found no significant interactions with diabetes.
Table 3.
Association of recent hospitalization for infection with risk of ischemic stroke based on survival analyses
| Risk of ischemic stroke during the time interval after hospitalization for infection, using survival analysis |
|||
|---|---|---|---|
| Hazard ratio (95% confidence interval) | |||
| P value | |||
| 14 days | 30 days | 90 days | |
| Unadjusted | 4.4 (2.2– 9.3) | 2.9 (1.6–5.3) | 2.9 (2.0–4.2) |
| p<0.001 | p<0.001 | p<0.001 | |
| Adjusted for demographics (age, sex, African American race) | 4.0 (2.0 – 8.2) | 2.5 (1.4–4.6) | 2.5 (1.7–3.6) |
| p<0.001 | p=0.002 | p<0.001 | |
| Adjusted for above and diabetes and smoking | 3.9 (1.9–8.0) | 2.5 (1.4–4.5) | 2.4 (1.7–3.5) |
| p<0.001 | p=0.003 | p<0.001 | |
| Adjusted for above and ln(common carotid intima-media thickness) | 3.9 (1.9–7.9) | 2.4 (1.3–4.4) | 2.4 (1.6–3.5) |
| p<0.001 | p=0.004 | p<0.001 | |
| Adjusted for demographics, diabetes, smoking, and ln(internal carotid intima-media thickness) | 3.9 (1.9–7.9) | 2.4 (1.3–4.4) | 2.4 (1.6–3.4) |
| p<0.001 | p=0.004 | p<0.001 | |
Discussion
We found complementary evidence from case-crossover and survival analyses of an association between hospitalization for infection and stroke risk. A graded temporal association was evident such that risk of stroke was highest within 14 days after hospitalization for infection with a decreasing but still elevated risk during the subsequent 90 days. The effect was attenuated in survival analyses treating hospitalization for infection as a time-varying exposure, but remained significant. Many infections in the cohort were either respiratory or urinary tract infections. These findings support hypotheses that stroke is not merely a stochastic event but associated with particular triggers, that acute infection is one trigger, and that risk of stroke may vary by time since infection.11,12,13
Moreover, we found no evidence of effect modification by diabetes, although diabetes was a significant covariate in all models. We also found consistent evidence that the association between hospitalization for infection and risk of stroke is modified by carotid IMT, a subclinical measure of atherosclerosis, although our finding that hospitalization for infection is less strongly associated with incident stroke in the presence of increased carotid IMT may be counter-intuitive. Our initial hypothesis was that the risk would be greater among those with pre-existing vascular disease. Those with less atherosclerosis, however, may be at higher risk than those with more advanced disease, in whom an acute trigger carries less weight than their intrinsic disease. This finding is consistent with analyses of chronic inflammation as a risk factor for stroke or atherosclerosis, whereby the effect of inflammation is greater among those with fewer atherosclerotic risk factors.14 A case-control analysis of recent respiratory infection as a trigger for stroke similarly provided evidence that the effect of infections was attenuated among those at higher underlying risk.15
Previous case-control studies have reported that recent infection (e.g., within 1 week), primarily upper respiratory infection, is associated with stroke.16,17,18,19,20,21 Such studies are limited, however, by potential confounding due to underlying risk factors, such as smoking, that could lead to both infection and stroke. In a prospective analysis of more than 50,000 stroke patients using the case-series method, both recent upper respiratory and urinary tract infections were associated with an increased risk of stroke.22 The risk of stroke in the three days after infection was approximately three times as high as during infection-free periods, and gradually diminished during the following three months. Our results are consistent with those findings and extend them to incident strokes among a biracial group of elderly US residents.
Specific viral infections, such as influenza, have been associated with increased short-term stroke risk. Case-control studies provide evidence that influenza vaccination is associated with a 50% reduction in risk of stroke.23 Pilot clinical trials in patients with coronary disease also suggest that vaccination reduces cardiovascular risk.24 Recent guidelines recommend annual flu vaccination for cardiovascular patients to prevent not only flu but also cardiovascular disease.25 Further indirect evidence that acute infection precipitates stroke is available from studies of leukocyte count and stroke risk. In the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial, for instance, patients were monitored for neutropenia, and increases in leukocyte count were associated with a short-term increased stroke risk.26 Among 211 patients who had an ischemic stroke during follow-up, leukocyte levels in the prior week, but not earlier, were significantly increased above baseline (mean difference + 0.5 × 109 cells/L).
Several direct biological mechanisms could account for the increased risk of stroke associated with infections. Severe infections are associated with hypercoagulability and platelet activation that contribute to tissue ischemia and necrosis of many organs during sepsis.27,28 Even sub-septic infections increase platelet reactivity and platelet-leukocyte interactions, leading to an increased risk of platelet aggregation, potentially precipitating stroke. Platelet activation assessed by P-selectin expression, and platelet-leukocyte aggregates, were both increased in stroke patients compared to controls.29 These effects are even greater among stroke patients with a history of infection within 1 week prior to stroke. Organisms implicated in causing atherosclerosis and ischemic events have also been associated with platelet aggregation, including C. pneumoniae, H. pylori and periodontal infections. 30,31,32
Infections may also impair endothelial function. Leukocyte count has been related to reduced endothelial reactivity in cross-sectional studies.33 Acute upper respiratory infections may also transiently impair endothelium-dependent relaxation in children.34 Among 135 children with acute infection, brachial artery flow-mediated dilation was reduced compared with children 2 weeks out from infection and control children. Brachial artery reactivity of the acutely infected children returned to normal by one year later.
More general reasons may also explain why patients hospitalized for infection have stroke. Patients with acute infections may become dehydrated, either because of fever and increased insensible fluid losses, or because of decreased appetite and thirst. Pulmonary infections may increase the chance for cardiac dysfunction and atrial arrhythmias that could lead to embolism. More severely infected patients may become immobilized when hospitalized, increasing the risk for deep venous thrombosis and paradoxical embolism. Data allowing us to classify the specific etiologic subtypes of ischemic stroke were limited. Also, factors that influence admission, rather than infection itself, may be related to risk of stroke. Patients who are frail or have comorbidities may be more likely to be hospitalized for infection than healthier patients.
Our study has limitations. The number of participants in the time interval groups is small, and thus our findings should be interpreted with caution. We relied on hospital discharge codes to identify infections. Thus our findings cannot be generalized to non-hospitalized infections. We also did not have data on whether patients discontinued use of stroke protective medications, such as antiplatelet agents, when hospitalized. In addition, our case-crossover analyses may be confounded by participant aging, since both risk of stroke and hospitalization increase with age.35 We limited this bias by analyzing only the 2 years before stroke, rather than more remote time intervals, and by using a confirmatory study design less susceptible to this bias. We also cannot exclude the possibility that hospitalization itself or factors associated with hospitalization, rather than infection per se, are responsible for the association with stroke. Our study cannot establish causality.
Strengths of our study include its well-characterized cohort with long follow-up and large number of incident ischemic stroke events. Events were adjudicated by a group of specialists in cerebrovascular disease and hospital discharge summaries were available in all cases.
The identification of a short-term state of elevated stroke risk after infection could have therapeutic implications. For example, the period during and soon after hospitalization for infection could constitute a “treatable moment,” during which patients can be evaluated for cardiovascular risk and standard preventive strategies instituted, including anti-platelet agents and statins. Although we were unable to confirm effect modification by diabetes and did identify potential effect modification by atherosclerotic burden, additional studies are needed to determine which patients with acute infection may be at greatest risk. Among those at high risk, there may be a role for increased doses of antiplatelet agents or statins during times of fever or infection, though this approach would require testing in a clinical trial. Alternatively, therapies directed at preventing infectious stressors could be targeted to stroke patients. Currently, guidelines for influenza vaccine36 include patients over age 50 and debilitated stroke patients at increased risk of respiratory complications. Our results, as well as other recent evidence,23,24,25 suggest that prevention of influenza in high risk patients may prevent not only influenza but stroke.
Supplementary Material
Acknowledgments
Acknowledgements and Funding
The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Dr. Elkind had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no disclosures relevant to this study.
References
- 1.Maclure M, Mittleman MA. Should we use a case-crossover design. Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 2.Koton S, Tanne D, Bornstein NM, Green MS. Triggering risk factors for ischemic stroke: a case-crossover study. Neurology. 2004;63:2006–2010. doi: 10.1212/01.wnl.0000145842.25520.a2. [DOI] [PubMed] [Google Scholar]
- 3.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 4.Muller JE, Mittleman MA, Maclure M, Sherwood JB, Tofler GH. Triggering myocardial infarction by sexual activity. Low absolute risk and prevention by regular physical exertion. Determinants of Myocardial Infarction Onset Study Investigators. JAMA. 1996;275:1405–1409. doi: 10.1001/jama.275.18.1405. [DOI] [PubMed] [Google Scholar]
- 5.Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863–1872. doi: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 7.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 8.Manolio TA, Kronmal RA, Burke GL, O'Leary DH, Price TR. Short-term predictors of incident stroke in older adults: the Cardiovascular Health Study. Stroke. 1996;27:1479–1486. doi: 10.1161/01.str.27.9.1479. [DOI] [PubMed] [Google Scholar]
- 9.Longstreth WT, Jr., Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, Furberg CD. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56:368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary DH, Polak JF, Wolfson SK, Bond MG, Bommer W, Sheth S, Psaty BM, Sharrett AR, Manolio TA. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 11.Elkind MSV. Why Now? Moving from Stroke Risk Factors to Stroke Triggers. Curr Opin Neurol. 2007;20:51–57. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]
- 12.Mostofsky E, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Coffee and acute ischemic stroke onset: the Stroke Onset Study. Neurology. 2010;75:1583–1588. doi: 10.1212/WNL.0b013e3181fb443d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostofsky E, Burger MR, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Alcohol and acute ischemic stroke onset: the stroke onset study. Stroke. 2010;41:1845–1849. doi: 10.1161/STROKEAHA.110.580092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curb JD, Abbott RD, Rodriguez BL, Sakkinen P, Popper JS, Yano K, Tracy RP. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation. 2003;107:2016–2020. doi: 10.1161/01.CIR.0000065228.20100.F7. [DOI] [PubMed] [Google Scholar]
- 15.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29:96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 16.Syrjanen J, Valtonen VV, Iivanainen M, Kaste M, Huttunen JK. Preceding infection as an important risk factor for ischaemic brain infarction in young and middle aged patients. Br Med J. 1988;296:1156–1160. doi: 10.1136/bmj.296.6630.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grau AJ, Buggle F, Heindl S, Steichen-Wiehn C, Banerjee T, Maiwald M, Rohlfs M, Suhr H, Fiehn W, Becher H. Recent infection as a risk factor for cerebrovascular ischemia. Stroke. 1995;26:373–379. doi: 10.1161/01.str.26.3.373. [DOI] [PubMed] [Google Scholar]
- 18.Bova IY, Bornstein NM, Korczyn AD. Acute infection as a risk factor for ischemic stroke. Stroke. 1996;27:2204–2206. doi: 10.1161/01.str.27.12.2204. [DOI] [PubMed] [Google Scholar]
- 19.Grau AJ, Buggle F, Becher H, Zimmermann E, Spiel M, Fent T, Maiwald M, Werle E, Zorn M, Hengel H, Hacke W. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia. Neurology. 1998;50:196–203. doi: 10.1212/wnl.50.1.196. [DOI] [PubMed] [Google Scholar]
- 20.Macko RF, Ameriso SF, Gruber FA, Griffin JH, Fernandez JA, Barndt R, Quismorio FP, Jr, Weiner JM, Fisher M. Impairment of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 21.Grau AJ, Preusch MR, Palm F, Lichy C, Becher H, Buggle F. Association of symptoms of chronic bronchitis and frequent flu-like illnesses with stroke. Stroke. 2009;40:3206–3210. doi: 10.1161/STROKEAHA.109.561019. [DOI] [PubMed] [Google Scholar]
- 22.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 23.Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36:1501–1506. doi: 10.1161/01.STR.0000170674.45136.80. [DOI] [PubMed] [Google Scholar]
- 24.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation. 2002;105:2143–2147. doi: 10.1161/01.cir.0000016182.85461.f4. [DOI] [PubMed] [Google Scholar]
- 25.Davis MM, Taubert K, Benin AL, Brown DW, Mensah GA, Baddour LM, Dunbar S, Krumholz HM. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. Circulation. 2006;114:1549–1553. doi: 10.1161/CIRCULATIONAHA.106.178242. [DOI] [PubMed] [Google Scholar]
- 26.Grau AJ, Boddy AW, Dukovic DA, Buggle F, Lichy C, Brandt T, Hacke W CAPRIE Investigators. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke. 2004;35:1147–1152. doi: 10.1161/01.STR.0000124122.71702.64. [DOI] [PubMed] [Google Scholar]
- 27.Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128:2864–2875. doi: 10.1378/chest.128.4.2864. [DOI] [PubMed] [Google Scholar]
- 28.Yaguchi A, Lobo FL, Vincent JL, Pradier O. Platelet function in sepsis. J Thromb Haemost. 2004;2:2096–2102. doi: 10.1111/j.1538-7836.2004.01009.x. [DOI] [PubMed] [Google Scholar]
- 29.Zeller JA, Lenz A, Eschenfelder CC, Zunker P, Deuschl G. Platelet-leukocyte interaction and platelet activation in acute stroke with and without preceding infection. Arterioscler Thromb Vasc Biol. 2005;25:1519–1523. doi: 10.1161/01.ATV.0000167524.69092.16. [DOI] [PubMed] [Google Scholar]
- 30.Kalvegren H, Majeed M, Bengtsson T. Chlamydia pneumoniae binds to platelets and triggers P-selectin expression and aggregation: a causal role in cardiovascular disease? Arterioscler Thromb Vasc Biol. 2003;23:1677–1683. doi: 10.1161/01.ATV.0000084810.52464.D5. [DOI] [PubMed] [Google Scholar]
- 31.Byrne MF, Kerrigan SW, Corcoran PA, Atherton JC, Murray FE, Fitzgerald DJ, Cox DM. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology. 2003;124:1846–1854. doi: 10.1016/s0016-5085(03)00397-4. [DOI] [PubMed] [Google Scholar]
- 32.Herzberg MC, Nobbs A, Tao L, Kilic A, Beckman E, Khammanivong A, Zhang Y. Oral streptococci and cardiovascular disease: searching for the platelet aggregation-associated protein gene and mechanisms of Streptococcus sanguis-induced thrombosis. J Periodontol. 2005;76:2101–2105. doi: 10.1902/jop.2005.76.11-S.2101. [DOI] [PubMed] [Google Scholar]
- 33.Elkind MSV, Sciacca R, Boden-Albala B, Tondella ML, Feikin DR, Fields BS, Sacco RL, Di Tullio MR, Homma S. Leukocyte count is associated with reduced endothelial reactivity. Atherosclerosis. 2005;181:329–338. doi: 10.1016/j.atherosclerosis.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Charakida M, Donald AE, Terese M, Leary S, Halcox JP, Ness A, Davey Smith G, Golding J, Friberg P, Klein NJ, Deanfield JE ALSPAC (Avon Longitudinal Study of Parents and Children) Study Team. Endothelial dysfunction in childhood infection. Circulation. 2005;111:1660–1665. doi: 10.1161/01.CIR.0000160365.18879.1C. [DOI] [PubMed] [Google Scholar]
- 35.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 36.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–52. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.