Summary
Early rapid changes in response to the phytohormone abscisic acid (ABA) have been observed at the transcript level, but little is known how these transcript changes translate to changes in protein abundance under the same conditions. Here we have performed a global quantitative analysis of transcript and protein changes in Arabidopsis suspension cells in response to ABA using microarrays and quantitative proteomics. In summary, 3494 transcripts and 50 proteins were significantly regulated by ABA over a treatment period of 20–24h. ABA also caused a rapid and strong increase in extracellular reactive oxygen species (ROS) production with an average half rise time of 33 seconds. A subset of ABA-regulated transcripts were differentially regulated in the presence of the reactive oxygen species (ROS) scavenger Dimethylthiourea (DMTU) as compared to ABA alone, suggesting a role for ROS in the regulation of these ABA-induced genes. Transcript changes showed an overall poor correlation to protein changes (r=0.66). Only a subset of genes was regulated at the transcript and protein level, including known ABA marker genes. We furthermore identified ABA-regulation of proteins that function in a branch of glucosinolate catabolism previously not associated with ABA signaling. The discovery of genes that were differentially regulated at the transcript and at the protein level emphasizes the strength of our combined approach. In summary, our dataset not only expands previous studies on gene and protein regulation in response to ABA, but rather uncovers unique aspects of the ABA regulon and gives rise to additional mechanisms regulated by ABA.
Keywords: Abscisic acid, Reactive oxygen species (ROS), quantitative proteomics
Introduction
The phytohormone abscisic acid (ABA) is a major regulator of plant development and stress responses, including seed dormancy, germination, stomatal aperture regulation and drought resistance responses (Cutler et al., 2010; Kim et al., 2010; Raghavendra et al., 2010). Recently, basic signaling mechanisms mediating ABA perception have been identified (Ma et al., 2009; Nishimura et al., 2010; Park et al., 2009; Santiago et al., 2009). ABA binds to several members of the PYR/RCAR protein family, a class of recently discovered ABA receptors (Ma et al., 2009; Park et al., 2009). Binding of ABA to the receptor leads to inactivation of PROTEIN PHOSPHATASE 2C (PP2C) proteins. PP2Cs have been identified as negative regulators of SUCROSE NON-FERMENTING PROTEIN (SNF1)-RELATED KINASE 2 (SNRK2) activation (Fujii and Zhu, 2009; Park et al., 2009; Rubio et al., 2009; Umezawa et al., 2009; Vlad et al., 2009; Yoshida et al., 2006). According to current models, SNRK2 proteins have three primary roles during ABA signaling. SNRK2.6 activates the NADPH oxidase RESPIRATORY BURST OXIDASE PROTEIN F (RBOHF) leading to production of reactive oxygen species (ROS) (Kwak et al., 2003; Mustilli et al., 2002; Sirichandra et al., 2009). SNRK2 proteins furthermore interact with anion- and K+-channels in the plasma membrane and regulate ion transport and they interact with and phosphorylate transcription factors of the ABA responsive element binding factor family (AREB/ABF) thereby mediating ABA-induced transcription (Fujita et al., 2009; Geiger et al., 2009; Kwak et al., 2001; Lee et al., 2009; Nakashima et al., 2009; Sirichandra et al., 2009; Vahisalu et al., 2010).
T87 suspension cells, originally derived from whole seedlings (Axelos, 1992), have been shown to respond to ABA, with SNRK2 kinase activation (Yoshida et al., 2002), rapid ROS-production (Trouverie et al., 2008), and ABA- and Ca2+-induced regulation of ion channels (Brault et al., 2004; Ghelis et al., 2000; Trouverie et al., 2008; Zalejski et al., 2006). In this study we show that T87 suspension cells respond not only to ABA, but also to the ABA analog pyrabactin with SNRK2 kinase activation. We show rapid ROS production in response to ABA and pyrabactin and detected gene regulation in response to ABA, including many marker genes previously identified as upregulated in guard cells.
Besides the direct link between early ABA-perception and ABA-regulated transcription through SNRK2s, it has not been considered yet whether secondary stimuli, including the production of ROS have an effect on gene regulation. In addition, gene expression changes in response to ABA have been extensively studied (Hoth et al., 2002; Leonhardt et al., 2004; Li et al., 2006; Okamoto et al., 2010; Seki et al., 2002; Yang et al., 2008). Furthermore, proteomic analyses of guard cell-expressed proteins, ABA-regulated proteins in guard cells and ABA-induced protein phosphorylation have been recently studied (Chen et al., 2010; Kline et al., 2010; Zhao et al., 2010; Zhao et al., 2008). However, very little is known how changes in transcript abundance translate to changes in protein levels under identical experimental conditions. In recent years quantitative proteomics techniques have evolved that allow relative quantification of cellular proteins (Gygi et al., 1999; Lahm and Langen, 2000; Oda et al., 1999; Ross et al., 2004).
Having thus established T87 as an ABA model system, we performed a detailed quantitative proteomic and transcriptomic analysis of A. thaliana T87 suspension cells in response to ABA. We used this model system to study the effect of ROS production on transcriptional regulation in response to ABA, analyzing transcript regulation in response to ABA and the H2O2 scavenger Dimethylthiourea (DMTU) by microarray analysis. Furthermore, we comparatively studied the effect of ABA on gene regulation and protein regulation in order to identify components linked to ABA signaling in plants, including components previously not associated with ABA responses.
Results
ABA perception and SNRK2 activation in suspension cells
In guard cells, ABA is perceived by the PYR/RCAR family of ABA receptors that has been recently identified (Ma et al., 2009; Park et al., 2009). Though it has previously been shown that in T87 suspension cells SNRK2 kinase activity is activated in an ABA-dependent manner (Yoshida et al., 2002), it was unclear whether the same ABA perception mechanism is functional in suspension cells as compared to intact plants. We therefore tested SNRK2 activation in response to ABA and pyrabactin, a chemical ABA agonist that binds to a subset of PYR/RCAR receptors (Park et al., 2009).
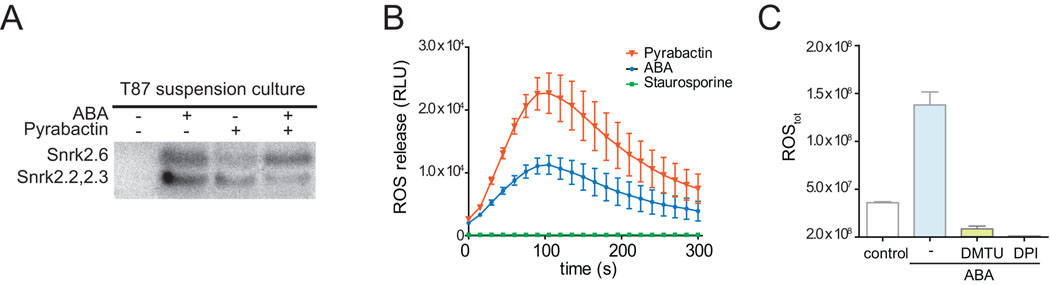
In in-gel kinase assays, two phosphorylation signals were detected in response to ABA and pyrabactin (Figure 1A). The apparent molecular weight of these signals is consistent with previous reports of SNRK2 kinases (Mustilli et al., 2002; Yoshida et al., 2002). No difference between ABA- and pyrabactin-activation of these two signals was seen in repeated experiments.
Figure 1.
A. In-gel SNRK2-kinase activity assay in response to abscisic acid and pyrabactin. Lysate of 5-day old T87 suspension cells incubated for 30 minutes in the presence of ABA (50 µM), pyrabactin (50 µM) or ethanol, were separated on a 12.5% SDS gel containing Histone-IIIS as phosphorylation substrate. ABA and pyrabactin, but not the control treatment, induce kinase activity at approximately 41 kDa and 44 kDa. ABA-specific kinase activity at this molecular weight has previously been identified as SNRK2.6 and SNRK2.2/SNRK2.3 activity (Mustilli et al., 2002; Yoshida et al., 2002). B. ABA-induces a rapid extracellular oxidative burst as recorded in suspension cells using a Luminol-derivate-based plate reader assay. Depicted are average values for cells treated with 50 µM ABA (n=8), 100 µM pyrabactin (n=8) and 50 µM ABA + 50 µM Staurosporine (n=8) over a time course of 6 minutes. C. Total ROS production as determined by area under curve for 10% ethanol as control, 50 µM ABA (n=8), 50 µM ABA + 5 mM DMTU (n=8) and 50 µM ABA + 250 µM DPI (n=8). Bars represent standard error.
Furthermore, ABA causes production of reactive oxygen species (ROS) in guard cells and T87 suspension cells (Kwak et al., 2003; Miao et al., 2006; Pei et al., 2000; Suhita et al., 2004; Trouverie et al., 2008; Zhang et al., 2001). We tested ROS production in T87 suspension cells in response to ABA and pyrabactin using a modified Luminol-based plate assay. Within seconds after ABA and pyrabactin treatment, a large and rapid increase in ROS production that peaked at about 2 minutes (t1/2=33 s) was observed (Figure 1B). The general kinase inhibitor Staurosporine (Tamaoki et al., 1986) abolished ABA-induced ROS production (Figure 1B). These data show rapid ABA-induced ROS production in T87 cells and furthermore show pyrabactin-induced ROS production, suggesting that in T87 cells ABA is perceived by the PYR/RCAR family of receptors. We then tested the NADPH-oxidase inhibitor Diphenyliodonium (DPI) (Hancock and Jones, 1987) and the H2O2 scavenger Dimethylthiourea (DMTU) (Tate et al., 1982) and quantified their effect on ROS production by comparing total ROS production (area under curve) of inhibitor + ABA-treated with ABA- and control-treated suspension cells (Figure 1C). DPI and DMTU completely abolished ABA-induced ROS production. Taken together these data suggest that T87 cells are a suitable model cell system for ABA-induced and ROS-mediated signaling.
Gene expression profiling in response to exogenous ABA
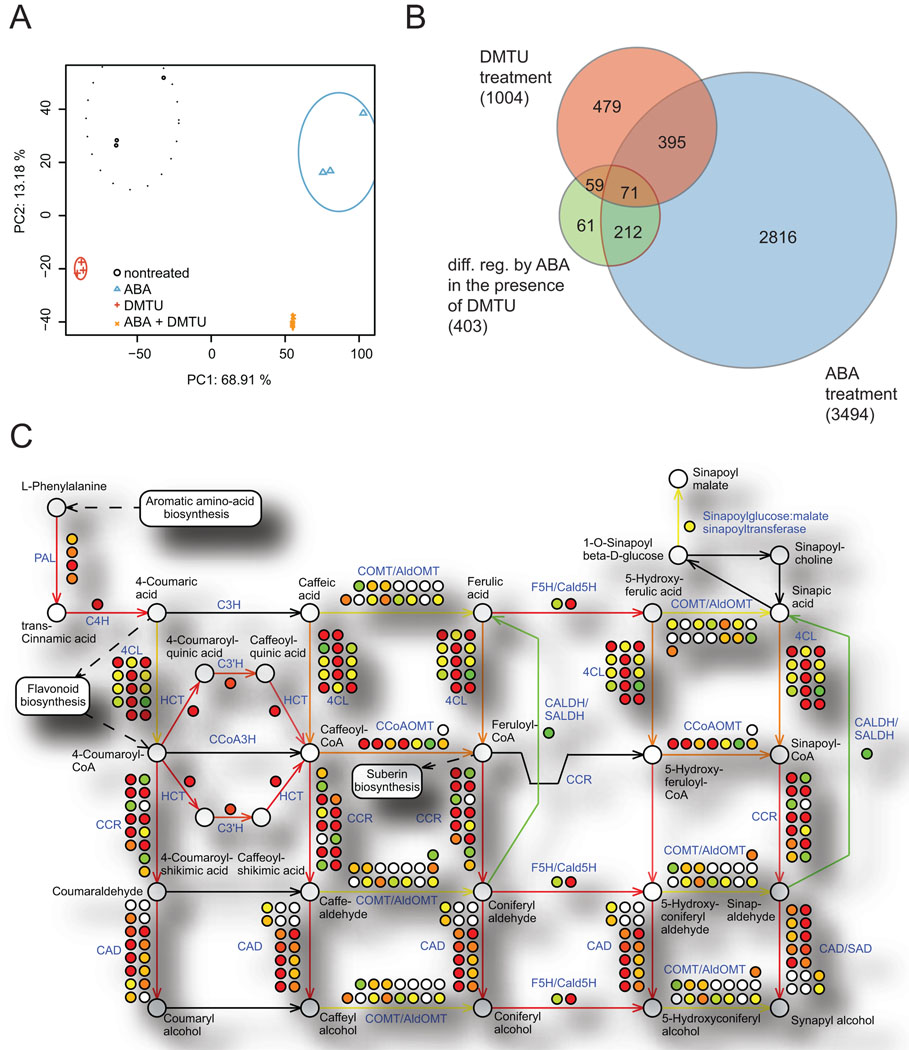
To study transcript changes in response to exogenous ABA and ABA-induced endogenous ROS production we carried out microarray analysis using Affymetrix ATH1 chips. Total RNA was extracted from 5 day old suspension cultures treated for 20h with 50 µM ABA, with 5 mM DMTU or ABA+DMTU and ethanol treated control cultures. Principal component analysis shows that the biological replicates of each treatment group cluster closely together (Figure 2A) and indicates that the variance between the 4 treatment groups is explained by the factors ABA and DMTU.
Figure 2.
A. Principal component analysis of all microarray samples. B. Venn Diagram of genes regulated by ABA (blue), DMTU (red) and of genes differentially regulated by ABA in the presence of DMTU (green). C. Graphical representation of gene regulation in the phenylpropanoid pathway in response to ABA. Changes in expression levels in individual genes (dots) and presumed regulation of the catalyzed pathways (arrows) in response to ABA as compared to control cells are color coded: red represents upregulation, green downregulation, yellow unchanged and white and black that no data were available. Genes are labeled in blue, metabolites in black. Figure is based on data from KaPPA-view 4 (Tokimatsu et al., 2005).
In summary, 3494 genes were regulated more than 2-fold in response to ABA (p<0.05), thereof 1512 genes were up- and 1982 genes were down-regulated (Figure 2B, supplementary file 1). Known ABA signaling components or ABA-targets were identified, e.g. RESPONSIVE TO DESSICATION 20 (RD20), RESPONSIVE TO ABA 18 (RAB18) and EARLY METHIONINE LABELED 6 (ATEM6). The strongest upregulated gene was the ALUMINIUM ACTIVATED MALATE TRANSPORTER 12 (ALMT12), a component of the R-type anion channel essential for stomatal closure (Meyer et al., 2010; Sasaki et al., 2010) (Table 1). Furthermore, we identified regulation of potassium channels, including the GATED OUTWARDLY-RECTIFYING K+ CHANNEL (GORK) that functions in stomatal regulation (Ache et al., 2000; Hosy et al., 2003) (Supplemental table 1). Other ABA-regulated potassium channels include Ca2+ ACTIVATED OUTWARD RECTIFYING K+ CHANNEL 5 (KCO5), POTASSIUM TRANSPORTER 1 (ATKT1), ARABIDOPSIS THALIANA K+ RECTIFYING CHANNEL 1 (ATKC1) and K+ UPTAKE PERMEASEs KUP6 and KUP11 (Supplemental table 1). We also identified downregulation of inward rectifying K+ channels, including ARABIDOPSIS K TRANSPORTER 1 (AKT1) and POTASSIUM TRANSPORTER 2 (ATKT2) (Supplemental table 1).
Table 1.
Selection of most strongly up-regulated genes in response to ABA treatment (50 µM, 20h) in the presence and absence of DMTU (5 mM)
| ABA | ABA + DMTU | DMTU | ||||||
|---|---|---|---|---|---|---|---|---|
| AGI | FC | P1 | FC | P1 | FC | P1 | sub | gene description |
| At4g17970 | 398.3 | 0.0 | 82.4 | 0.0 | 0.9 | 0.8 | cyto | ALUMINUM-ACTIVATED, MALATE TRANSPORTER 12 (ALMT12) |
| At5g09530 | 371.2 | 0.0 | 63.7 | 0.0 | 0.4 | 0.3 | sec | hydroxyproline-rich glycoprotein family protein |
| At3g22620 | 355.7 | 0.0 | 36.9 | 0.0 | 0.9 | 0.8 | LTP family protein | |
| At5g41040 | 304.4 | 0.0 | 35.9 | 0.0 | 0.8 | 0.8 | cyto | transferase family protein |
| At5g06760 | 282.6 | 0.0 | 121.7 | 0.0 | 1.0 | 1.0 | cyto | LATE EMBRYOGENESIS ABUNDANT 4–5 (LEA4-5) |
| At2g38530 | 273.6 | 0.0 | 19.5 | 0.0 | 0.9 | 0.8 | sec | LIPID TRANSFER PROTEIN 2 (LTP2) |
| At3g02480 | 242.7 | 0.0 | 14.2 | 0.0 | 10.0 | 0.0 | ABA-responsive protein-related | |
| At2g33380 | 241.9 | 0.0 | 427.4 | 0.0 | 0.5 | 0.1 | RESPONSIVE TO DESSICATION 20 (RD20) | |
| At5g09520 | 186.8 | 0.0 | 7.5 | 0.0 | 0.9 | 0.9 | sec | hydroxyproline-rich glycoprotein family protein |
| At4g17280 | 176.2 | 0.0 | 9.7 | 0.0 | 1.0 | 1.0 | sec | unknown protein |
| At5g59220 | 169.7 | 0.0 | 46.3 | 0.0 | 1.1 | 0.7 | chl | HIGHLY ABA-INDUCED PP2C GENE 1 (HAI1) |
| At5g66400 | 148.0 | 0.0 | 47.9 | 0.0 | 1.0 | 0.9 | cyto | RESPONSIVE TO ABA 18 (RAB18) |
| At1g49450 | 147.7 | 0.0 | 21.1 | 0.0 | 1.1 | 0.8 | transducin family protein / WD-40 repeat family protein | |
| At5g13170 | 137.8 | 0.0 | 27.8 | 0.0 | 1.0 | 0.9 | sec | SENESCENCE-ASSOCIATED PROTEIN 29 (SAG29) |
| At4g21440 | 129.6 | 0.0 | 52.9 | 0.0 | 0.9 | 0.7 | cyto | ARABIDOPSIS MYB-LIKE 102 (ATMYB102) |
| At3g48520 | 128.1 | 0.0 | 78.6 | 0.0 | 1.0 | 0.9 | sec | CYTOCHROME P450, FAMILY 94, SUBFAMILY B, POLYPEPTIDE 3 (CYP94B3) |
| At1g54540 | 111.7 | 0.0 | 5.6 | 0.0 | 1.1 | 0.9 | unknown protein | |
| At4g24130 | 111.5 | 0.0 | 5.4 | 0.0 | 1.2 | 0.8 | cyto | unknown protein |
| At3g50400 | 109.7 | 0.0 | 3.9 | 0.0 | 0.9 | 0.9 | sec | GDSL-motif lipase/hydrolase family protein |
| At2g40170 | 109.2 | 0.0 | 9.0 | 0.0 | 0.9 | 0.8 | ARABIDOPSIS EARLY METHIONINE-LABELLED 6 (ATEM6) | |
AGI, Arabidopsis gene identifier; p1, posthoc p-value of transcript changes calculated by Robin v1.1.5 with standard settings; FC, fold change ratios; sub, subcellular localization as predicted by GO term annotation (http://www.arabidopsis.org/tools/bulk/protein/index.jsp): cyto, cytosol or other; sec, secreted.
In order to functionally classify the genes regulated by ABA we performed GO term enrichment analysis against the ATH1 background using the DAVID Bioinformatics Resources (Huang da et al., 2009, details shown in supplementary table 3). The most highly enriched GO terms in the upregulated gene group were related to ABA stimulus (enrichment score 11.01x), osmotic stress (enrichment score 9.3x), peroxisomes (enrichment score 7.94x) and a group containing CBL interacting protein kinases (enrichment score 5.21x). Analysis for the enrichment of metabolic functions using MAPMAN (Thimm et al., 2004) identified ABA-regulation of lipid metabolism (p<1e-20), secondary metabolite production (p=1.1e-12) and the phenylpropanoid pathway (p=9.8e-07) (Figure 2C). Among the genes downregulated by ABA we found nucleolus-associated genes (enrichment score 27.7x), ribosomal proteins (enrichment score 27.63x), RNA processing (enrichment score 18.69x) and the cell cycle (enrichment score 10.28x).
Gene expression profiling in response to ABA and ROS
In order to test the effect of ABA-induced ROS production on gene regulation, we tested ABA-induced gene regulation in the presence of the ROS scavenger DMTU. DMTU treatment alone led to the differential regulation of 1004 genes, 554 genes being downregulated and 450 genes being upregulated (Figure 2B). GO term enrichment analysis (Huang da et al., 2009) against the ATH1 background identified genes involved in the regulation of protein kinases, including cyclins (enrichment score 4.62x) and cell cycle (enrichment factor 2.18x). Among the downregulated proteins we identified genes associated with response to hormone stimulus (enrichment factor 4.49x), response to jasmonic acid, salicylic acid and gibberelic acid stimulus (enrichment factor 2.44x) and genes responding to ROS (enrichment factor 2.37x) (details shown in Supplementary table 3).
Comparing the changes in gene regulation induced by ABA alone with the changes in gene regulation in the presence of ABA and DMTU (Figure 2B), we found that depletion of H2O2 by DMTU had a significant effect on ABA-regulated gene expression (Figure 2B, Table 1). Of the 3494 ABA-regulated genes, 283 were differentially regulated more than 2-fold in the presence of DMTU. Of these, 71 genes were significantly regulated by DMTU also in the absence of ABA and for the remaining 212 genes DMTU only affected ABA-induced gene regulation. The 283 genes differentially regulated by ABA in the presence of DMTU were clustered based on expression ratios from DMTU-treated versus control samples, ABA-treated versus control samples and DMTU+ABA-treated versus DMTU-treated samples (Figure 3A) into eight clusters with respective expression profiles (Figure 3B).
Figure 3.
A. Probesets differentially regulated by ABA in the presence of DMTU were hierarchically clustered based on their fold-change ratios in response to ABA, DMTU and ABA + DMTU. 283 genes with differentially expressed profiles were used in this analysis. Normalized logarithmic expression ratios for each treatment versus control are indicated by different colors, green and red represent low and high ratios, black indicating no change. B. Eight clusters have been created by k-means clustering based on transcript ratios under the displayed conditions. C. Microarray validation by qRT-PCR of selected genes of the Fatty acid/lipid metabolism, Phenylpropanoid pathway and ROS sensitive genes that are differentially regulated by ABA in the presence of DMTU.
The clusters A, B, C, F and H contained genes that were regulated by ABA and the regulation was decreased or abolished in the presence of DMTU. In clusters C and F, DMTU reduced expression changes in response to ABA back to levels in control samples. DMTU alone did not change transcript level in clusters A, C, F and H. Functional characterization of cluster A identified an enrichment of genes responsive to ABA (enrichment factor 1.76x). Clusters B was enriched in glycoproteins (1.98x), cluster C in genes of the phenylpropanoid pathway (2.5x), and cluster F constitutes of genes that are involved in fatty acid metabolic processes (2.21x) and cluster H was enriched in acyltransferases (2.47x). Clusters D and E which contained genes whose transcriptional regulation by ABA was unaffected by DMTU treatment, displayed an enrichment of microtubuli, cytoskeleton, kinesin and cell cycle genes (2.95x). Cluster G contained no significant enrichments.
We further identified ABA-regulation of a small number of genes of the phenylpropanoid and lignin pathway as well as the lipid metabolism by qRT-PCR. We confirmed the effect of DMTU on these highly regulated genes by qRT-PCR (Figure 3C).
Quantitative proteomic profiling in response to ABA
Since changes at the transcript level are not necessarily reflected at the protein and therefore functional level, we pursued a proteomic approach to quantify protein changes in response to ABA. During protein extraction we tried not to emphasize a particular subcellular compartment. Protein extraction was therefore performed by using phenol/SDS/ammonium acetate precipitation (Wang et al., 2003). Rubisco, which constitutes about 30% of total protein in leaves, covered only 1–1.5% of spectrum ID’s in each run. ATMS1, methionine synthase 1, is the protein with the most identifications in our datasets and covered only 1.7% of spectrum ID’s. The low coverage of highly abundant housekeeping proteins allowed for an in depth proteomic analysis. Comparison of subcellular localizations between our data set and the whole genome shows that proteins identified in this study covered all subcellular localizations without bias (Supplementary file 4).
We identified on average 500 proteins per mass spec run with a Proteinprophet probability > 95% (Nesvizhskii et al., 2003). Normalized comparison of protein identifications from all mass spectrometry runs led to a total of 930 proteins (see supplementary table 2). Of these, 761 proteins fulfilled our criteria for reliable iTRAQ quantification. Of the 761 proteins we quantified, 188 were observed in only one biological replicate and were not considered for further analysis. The remaining 573 protein quantifications were analyzed for ABA-induced protein responses. Using the described criteria (see experimental procedures) we identified 42 proteins as up-regulated and 8 proteins as down-regulated by ABA (Table 2). The upregulated proteins could be classified into phenylpropanoid synthesis, ROS detoxification, sugar metabolism, fatty acid metabolism, protein degradation, amino acid metabolism and abiotic stress. Interestingly, among the proteomic responses to ABA we also detected a group of proteins that functions in the degradation of sulfur-containing glucosinolates. We measured an upregulation of NITRILE SPECIFIER PROTEIN 5 (NSP5), NITRILASE 1 (NIT1) and NITRILASE 2 (NIT2) at the protein level.
Table 2.
List of proteins significantly regulated by more than 20% in protein abundance in reponse to ABA treatment (50 µM, 24h) along with corresponding mRNA changes
| Biological Replicates | Protein | mRNA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AGI | 1 | 2 | 3 | 4 | 5 | 6 | 7 | FC | p1 | FC | p2 | gene description |
| upregulated proteins | ||||||||||||
| Glucosinolate catabolism | ||||||||||||
| AT5G48180 | 1.56 | 1.64 | 2.26 | 1.50 | 1.42 | 2.15 | 1.76 | 0.00 | 3.46 | 0.00 | NITRILE SPECIFIER PROTEIN 5 (NSP5) | |
| AT3G44300 | 1.66 | 1.45 | 1.10 | 1.66 | 1.47 | 0.03 | ≠*1.26 | ≠*0.09 | NITRILASE 2 (NIT2) | |||
| AT3G44310 | 1.47 | 1.45 | 1.30 | 0.97 | 1.24 | 1.44 | 1.31 | 0.01 | ≠*1.26 | ≠*0.09 | NITRILASE 1 (NIT1) | |
| Phenylpropanoid / lignin biosynthesis | ||||||||||||
| AT5G41040 | 2.63 | 1.37 | 2.22 | 1.57 | 1.95 | 0.02 | 304.37 | 0.00 | Feruloyl-CoA transferase | |||
| AT2G30490 | 2.02 | 1.68 | 1.60 | 1.80 | 1.77 | 0.00 | 4.64 | 0.00 | CINNAMATE-4-HYDROXYLASE (ATC4H) | |||
| AT4G34050 | 1.59 | 1.58 | 1.50 | 1.30 | 1.49 | 0.00 | 1.72 | 0.00 | caffeoyl-CoA 3-O-methyltransferase | |||
| AT5G19440 | 1.25 | 1.37 | 1.33 | 1.16 | 1.28 | 1.57 | 1.33 | 0.00 | 1.18 | 0.09 | alcohol dehydrogenase | |
| AT3G48990 | 1.25 | 1.32 | 1.27 | 1.30 | 1.29 | 0.00 | 1.70 | 0.00 | 4-COUMARATE-COA LIGASE LIKE 2 | |||
| AT3G19450 | 1.33 | 0.93 | 1.54 | 1.10 | 1.09 | 1.26 | 1.21 | 0.06 | 1.26 | 0.61 | cinnamyl-alcohol dehydrogenase (ATCAD5) | |
| AT4G34230 | 1.58 | 0.00 | cinnamyl-alcohol dehydrogenase (ATCAD4) | |||||||||
| ROS detoxification | ||||||||||||
| AT1G20630 | 1.39 | 1.54 | 1.50 | 1.46 | 1.66 | 1.51 | 0.00 | 2.24 | 0.01 | CATALASE 1 (CAT1) | ||
| AT4G35090 | 1.79 | 0.02 | CATALASE 2 (CAT2) | |||||||||
| Sugar metabolism | ||||||||||||
| AT1G23190 | 1.36 | 1.23 | 1.50 | 1.03 | 1.28 | 0.06 | 2.34 | 0.00 | PHOSPHOGLUCOMUTASE 2 (PGM2) | |||
| AT5G40760 | 1.25 | 1.40 | 1.09 | 1.19 | 1.20 | 1.23 | 0.01 | 1.82 | 0.00 | GLUCOSE-6-P DEHYDROGENASE 6 (G6PD6) | ||
| AT3G46440 | 1.20 | 1.15 | 1.36 | 1.12 | 1.26 | 1.22 | 0.00 | 0.9 | 0.41 | UDP-XYL SYNTHASE (UXS6) | ||
| AT2G28760 | 0.59 | 0.06 | UDP-XYL SYNTHASE (UXS5) | |||||||||
| AT5G59290 | 4.64 | 0.00 | UDP-XYL SYNTHASE (UXS3) | |||||||||
| AT2G47650 | 1.24 | 1.18 | 1.14 | 1.25 | 1.20 | 0.00 | *1.68 | *0.00 | UDP-XYL SYNTHASE (UXS4) | |||
| AT3G62830 | UDP-XYL SYNTHASE (UXS2) | |||||||||||
| Fatty acid metabolism | ||||||||||||
| AT2G33150 | 1.31 | 1.36 | 1.45 | 1.45 | 1.56 | 1.43 | 0.00 | 1.86 | 0.00 | PEROXI. 3-KETOACYL-COA THIOLASE 3 (PKT3) | ||
| AT2G04350 | 1.33 | 1.30 | 1.09 | 1.48 | 1.30 | 0.03 | 2.70 | 0.00 | LONG-CHAIN-FA--COA LIGASE 8 (LACS8) | |||
| Protein degradation | ||||||||||||
| AT2G05840 | 1.25 | 1.40 | 1.15 | 1.27 | 0.06 | *0.86 | *0.30 | 20S proteasome subunit PAA2 | ||||
| AT4G01610 | 1.30 | 1.21 | 1.67 | 1.35 | 1.19 | 1.53 | 1.37 | 0.00 | 1.59 | 0.00 | cathepsin B-like cysteine protease | |
| AT1G47128 | 1.16 | 1.11 | 1.45 | 1.50 | 1.30 | 0.04 | 1.98 | 0.00 | RESPONSIVE TO DESICCATION 21 (RD21) | |||
| Amino acid metabolism | ||||||||||||
| AT5G11520 | 1.59 | 1.70 | 1.39 | 1.36 | 1.20 | 1.45 | 0.00 | 1.56 | 0.04 | ASPARTATE AMINOTRANSFERASE 3 (ASP3) | ||
| AT2G41220 | 1.41 | 1.39 | 1.40 | 0.02 | 1.63 | 0.00 | GLUTAMATE SYNTHASE 2 (GLU2) | |||||
| AT5G04740 | 1.23 | 1.16 | 1.23 | 1.37 | 1.45 | 1.37 | 1.30 | 0.00 | 2.04 | 0.00 | ACT domain-containing protein | |
| AT1G66200 | 1.33 | 1.37 | 1.18 | 1.13 | 1.25 | 0.02 | glutamate-ammonia ligase GLN1;4 | |||||
| AT5G37600 | glutamate-ammonia ligase ATGSR1 | |||||||||||
| AT5G16570 | glutamate-ammonia ligase ATGSR2 | |||||||||||
| AT5G07440 | 1.20 | 1.25 | 1.23 | 0.05 | 1.87 | 0.01 | GLUTAMATE DEHYDROGENASE 2 (GDH2) | |||||
| abiotic stress | ||||||||||||
| AT3G02480 | 2.12 | 1.68 | 1.90 | 0.11 | 242.71 | 0.00 | ABA-responsive protein-related | |||||
| AT1G13930 | 1.53 | 1.84 | 1.69 | 0.11 | 3.44 | 0.00 | Involved in response to salt stress | |||||
| AT1G76180 | 1.37 | 1.39 | 1.53 | 1.33 | 1.68 | 1.49 | 1.53 | 1.47 | 0.00 | 2.19 | 0.00 | EARLY RESPONSE TO DEHYDRATION 14 (ERD14) |
| AT3G02360 | 1.31 | 1.15 | 1.27 | 1.24 | 0.03 | 1.79 | 0.00 | ABA-responsive protein-related | ||||
| undefined | ||||||||||||
| AT2G45570 | 2.50 | 2.25 | 1.91 | 6.29 | 3.24 | 0.03 | 51.51 | 0.00 | CYTOCHROME P450 76C2 (CYP76C2) | |||
| AT2G45550 | 1.11 | 0.29 | CYTOCHROME P450 76C4 (CYP76C4) | |||||||||
| AT5G14780 | 1.63 | 1.35 | 2.66 | 2.44 | 2.02 | 0.03 | 15.40 | 0.00 | FORMATE DEHYDROGENASE (FDH) | |||
| AT5G06760 | 1.80 | 1.87 | 2.10 | 1.44 | 1.80 | 0.01 | 282.61 | 0.00 | LATE EMBRYOGENESIS ABUNDANT 1 (LEA1 ) | |||
| AT5G16970 | 1.70 | 1.53 | 2.00 | 1.44 | 1.42 | 1.80 | 1.65 | 0.00 | 1.99 | 0.01 | 2-alkenal reductase (AT-AER) | |
| AT3G49120 | 1.13 | 1.59 | 1.16 | 2.10 | 1.50 | 1.84 | 1.67 | 1.57 | 0.00 | *1.30 | *0.25 | PEROXIDASE 34 (PERX34) |
| AT2G44100 | 1.59 | 1.45 | 1.52 | 0.07 | 0.75 | 0.00 | GDP DISSOCIATION INHIBITOR 1 (ATGDI1) | |||||
| AT1G74020 | 1.74 | 1.50 | 1.39 | 1.46 | 1.52 | 0.00 | 2.78 | 0.00 | STRICTOSIDINE SYNTHASE 2 (SS2) | |||
| AT1G77120 | 1.58 | 1.61 | 1.39 | 1.19 | 1.56 | 1.47 | 0.00 | 7.99 | 0.00 | ALCOHOL DEHYDROGENASE 1 (ADH1) | ||
| AT2G01490 | 1.24 | 1.50 | 1.24 | 1.42 | 1.35 | 0.01 | 2.09 | 0.00 | PHYTANOYL-CoA DIOXYGENASE H (PHYH) | |||
| AT1G24180 | 1.47 | 1.12 | 1.27 | 1.29 | 0.09 | 1.45 | 0.10 | oxidoreductase (AT1G13930) | ||||
| AT4G21580 | 1.31 | 1.17 | 1.35 | 1.10 | 1.50 | 1.28 | 0.01 | 1.58 | 0.02 | oxidoreductase | ||
| AT5G53120 | 1.20 | 1.30 | 1.26 | 1.25 | 0.01 | 3.25 | 0.00 | SPERMINE SYNTHASE (SPMS) | ||||
| AT1G48030 | 1.30 | 1.77 | 1.12 | 1.00 | 1.10 | 1.16 | 1.24 | 0.06 | 1.05 | 0.65 | LIPOAMIDE DEHYDROGENASE 1 (mtLPD1) | |
| AT4G18100 | 1.23 | 1.19 | 1.21 | 0.05 | *0.58 | *0.00 | 60S ribosomal protein L32 (RPL32A) | |||||
| AT5G46430 | 60S ribosomal protein L32 (RPL32B) | |||||||||||
| downregulated proteins | ||||||||||||
| RNA binding | ||||||||||||
| AT4G17520 | 0.64 | 0.75 | 1.03 | 0.84 | 0.72 | 0.80 | 0.04 | 0.32 | 0.00 | nuclear RNA-binding protein | ||
| AT2G21660 | 0.94 | 0.67 | 0.64 | 0.91 | 0.92 | 0.60 | 0.78 | 0.03 | *0.17 | *0.08 | GLYCINE RICH PROTEIN 7 (GRP7) | |
| undefined | ||||||||||||
| AT5G24650 | 0.91 | 0.65 | 0.90 | 0.70 | 0.79 | 0.07 | 0.73 | 0.05 | TRANSLOCASE OF THE INNER MEMBRANE protein | |||
| AT1G02920 | 0.76 | 0.79 | 0.78 | 0.05 | GLUTATHIONE S-TRANSFERASE (GST1, ERD11) | |||||||
| AT2G02930 | GLUTATHIONE S-TRANSFERASE (GST2) | |||||||||||
| AT4G02520 | GLUTATHIONE S-TRANSFERASE (GST11) | |||||||||||
| AT1G02930 | GLUTATHIONE S-TRANSFERASE (GST16) | |||||||||||
| AT2G29550 | 1.07 | 0.40 | 0.79 | 0.85 | 0.82 | 0.70 | 0.77 | 0.08 | Tubulin TUB1 | |||
| AT1G20010 | Tubulin TUB2 | |||||||||||
| AT1G75780 | Tubulin TUB5 | |||||||||||
| AT5G62700 | Tubulin TUB6 | |||||||||||
| AT5G12250 | Tubulin TUB7 | |||||||||||
| AT5G23860 | Tubulin TUB8 | |||||||||||
| AT5G64120 | 1.00 | 0.43 | 1.07 | 0.69 | 0.71 | 0.72 | 0.77 | 0.07 | 0.06 | 0.00 | cell wall bound peroxidase | |
| AT2G03440 | 0.80 | 0.70 | 0.83 | 0.72 | 0.78 | 0.65 | 0.74 | 0.00 | 0.00 | 0.00 | NAP1-RELATED PROTEIN 1 (NRP1) | |
| AT4G01700 | 1.07 | 0.62 | 0.51 | 0.71 | 0.77 | 0.74 | 0.05 | 0.13 | 0.00 | chitinase | ||
AGI, Arabidopsis gene identifier; p1, p-value of students t-test of log10-transformed protein ratios; p2, posthoc p value; FC, fold change ratios of ABA-treated versus untreated samples at the protein or mRNA level; * transcript changes are based on probe sets mapping to multiple genes on the ATH1 array; ≠ two gene products that share probe sets on the ATH1 array were identified as individual proteins in the iTRAQ experiment.
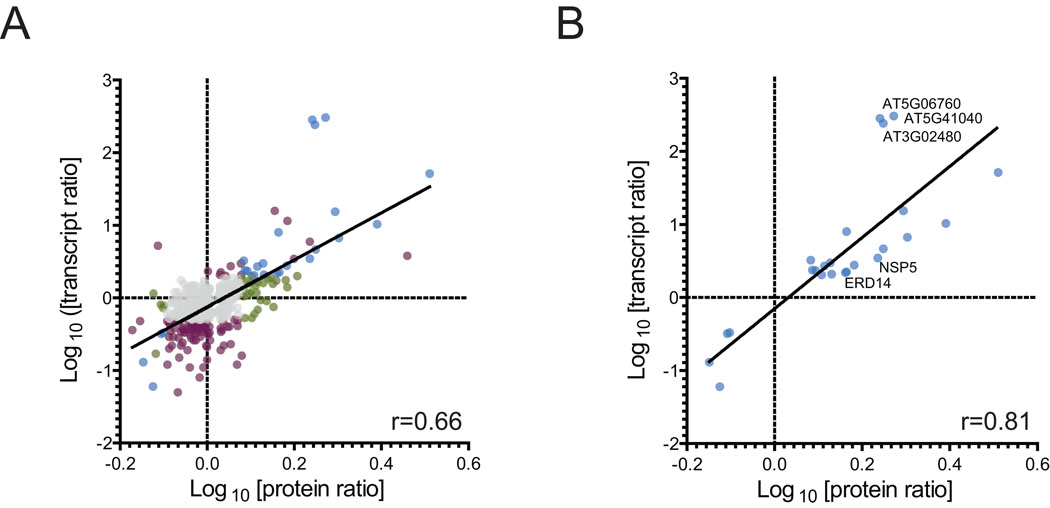
24 of the 50 proteins regulated by ABA at the protein level have also been identified as being transcriptionally regulated by ABA in this study (Figure 5AB, blue). These genes include EARLY RESPONSE TO DEHYDRATION (ERD14; 1.47-fold upregulated), ABA-responsive protein related (AT3G02480; 1.90-fold upregulated) and NSP5 (1.76-fold upregulated).
Our proteomic dataset also includes a number of proteins not regulated at the protein level, but only at the transcript level (Figure 5A, purple). These genes include the vacuolar Ca2+ binding protein At1g62480 (12.5-fold downregulated), the malate dehydrogenase AT5G58330 (2.32-fold upregulated) or the RNA-binding protein NOP10 (5.26-fold downregulated). We also identified genes that were only regulated at the protein level (Figure 5A, green), including the GDP DISSOCIATION INHIBITOR 1 (ATGDI1; 1.52-fold upregulated) (Table 2). Identification of these discrepancies between transcript and protein responses demonstrates the strength of our combined approach.
The relationship between transcriptomic and proteomic responses to ABA that we identified for some exemplary genes were then compared on a more general level. We compared 433 genes that were quantified in at least two biological replicates at the protein level and in three biological replicates at the transcript level and had unique probe sets on the microarray. A scatterplot of ABA-induced protein- and transcript-responses displayed a poor correlation with a Pearson correlation coefficient of r=0.66 (Figure 5A). This group of genes contained 95 genes that changed only at the transcript (Figure 5A, purple), of which 88 were downregulated and 7 were upregulated, 31 genes that only changed at the protein level (Figure 5A, green), 34 genes that changed at both levels (Figure 5A, blue) and genes that showed no changes towards ABA (Figure 5A, grey). A comparison of genes that showed significant ABA-induced protein responses and corresponding changes at the transcript level displayed a relatively high correlation coefficient of r=0.81 (Figure 5B). The three genes for which transcript responses most strongly deviated from protein responses, AT5G41040, AT3G02480 and AT5G06760, had very low signal values in the control sample which likely affected an accurate determination of transcript changes in response to ABA (Figure 5B).
Discussion
In this work we analyzed T87 suspension cells as a model system for ABA-regulated gene and protein expression. By analyzing SNRK2 kinase activation in response to pyrabactin and ABA, we could show that perception of ABA in suspension cells is mediated by the same family of ABA receptors as in plants (Figure 1). Furthermore, we detected a rapid NADPH-oxidase-dependent production of ROS in response to ABA as had previously been shown in guard cells and T87 suspension cells (Kwak et al., 2003; Miao et al., 2006; Murata et al., 2001; Pei et al., 2000; Suhita et al., 2004; Zhang et al., 2001). In addition, we could show that pyrabactin also induced ROS production in these cells with similar rapid kinetics as ABA. Taken together, these results underline that T87 suspension cells provide a good model system for the analysis of ABA signaling.
Comparison of transcript and protein changes
In this study we identified regulation of 50 proteins in response to ABA, of which 42 were up- and 8 were downregulated. The protein with the highest change in protein abundance we observed was a Cytochrome P450 protein that was upregulated 3.24-fold. The most strongly downregulated protein was a chitinase that was downregulated 0.74-fold (Table 2). The dynamic range of protein responses was therefore far less than what we measured in gene regulation (398-fold upregulated to 81-fold downregulated). This brings up a long standing question, as to what extent changes in transcriptomic data reflect actual changes at the protein level. The presented data show a poor correlation of r=0.66 comparing all genes quantified at the transcript and protein level in this study. Only considering genes that showed an ABA-induced response at the transcript and protein level, however, displayed an overall good correlation (r= 0.81) between transcript and protein changes in response to ABA (Figure 4). The correlation between transcript and protein responses can vary significantly in different systems. In a recent study in Drosophila melanogaster a similarly good Pearsson correlation coefficient of r=0.8 was identified when genes not responding to the applied stimulus were removed (Bonaldi et al., 2008). The slope of the linear regression line in the log/log-scaled scatter plot in Figure 4 reflects the differences in dynamic range between transcript and protein changes.
Figure 4.
A. Scatterplot of log10-transformed protein ratios versus their corresponding transcript ratios. 433 individual proteins that were quantified in at least two replicates and for which transcript ratios from unique probesets on the microarray were available were analyzed. Blue, significantly regulated at mRNA and protein levels; green, significantly regulated at protein level only; purple, significantly regulated at mRNA level only; and grey, not regulated. B. Scatterplot of ratios only from genes that were ABA-regulated at the protein (p<0.11, 0.8<FC>1.2) and transcript level (p<0.05; 0.5<FC>2). Pearson correlation coefficients (r) are shown.
Furthermore, the data show that individual protein changes are not predictable based on transcript changes. In particular downregulation in transcript levels often did not correlate well to downregulation in protein levels. This was shown for the RNA-binding protein NOP10 (5.26-fold downregulated at the transcript level) or the vacuolar Ca2+-binding protein At1g62480 (12.5-fold downregulated at the transcript level) that did not show any response at the protein level. Protein stability determines the time to reach a new equilibrium between protein and transcript abundance. Three proteins involved in protein degradation were upregulated at the protein level in response to ABA. These were a subunit of the 20S proteasome (AT2G05840), RD21 and a cathepsin B-like cysteine protease (AT4G01610) (Table 2). These proteases might be involved in actively degrading target proteins. On the other hand, lower transcription rates might also reflect a negative feedback loop where increases in protein abundance negatively regulate transcription. In addition, posttranscriptional regulation can affect the protein abundance without affecting transcript levels, as we have found for GDI1, where there is a reverse correlation between transcript and protein regulation (Table 2).
Comparison with recent proteomic studies
Two recent proteomic studies have analyzed proteins expressed in guard cells (Zhao et al., 2010; Zhao et al., 2008). The effect of ABA on protein regulation was quantified by iTRAQ followed by LC-MS/MS showing ABA-regulation of 8 proteins (Zhao et al., 2010). Two proteins, a mitochondrial MALATE DEHYDROGENASE (MDH, At1g53240) and RUBISCO SMALL SUBUNIT 2B (RBCS2B), were identified to be upregulated 1.37-fold and 1.18-fold, respectively, in response to ABA in wildtype guard cells. Another six proteins were regulated by ABA in guard cells of a mutant of the Gα-subunit gpa1–4 that functions in ABA-regulated stomatal movement. Four proteins of the photosynthesis machinery (e.g. PSBC) were upregulated 1.32- to 1.58-fold, and two proteins, CP24 and ERD14, were downregulated by ABA 0.74- and 0.75-fold, respectively (Zhao et al., 2010).
In our study in T87 cells none of the five quantified MDHs, including At1g53240 showed a response towards ABA at the protein level which is consistent with no significant regulations at the transcript level in these cells. For RBCS2B we did not have sufficiently specific peptide information to differentiate between RBCS2B and RBCS3B. No change in protein abundance was detected for these peptides as well as for PSBC in response to ABA in T87 cells. Interestingly, we detected an upregulation of ERD14 in response to ABA in T87 suspension cells. Further studies will be necessary to conclude whether any observed differences between studies are sample specific or due to differences in experimental conditions.
The role of ROS in ABA-induced gene regulation
The NADPH oxidases RBOHF and RBOHD function in ABA signaling (Kwak et al., 2003; Sirichandra et al., 2009; Suhita et al., 2004). RBOHF is regulated by SNRK2.6 and there is increasing evidence that RBOHD/F can also be activated by CDPKs (Kobayashi et al., 2007; Mori et al., 2006). ROS production takes place upstream of Ca2+-permeable ICa channel activation (Murata et al., 2001; Pei et al., 2000). Here we present evidence that ROS production is furthermore involved in the regulation of a subset of ABA-regulated genes (Figures 2B, 2D, 4, Supplementary table 1). Clustering and functional classification of this subset of genes identified the fatty acid metabolism, phenylpropanoid synthesis and ROS-sensitive genes that are regulated by ABA only in the presence of ROS (Figure 4). Among the ROS sensitive genes that we identified and validated further by qRT-PCR are a number of peroxidases that respond to increases in ROS and presumably function in ROS detoxification.
ABA has been shown to modulate fatty acid synthesis towards long-chain fatty acids in Brassica embryos (Finkelstein and Somerville, 1989). Changes in gene expression of fatty acid synthesis genes that we observed in this study might reflect these metabolic changes. On the other hand, fatty acids are oxidized by ROS species. Activation of fatty acid synthesis might therefore reflect a positive feedback loop in response to fatty acid oxidation.
ABA-activation of a substantial number of genes of the phenylpropanoid pathway is affected by DMTU treatment in our dataset. Transcriptional regulation of the phenylpropanoid pathway is activated by a number of abiotic stimuli, including ABA and cold stress (Christie et al., 1994; Grimplet et al., 2007) and might be activated as a general stress response, rather than having a specific function in ABA signaling. The DMTU experiments indicate that ROS production in response to these stress stimuli might be the point of convergence for this gene regulation.
ABA-regulation of glucosinolate catabolism
NITRILE SPECIFIER PROTEIN 5 (NSP5), NITRILASE 1 (NIT1) and NITRILASE 2 (NIT2) were upregulated at the protein level in response to ABA (Table 2). NSP5 was furthermore detected in this study as upregulated at the transcript level.
NIT1 and NIT2 belong to a family of NIT1-homologuous nitrilases that has evolved in Brassicaceae (Janowitz et al., 2009). NIT1 and NIT2 were initially described as the final enzymes for the generation of indole-3-acetic acid (IAA, Auxin) from indole-3-acetonitrile (Bartling et al., 1992). Enzymatic characterization, however, shows that indole-3-acetonitrile is a poor substrate for these nitrilases (Vorwerk et al., 2001). It has therefore been hypothesized that this group of nitrilases may have been neofunctionalized for the endogenous controlled catabolism of glucosinolates (Janowitz et al., 2009). The two thioglucosidase myrosinases expressed in leaves, TGG1 and TGG2, catalyze the breakdown of glucosinolates into isothiocyanates as herbivore repellents. Interestingly, tgg1 single and tgg1tgg2 double mutant plants share ABA hyposensitivity and ABA insensitivity in induction of stomatal closure (Islam et al., 2009; Zhao et al., 2008). Furthermore, addition of glucosinolates to wildtype guard cells, but not to tgg1 mutant guard cells resulted in inhibition of inward K+-channels, a prerequisite for stomatal closure (Zhao et al., 2008). Simultaneous addition of glucosinolates and myrosinases to guard cells led to K+−channel inhibition of both, wildtype and tgg1 mutant plants, indicating that the functional compound is a product of myrosinase-catalyzed glucosinolates degradation (Zhao et al., 2008).
The upregulation of NIT1, NIT2 and NSP5 that we identified might indicate that isothiocyanate is not the final product of glucosinolates degradation during stomatal closure, but the formation of nitriles. It is possible that Brassicaceae are more dependent on recycling their sulfur and nitrogen than other plant families that do not synthesize glucosinolates. Glucosinolates represent a large store for nitrogen and sulfur in the cell. At any given point up to 30 % of cellular sulfur can be stored in the form of glucosinolates (Falk et al., 2007). We therefore speculate that under stress conditions where there might be an increased demand for nitrogen and sulfur, a controlled endogenous catabolism of glucosinolates makes nitrogen and sulfur available for primary metabolism (Janowitz et al., 2009). The most efficient breakdown of glucosinolates is the formation of nitriles by TGG proteins in combination with NSPs, e.g. NSP5, which leads to the release of both sulfur atoms (Janowitz et al., 2009). Nitriles are then further broken down by NITRILASES (NIT), e.g. NIT1 and NIT2, recovering nitrogen as ammonia.
Conclusion
In summary, with a combined quantitative transcriptomic and proteomic approach we could generate a dataset that is not only complementary to transcriptomic approaches, but significantly enhances our current knowledge of ABA-induced responses and signaling components. ABA-induced ROS production is required for a subset of ABA-induced genes. Overall we have demonstrated a poor correlation between transcript and protein changes in response to ABA. A good correlation, however, exists between ABA-induced transcriptomic and proteomic responses for genes that are regulated on both levels. But even for these genes changes in protein abundance cannot be predicted based on transcript changes. We have identified unique candidates for further in-depth analyses and discuss pathways identified here that are strongly regulated by ABA.
Experimental procedures
Culture conditions and treatment
Arabidopsis thaliana (Columbia ecotype) T87 suspension cells were obtained from RIKEN BioResource Center (Tsukuba, Japan) and were cultured at 20 °C under 16 h light, 8h dark illumination (26 µE s−1 m−2) with shaking at 120 rpm (Supplementary figure 5). Cells were subcultured every 7 days by adding 10 ml of the cell suspension to a 500 ml flask containing 90 ml of Gamborg B5 medium with 3% sucrose and 0.5 µM 1-naphthaleneacetic acid (NAA). Experiments were carried out in the exponential phase of growth at about 120 h of cell cultivation. ABA was added to the medium containing the suspension cells to a final concentration of 50 µM. ABA treatment was continued for 20 h before RNA extraction and for 24 h before protein extraction. Cells were harvested by vacuum filtration through Miracloth (Calbiochem) and immediately placed in liquid nitrogen before storage at −80 °C. DMTU was used at a final concentration of 5 mM.
RNA extraction and microarrays
Three independent biological experiments were performed for the transcriptome comparison of ABA-treated and untreated suspension cells. RNA was isolated with the RNeasy Plant Mini Kit (Qiagen) following the manufacturer's instructions. Arabidopsis ATH1 oligonucleotide-based genome arrays were used, and hybridization and washing were performed by the Genechip Microarray Core facility (University of California, San Diego) using the standard Affymetrix protocol for RNA analyses (www.affymetrix.com/products/arrays/specific/arab.affx). RNA/DNA yield was quantified by UV absorption (NanoDrop ND-1000 Spectrophotometer) and purity was estimated by the 260 and 280 nm absorbance ratio. Acceptable values were between 1.7 and 2.1. The quality of sample RNA was assessed using RNA 6000 Labchip Kit on the Agilent 2100 Bioanalyzer.
Microarray data were analyzed using Robin v1.1.5 (Lohse et al., 2010) using the standard parameters (RMA, posthoc p-value cutoff p<0.05 (Benjamini-Hochberg correction), nestedF multiple testing strategy and a log2-fold change minimum of 1). The R script that was generated is available (Supplementary file 5). Probesets were mapped to genome loci using the Tair9 annotation file from January 2010. Of a total of 22810 probe sets on the ATH1 chip, 13098 genes had a normalized log2-transformed signal intensity of at least 5.9 under at least one of the tested conditions and were considered expressed. Three biological replicates for each of the four analyzed conditions were compared. Metagroups of ABA-treated versus control samples and ABA + DMTU versus DMTU samples were generated to compare changes in gene expression in the presence and in the absence of DMTU (Figure 2A). The dataset of this microarray study was deposited in Gene Expression Omnibus (GEO) with the series accession number GSE23301.
Microarray data validation by qRTPCR
Total RNA was isolated with the RNeasy Plant Mini Kit (Qiagen). 2 µg total RNA for each sample was treated with Amp-grade DNase I (Invitrogen). cDNA was generated from 1 µg DNase I-treated RNA using Superscript III and oligo-dT-V primer. All steps followed manufacturer’s instructions. qPCR was performed using SYBR Green JumpStart Taq ReadyMix for QPCR, Capillary formulation (Sigma). The following primer pairs were used for PCR amplification: AT3G50400 (5’-gtaccatagctgacatcgttggag-3’ and 5’-caccacctgatgcatagttcacac-3’), AT3G56240 (5’-tcggtccatcgatctcaaaggc-3’ and 5’-ctttgaggacaacggtctgagc-3’), AT1G04220 (5’-tcgctaaacagcttcttcaggttc-3’ and 5’-ttgatcggtcgttgcctaaatacc-3’), AT5G13930 (5’-ttccgcatcaccaacagtgaac-3’ and 5’-cgcacatgcgcttgaacttctc-3’), AT4G26010 (5’-aatgcaagcgtgagaggctacg-3’ and 5’-atgcagcctcgagctgtctcttag-3’), AT1G68850 (5’-gctagagatgctacaatcctggtg-3’ and 5’-tggaaggtttgttgtggcaagc-3’), AT1G01120 (5’-tgcggtttggaaagcgttacgac-3’ and 5’-tcgaaccagcccaagcattacc-3’), AT2G45970 (5’-tacgccgttctttggccaactc-3’ and 5’-tgaattatgcagccaaccaaccg-3’), AT4G14440 (5’-gcatgatagtgcggaaggtgtg-3’ and 5’-agcggccaaactttctccaagg-3’). PCR amplification was monitored in triplicate using a Lightcycler (Roche). Amplification of Clathrin served as an internal control for normalization. Clathrin transcript levels were not affected by ABA in our microarray data. Gene transcript levels were then calculated with reference to expression in the untreated sample and averaged to 1. Primer efficiencies were calculated by LinRegPCR (Ramakers et al., 2003), and ct values were determined by Roche Quantification Software using the second deviation method. Fold changes were calculated with the ΔΔct method using calculated primer efficiencies. Statistical analysis was done by Student’s t test using Prism v5.03 (Graphpad).
In-gel kinase assay
The in-gel kinase assay was performed as described previously (Romeis et al., 1999) with modifications. Protein was extracted from T87 suspension cells by homogenization in liquid nitrogen and addition of extraction buffer (100 mM HEPES, pH 7.5, 5 mM EDTA, 5 mM EGTA, 0,5% Triton X-100, 150 mM NaCl, 10 µM DTT, 10mM NaF, 1/200 Vol. protease inhibitor (P9599, Sigma-Aldrich), 1/200 Vol. phosphatase inhibitor (P2580, Sigma-Aldrich), 5 mM Na3VO4). 20 µg of protein per lane was subjected to electrophoresis on a 10.5% polyacrylamide gel that contained SDS and 0.25 mg of Histone III-S per ml. After electrophoresis, the gel was washed three times with washing buffer (25 mM Tris–HCl pH 8.0, 0.5 mM DTT, 0.1 mM Na3VO4, 5 mM NaF, 0.5 mg ml=1 BSA, 0.1% Triton X-100) for 30 min at room temperature, followed by two washes with renaturation buffer (25 mM Tris–HCl pH 8.0, 1 mM DTT, 0.1 mM Na3VO4, 5 mM NaF) for 30 min at room temperature and one wash at 4°C overnight. The gel was equilibrated with reaction buffer (25 mM HEPES pH 7.5, 2 mM EGTA, 12 mM MgCl2, 1 mm DTT, 0.1 mM Na3VO4) for 30 min at room temperature and then incubated in 20 ml of reaction buffer with 50µCi [γ-32P]ATP (3000 Ci mmol−1) for 90 min at room temperature. The gel was washed with washing solution (5% TCA, 1% pyrophosphoric acid) until no radioactivity was detected in the washing solution anymore. The gel was dried on Whatman 3MM paper, exposed to a storage phosphor screen (Molecular Dynamics) and quantified using a Typhoon Imager (GE Lifesciences).
Oxidative Burst Measurements
T87 suspension cells were grown as described. ROS production was triggered with 50 µM ABA applied together with 20 µM L-012 and 1 µg per 100 µL of horseradish peroxidase. Luminescence was recorded by a Spectramax L luminescence plate reader (Molecular Devices) at 470nm for 6 minutes, 1 s integration time and 15 s intervals. All ROS measurements were repeated at least four times with similar results. The areas under curves were calculated and referred to RLUtot (where RLU = relative light units). Statistical analysis was done by Student’s t test using Prism v5.03 (Graphpad).
Protein extraction, trypsin digestion and iTRAQ labeling
Vacuum filtered suspension cells were ground in liquid nitrogen. Protein was extracted from 200 mg of tissue for each sample using the phenol/SDS/ammonium acetate method with one modification (Wang et al., 2003). Instead of a second grinding step with quartz sand, 10% TCA in acetone was added to the tissue and the mixture was sonicated. Protein concentration was determined using the BCA assay (Pierce) and protein was stored in 80% Acetone at −20°C. 50 or 100 µg of proteins were used for further reduction, alkylation, digestion and iTRAQ labeling using iTRAQ Reagents Multiplex Kit (Applied Biosystems) according to manufacturer's protocol. Briefly, protein samples were reduced with 5 mM tris-(2-carboxyethyl)phosphine (TCEP) at 60°C for 1 h and the cysteine-groups were blocked using a 10 mM methyl methanethiosulfonate (MMTS) solution at room temperature for 10 min. The proteins were then digested by 10 µg of trypsin at 37°C for 16 h. Each peptide solution was labeled at room temperature for 1 h with one iTRAQ reagent vial (mass tag 114, 115, 116 or 117) previously reconstituted with 70 µl of ethanol. Samples of the same protein content, and labeled respectively with iTRAQ114, iTRAQ 115, iTRAQ 116 and iTRAQ117 reagents, were combined and labeling reaction stopped by evaporation in a Speed Vac.
Protein extractions from seven independent biological replicates of ABA treatment were fractionated into 8–13 individual strong cation exchange (SCX) fractions each to reduce the complexity of the peptide mixture. Therefore the iTRAQ-labeled samples were loaded onto an SCX column (PolysulfoethylA, 1×100, 5µM, 300Å) using an Äkta FPLC system (GE Healthcare). Peptides were separated by a linear gradient for 20 min from 100% buffer A (0.05% Formic acid, 25% Acetonitrile) to 100% buffer B (250 mM NH4Cl, 0.05% Formic acid, 25% Acetonitrile) with a flow rate of 0.1 ml/min. Fractions were collected every minute using a fraction collector. In total, 27 SCX fractions including the column flow-through were collected and combined into 9 fractions for further analysis. Fractions were dried in a speed vac and resuspended in 5% acetonitrile/5% formic acid to an end volume of 10 µL and analyzed by on-line nanoflow LC-MS/MS.
LC-MS/MS
All nano-LC-MS/MS experiments were performed on an Eksigent Tempo 2D nano-HPLC system connected to a QSTAR Elite Hybrid mass spectrometer (ABSCIEX) through a nanoelectrospray ion source. Samples were injected, and captured onto a 5 µm, 100 Å trapping column, 5 mm × 300 µm (LC Packings). Trapped samples were then eluted onto a 0.18 × 100 mm analytical column (5 µm C18 Zorbax™ beads, Agilent Technologies) using an automated binary gradient with a flow of 400 nL/min from 5% to 60% Acetonitrile. The buffers used to create the ACN gradient were: Buffer A (98% H2O, 2% ACN, 0.2% formic acid, and 0.005% TFA) and Buffer B (100% ACN, 0.2% formic acid, and 0.005% TFA). MS/MS data were acquired in a data-dependent manner in which the MS1 data was acquired at m/z of 400 to 1800 Da and the MS/MS data was acquired from m/z of 50 to 2,000 Da.
Mass spectrometry analysis pipeline
Mass spectra were analyzed against the TAIR9 representative model protein database from 06/19/2009 (27379 ORFs) with MASCOT 1.01 (Matrix Science). Peptide spectra were interrogated with an identical predefined QSTAR-ESI mass tolerance (0.5 Da MS, 0.6 Da MS/MS) and with Methylthio-cysteine and iTRAQ labels as defined modifications. As variable modifications we included NQ-deamidation and methionine-oxidation. Missed-cleavage tolerances were left undefined, thus allowing all cleavage points for consideration. The Transproteomic Pipeline was used for further statistical evaluation of the identified proteins and quantitation of iTRAQ results (Keller et al., 2005). As a cut-off for protein identification, a ProteinProphet probability of 0.95 was used. The parameters used in Libra for iTRAQ quantification were: mass tolerance of 0.2 Da, centroiding by intensity weighted mean, normalization against sum of reagent profiles and minimum threshold intensity for each channel of 20 counts. Calculated iTRAQ ratios for fold change calculation in response to ABA and median ratios were: replicate 1 (116/114: 1.127), replicate 2 (114/115: 0.707), replicate 3 (115/114: 1.605), replicate 4 (115/114: 2.252), replicate 5 (117/116: 0.333), replicate 6 (114/115: 1.406) and replicate 7 (117/116: 0.417). Bias normalization for whole proteome analyses was performed by correcting the bias median ratio of each comparison toward unity. In order to identify reliable protein biomarkers for ABA-induced changes in protein abundance, we calculated p values over all biological replicates for each protein using the student’s t-test. The student’s t-test has previously been described as a too strict measure of reproducibility for iTRAQ measurements (O'Brien et al., 2010). We therefore increased the cut-off to p<0.11 based on known ABA-regulated proteins and protein annotations. The median standard deviation of all proteins quantified at least in two biological replicates in this study was 0.06. We therefore set the minimum fold change for an ABA-regulated protein to 3Σmed = 20%. Proteins with a change in protein abundance of more than 20% and a p-value of p<0.11 were considered ABA-regulated. All mass spectrometry files were uploaded to Peptidome with the accession number PSE149.
Supplementary Material
Acknowledgement
We thank Prof. Dr. Elizabeth Komives, Dr. Justin Torpey and Dr. Majid Ghassemian (UCSD Biomolecular/Proteomics Mass Spectrometry Facility) as well as Dr. Larry Gross (UCSD) for advice on masss spectrometrical analyses. We thank Dr. Felix Hauser, Dr. Diego H. Sanchez for advice on the microarray analyses and discussion of the manuscript. This work was supported by grants from NIH (GM060396-ES010337), NSF (CMCB0918220) and the Energy Biosciences program of the Department of Energy (DE-FG02–03ER15449) to J.I.S and a Longterm Research fellowship of the Deutsche Forschungsgemeinschaft (DFG) to M.B.
References
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K(+)-selective, K(+)-sensing ion channel. FEBS Lett. 2000;486:93–98. doi: 10.1016/s0014-5793(00)02248-1. [DOI] [PubMed] [Google Scholar]
- Axelos M. A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol. Biochem. 1992;30:123. [Google Scholar]
- Bartling D, Seedorf M, Mithöfer A, Weiler EW. Cloning and expression of an Arabidopsis nitrilase which can convert indole-3-acetonitrile to the plant hormone, indole-3-acetic acid. Eur. J. Biochem. 1992;205:417–424. doi: 10.1111/j.1432-1033.1992.tb16795.x. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Straub T, Cox J, Kumar C, Becker PB, Mann M. Combined use of RNAi and quantitative proteomics to study gene function in Drosophila. Molecular Cell. 2008;31:762–772. doi: 10.1016/j.molcel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Brault M, Amiar Z, Pennarun AM, Monestiez M, Zhang ZS, Cornel D, Dellis O, Knight H, Bouteau FO, Rona JP. Plasma membrane depolarization induced by abscisic acid in Arabidopsis suspension cells involves reduction of proton pumping in addition to anion channel activation, which are both Ca2+ dependent. Plant Physiol. 2004;135:231–243. doi: 10.1104/pp.104.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hoehenwarter W, Weckwerth W. Comparative analysis of phytohormone-responsive phosphoproteins in Arabidopsis thaliana using TiO2-phosphopeptide enrichment and mass accuracy precursor alignment. The Plant Journal. 2010;63:1–17. doi: 10.1111/j.1365-313X.2010.04218.x. [DOI] [PubMed] [Google Scholar]
- Christie PJ, Alfenito MR, Walbot V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta. 1994;194:541–549. [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic Acid: Emergence of a Core Signaling Network. Annual Review of Plant Biology. 2010;61 doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Falk KL, Tokuhisa JG, Gershenzon J. The Effect of Sulfur Nutrition on Plant Glucosinolate Content: Physiology and Molecular Mechanisms. Plant Biology. 2007;9:573–581. doi: 10.1055/s-2007-965431. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Somerville C. Abscisic acid or high osmoticum promote accumulation of long-chain fatty acids in developing embryos of Brassica napus. Plant Sci. 1989;61:213–217. [Google Scholar]
- Fujii H, Zhu JK. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8380–8385. doi: 10.1073/pnas.0903144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Nakashima K, Yoshida T, Katagiri T, Kidokoro S, Kanamori N, Umezawa T, Fujita M, Maruyama K, Ishiyama K, Kobayashi M, Nakasone S, Yamada K, Ito T, Shinozaki K, Yamaguchi-Shinozaki K. Three SnRK2 Protein Kinases are the Main Positive Regulators of Abscisic Acid Signaling in Response to Water Stress in Arabidopsis. Plant Cell Physiol. 2009;50:2123–2132. doi: 10.1093/pcp/pcp147. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, Romeis T, Hedrich R. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelis T, Dellis O, Jeannette E, Bardat F, Cornel D, Miginiac E, Rona JP, Sotta B. Abscissic acid specific expression of RAB18 involves activation of anion channels in Arabidopsis thaliana suspension cells. FEBS Lett. 2000;474:43–47. doi: 10.1016/s0014-5793(00)01574-x. [DOI] [PubMed] [Google Scholar]
- Grimplet J, Deluc L, Tillett R, Wheatley M, Schlauch K, Cramer G, Cushman J. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics. 2007;8:187. doi: 10.1186/1471-2164-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nature Biotechnology. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987;242:103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Garmard F, Poree F, Boucherz J, Lebaudy A, Bouchez D, Very AA, Simoneau T, Thibaud JB, Sentenac H. The Arabidiopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA. 2003;100:5549–5554. doi: 10.1073/pnas.0733970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez J-P, Hanafey MK, Tingey SV, Chua N-H. Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. J Cell Sci. 2002;115:4891–4900. doi: 10.1242/jcs.00175. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Islam MM, Tani C, Watanabe-Sugimoto M, Uraji M, Jahan MS, Masuda C, Nakamura Y, Mori IC, Murata Y. Myrosinases, TGG1 and TGG2 redundantly function in ABA and MeJA signaling in Arabidopsis guard cells. Plant Cell Physiol. 2009;50:1171–1175. doi: 10.1093/pcp/pcp066. [DOI] [PubMed] [Google Scholar]
- Janowitz T, Trompetter I, Piotrowski M. Evolution of nitrilases in glucosinolate-containing plants. Phytochemistry. 2009;70:1680–1686. doi: 10.1016/j.phytochem.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Keller A, Eng J, Zhang N, Li XJ, Aebersold R. A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol Syst Biol. 2005;1 doi: 10.1038/msb4100024. 2005 0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signalling. Annual Review Plant Biology. 2010;61:13.1–13.31. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline KG, Barrett-Wilt GA, Sussman MR. In planta changes in protein phosphorylation induced by the plant hormone abscisic acid. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15986–15991. doi: 10.1073/pnas.1007879107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. Calcium-Dependent Protein Kinases Regulate the Production of Reactive Oxygen Species by Potato NADPH Oxidase. Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. Embo J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, Schroeder JI. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in arabidopsis. Plant Physiol. 2001;127:473–485. [PMC free article] [PubMed] [Google Scholar]
- Lahm HW, Langen H. Mass spectrometry: A tool for the identification of proteins separated by gels. Electrophoresis. 2000;21:2105–2114. doi: 10.1002/1522-2683(20000601)21:11<2105::AID-ELPS2105>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21419–21424. doi: 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell. 2004;16:596–615. doi: 10.1105/tpc.019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Res. 2006;16:414–427. doi: 10.1101/gr.4237406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M, Nunes-Nesi A, Kruger P, Nagel A, Hannemann J, Giorgi FM, Childs L, Osorio S, Walther D, Selbig J, Sreenivasulu N, Stitt M, Fernie AR, Usadel B. Robin: An Intuitive Wizard Application for R-Based Expression Microarray Quality Assessment and Analysis. Plant Physiol. 2010;153:642–651. doi: 10.1104/pp.109.152553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS, Geiger D, Marten I, Martionia E, Hedrich R. ATALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. The Plant Journal. 2010;9999 doi: 10.1111/j.1365-313X.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, Wang X-C, Chen J, Miao C, Song C-P. An Arabidopsis Glutathione Peroxidase Functions as Both a Redox Transducer and a Scavenger in Abscisic Acid and Drought Stress Responses. Plant Cell. 2006;18:2749–2766. doi: 10.1105/tpc.106.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang Y-F, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, Kwak JM, Schroeder JI. CDPKs CPK6 and CPK3 Function in ABA Regulation of Guard Cell S-Type Anion- and Ca2+ Permeable Channels and Stomatal Closure. PLoS Biology. 2006;4:e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell. 2001;13:2513–2523. doi: 10.1105/tpc.010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Fujita Y, Kanamori N, Katagiri T, Umezawa T, Kidokoro S, Maruyama K, Yoshida T, Ishiyama K, Kobayashi M, Shinozaki K, Yamaguchi-Shinozaki K. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 2009;50:1345–1363. doi: 10.1093/pcp/pcp083. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A Statistical Model for Identifying Proteins by Tandem Mass Spectrometry. Analytical Chemistry. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, Yates JR, Schroeder JI. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C–interacting proteins in Arabidopsis. Plant J. 2010;61:290–299. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RN, Shen Z, Tachikawa K, Lee PJ, Briggs SP. Quantitative proteome analysis of pluripotent cells by iTRAQ mass tagging reveals post-transcriptional regulation of proteins required for ES cell self-renewal. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M110.000281. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Huang K, Cross FR, Cowburn D, Chait BT. Accurate quantitation of protein expression and site-specific phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6591–6596. doi: 10.1073/pnas.96.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Tatematsu K, Matsui A, Morosawa T, Ishida J, Tanaka M, Endo TA, Mochizuki Y, Toyoda T, Kamiya Y, Shinozaki K, Nambara E, Seki M. Genome-wide analysis of endogenous abscisic acid-mediated transcription in dry and imbibed seeds of Arabidopsis using tiling arrays. Plant J. 2010;62:39–51. doi: 10.1111/j.1365-313X.2010.04135.x. [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF, Cutler SR. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling. Trends in Plant Science. 2010;15:395–401. doi: 10.1016/j.tplants.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JD. Rapid Avr9- and Cf-9 -dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell. 1999;11:273–287. doi: 10.1105/tpc.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed Protein Quantitation in Saccharomyces cerevisiae Using Amine-reactive Isobaric Tagging Reagents. Molecular & Cellular Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- Rubio S, Rodrigues A, Saez A, Dizon MB, Galle A, Kim TH, Santiago J, Flexas J, Schroeder JI, Rodriguez PL. Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiol. 2009;150:1345–1355. doi: 10.1104/pp.109.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Rodrigues A, Saez A, Rubio S, Antoni R, Dupeux F, Park SY, Marquez JA, Cutler SR, Rodriguez PL. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009;60:575–588. doi: 10.1111/j.1365-313X.2009.03981.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Mori IC, Furuichi T, Munemasa S, Toyooka K, Matsuoka K, Murata Y, Yamamoto Y. Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 2010;51:354–365. doi: 10.1093/pcp/pcq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Ishida J, Narusaka M, Fujita M, Nanjo T, Umezawa T, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K. Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct Integr Genomics. 2002;2:301. doi: 10.1007/s10142-002-0070-6. [DOI] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, Kwak JM. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009;583:2982–2986. doi: 10.1016/j.febslet.2009.08.033. [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem. Biophys. Res. Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tate RM, Vanbenthuysen KM, Shasby DM, McMurtry IF, Repine JE. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982;126:802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Thimm O, Blasing O, Gibon Y, Nagel A, Meyer S, Kruger P, Selbig J, Muller LA, Rhee SY, Stitt M. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004;37:914–939. doi: 10.1111/j.1365-313x.2004.02016.x. [DOI] [PubMed] [Google Scholar]
- Trouverie J, Vidal G, Zhang Z, Sirichandra C, Madiona K, Amiar Z, Prioul JL, Jeannette E, Rona JP, Brault M. Anion channel activation and proton pumping inhibition involved in the plasma membrane depolarization induced by ABA in Arabidopsis thaliana suspension cells are both ROS dependent. Plant Cell Physiol. 2008;49:1495–1507. doi: 10.1093/pcp/pcn126. [DOI] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17588–17593. doi: 10.1073/pnas.0907095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzorjova I, Brosche M, Valk E, Lepiku M, Moldau H, Pechter P, Wang YS, Lindgren O, Salojarvi J, Loog M, Kangasjarvi J, Kollist H. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010;62:442–453. doi: 10.1111/j.1365-313X.2010.04159.x. [DOI] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Lauriere C, Merlot S. Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell. 2009;21:3170–3184. doi: 10.1105/tpc.109.069179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorwerk S, Biernacki S, Hillebrand H, Janzik I, Muller A, Weiler EW, Piotrowski M. Enzymatic characterization of the recombinant Arabidopsis thaliana nitrilase subfamily encoded by the NIT2/NIT1/NIT3-gene cluster. Planta. 2001;212:508–516. doi: 10.1007/s004250000420. [DOI] [PubMed] [Google Scholar]
- Wang W, Scali M, Vignani R, Spadafora A, Sensi E, Mazzuca S, Cresti M. Protein extraction for two-dimensional electrophoresis from olive leaf, a plant tissue containing high levels of interfering compounds. Electrophoresis. 2003;24:2369–2375. doi: 10.1002/elps.200305500. [DOI] [PubMed] [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;4:6. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002;43:1473–1483. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem. 2006;281:5310–5318. doi: 10.1074/jbc.M509820200. [DOI] [PubMed] [Google Scholar]
- Zalejski C, Paradis S, Maldiney R, Habricot Y, Miginiac E, Rona JP, Jeannette E. Induction of abscisic acid-regulated gene expression by diacylglycerol pyrophosphate involves Ca2+ and anion currents in Arabidopsis suspension cells. Plant Physiol. 2006;141:1555–1562. doi: 10.1104/pp.106.080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–1448. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Stanley BA, Zhang W, Assmann SM. ABA-regulated G protein signaling in Arabidopsis guard cells: a proteomic perspective. J Proteome Res. 2010;9:1637–1647. doi: 10.1021/pr901011h. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhang W, Stanley BA, Assmann SM. Functional Proteomics of Arabidopsis thaliana Guard Cells Uncovers New Stomatal Signaling Pathways. Plant Cell. 2008;20:3210–3226. doi: 10.1105/tpc.108.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.