Abstract
The T-box transcription factor, Tbx1, an important regulatory gene in development, is highly expressed in hair follicle stem cells in adult mice. Because mouse models of skin carcinogenesis have demonstrated that hair follicle stem cells are a carcinogen target population and contribute significantly to tumor development, we investigated whether Tbx1 plays a role in skin carcinogenesis. We first assessed Tbx1 expression levels in mouse skin tumors, and found down-regulation in all tumors examined. To study the effect of Tbx1 expression on growth and tumorigenic potential of carcinoma cells, we transfected mouse Tbx1 cDNA into a mouse spindle cell carcinoma cell line that did not express endogenous Tbx1. Following transfection, two cell lines expressing different levels of the Tbx1/V5 fusion protein were selected for further study. Intradermal injection of the cell lines into mice revealed that Tbx1 expression significantly suppressed tumor growth, albeit with no change in tumor morphology. In culture, ectopic Tbx1 expression resulted in decreased cell growth and reduced development into multilayered colonies, compared to control cells. Tbx1-transfectants exhibited a reduced proliferative rate compared to control cells, with fewer cells in S and G2/M phases. The Tbx1 transfectants developed significantly fewer colonies in soft agar, demonstrating loss of anchorage independent growth. Taken together, our data show that ectopic expression of Tbx1 restored contact inhibition to the skin tumor cells, suggesting that this developmentally important transcription factor may have a novel dual role as a negative regulator of tumor growth.
Keywords: Skin cancer, stem cell, transcription factor, contact inhibition
Introduction
Nonmelanoma skin cancers (basal cell carcinomas and squamous cell carcinomas) are among the most prominent of human cancers, with more than one million new cases diagnosed yearly in the United States (http://www.cancer.gov/cancertopics/types/skin). The prevalence of these cancers represents an important clinical challenge, and drives the need to understand the earliest mechanisms of skin cancer development. The mouse model of epidermal skin carcinogenesis has provided valuable insights into the complexities of neoplasia [1-3]. This model has provided evidence that stem cells residing in the hair follicle (HF) are a major target for carcinogens and likely contribute directly to skin tumor development [4-6]. Gene expression analyses of mouse hair follicle stem cells have been conducted and the results suggest that a complex network of genetic pathways regulate the biological characteristics of this population [7-10]. Among the many genes that have emerged from these analyses, the T-box transcription factor, Tbx1, was highly expressed in HF stem cells [9,11]. Although biological roles for Tbx1 in normal or neoplastic skin have not been determined, during HF development, Tbx1 expression was shown in the epidermal placode in mouse skin, with progressive localization to the outer root sheath over time [12].
Tbx1 is part of a large family of developmentally important transcription factors characterized by a 180-200 amino acid conserved DNA binding domain termed the T-box [13]. This gene is located on human chromosome 22q11, and deletion of this region is found in individuals with DiGeorge syndrome (DGS)/Velocardiofacial syndrome (VCFS) [13]. Tbx1 was identified as a key gene in this syndrome after homozygous null mice replicated the primary features of DGS, including thymus/parathyroid hypoplasia, craniofacial abnormalities, cleft palate, and abnormalities in the cardiac outflow tract [14].Tbx1 is required for heart [15] and inner ear development [16], and has been shown to play a role in the regulation of heart progenitor cell proliferation and differentiation [15]. Tbx1 has recently been identified as a potential target gene for Ectodysplasin A (EDA) [17], which is part of a signaling pathway regulating the development of ectodermal appendages, including HFs, sweat glands, and teeth [18]. Interestingly, Tbx1 expression has been shown to be localized to the developing tooth bud [12]. Other signaling molecules that have been shown to interact with Tbx1 include Fgf, Shh, Bmp, Retinoic acid (reviewed in [13]) Smad [19], and most recently, Wnt [20,21].
Because Tbx1 is uniquely expressed in adult hair follicle stem cells, and regulates progenitor cell proliferation and differentiation in development, we undertook experiments to determine the contributions of Tbx1 to mouse skin tumor development. In our initial studies, we observed that while Tbx1 was expressed in skin stem cells, expression was not detected in benign papillomas or various cutaneous malignancies. Therefore, to elucidate the effects of Tbx1 on growth and tumorigenicity of carcinoma cells, we expressed a full-length mouse Tbx1 cDNA in a spindle cell tumor (SCT) line derived from an FVB/N mouse, which we had determined did not express endogenous Tbx1. The results demonstrated that Tbx1 expression suppressed the ability of these cells to grow into tumors in mice. Furthermore, in vitro studies indicated that Tbx1 expression restored contact inhibition to the SCTs and caused a reduction in anchorage independent growth. These findings provide the first evidence for a role of Tbx1 in skin tumor development, and suggest that this developmentally important transcription factor may function as a negative regulator of tumor cell growth.
Materials and Methods
Animals
Four-week old female FVB/N mice were purchased from Taconic Farms (Germantown, NY) and held in the NIEHS animal facility in accordance with an approved animal study proposal and institutional guidelines for the care and maintenance of experimental animals. Mice were provided food and water ad libitum and held under a 12-hour light/dark cycle.
Mouse skin carcinoma cell line
A mouse tumor cell line, designated FVB217, was originally derived from a cutaneous malignancy obtained from a 7,12-dimethylbenz(a)anthracene (DMBA) - initiated, 12-O-tetradecanoylphorbol-13-acetate (TPA)-promoted FVB/N mouse [22]. Cells were grown at 37°C and 5% CO2 in RPMI media supplemented with 10% fetal bovine serum, 1 × PenStrep, and 1 × L-Glutamine (all reagents from Invitrogen, Carlsbad, CA).
Semi-quantitative analysis of Tbx1 expression in mouse tissue
RNA was isolated from mouse tumor tissues [23] and mouse keratinocytes [24] using the Qiagen RNeasy kit (Qiagen, Valencia, CA), following the manufacturer's protocol. Reverse transcription (RT) was accomplished with the GenAmp Gold RNA PCR Reagent kit (Applied Biosystems, Foster City, CA), using 100 to 500 ng/μl total RNA per reaction and following the manufacturer's directions. Three μl of each RT reaction was combined with PCR mix, using ThermalAce DNA Polymerase (Invitrogen) and the GC-rich PCR protocol as suggested by the manufacturer (98°C for 3 min, 35 cycles each of 98°C for 30 s, 55°C for 30 s, and 72°C for 7 min). Primers for full-length mouse Tbx1 (NM_011532.1) were: Sense 5’-ATGATCTCCGCCGTGTCTAGTCC-3’, and antisense 5’-TCTGGGGCAGTAGTCGTAG-3’. Housekeeping primers were for mouse β-2 microglobulin (Mβ2) as described elsewhere [25]. Amplicon sizes were 1.5 kb for full length mouse Tbx1 and 212 bp for Mβ2.
Cloning of full-length mouse Tbx1 into the pcDNA3.1/V5-His expression vector
Mouse Tbx1 was amplified from mouse testis total RNA, using primers and conditions as described above and cloned into the pcDNA3.1/V5-His TOPO TA expression vector, which contained a CMV promoter and a C-terminal V5-His fusion tag (Invitrogen). Primer details and additional experimental information is available in supplemental materials and methods.
Transfection of parental cells with full-length Tbx1 and generation of stable cell lines
FVB217 parental cells were first grown in media as described above. Media was then changed to Opti-MEM (Invitrogen) containing no antibiotics for transfection with mTbx1/pcDNA3.1 or empty vector (1 μg), using FuGENE transection reagent (Roche Applied Science, Indianapolis, IN), following the manufacturer's directions. Selection of transfected cells was accomplished with Geneticin (G418; Invitrogen) at 400 μg/ml, starting at day 3 after transfection. Single colonies were isolated and expanded, then assayed for Tbx1 expression.
Western Blotting
Total protein lysates were prepared as described elsewhere [26]. After SDS gradient gel electrophoresis and transfer to membranes (Hybond; GE Healthcare, Piscataway, NJ), blots were incubated with mouse anti-V5 antibody (1:5000, Invitrogen), and rabbit anti-mouse actin (1:1000; Sigma-Aldrich). Signal was detected following incubation with HRP-conjugated secondary antisera (1:3000, Santa Cruz Biotechnologies, Santa Cruz, CA), followed by enhanced chemiluminescence (GE Healthcare). Relative levels of the Tbx1/V5 fusion protein for cell lines 14M and 11S were calculated from RT-PCR and Western blot images using NIH ImageJ software.
Immunofluorescent localization of Tbx1/V5 expression
Stably transfected cells were grown in 4-well chamber slides (BD Biosciences, San Jose, CA), then fixed in acetone:methanol (1:1) at -20°C for 10 min. Slides were incubated with mouse anti-V5 antibody (Invitrogen) or mouse IgG (DAKO Corporation, Carpinteria, CA) at 1:50. Following incubation with donkey anti-mouse IgG AlexaFluor-594 (Molecular Probes, Invitrogen), coverslips were affixed to slides with ProLong Gold with DAPI mounting media (Invitrogen), and images captured using confocal microscropsy (NIEHS Fluorescence Micropscopy and Imaging Center).
Growth of Tbx1-expressing skin tumor clones in vivo
Seven-week old female FVB/N mice were injected intradermally with 2 × 105 cells per clone, then tumor tissue collected on days 3, 5, 7, 9, 11, and 13 post-injection from three mice per clone per time point. Tumors were first measured with digital calipers (Fisher Scientific, Pittsburg, PA) to determine volume (mm3; length × width × depth), then fixed in 10% neutral buffered formalin (10% NBF) for histological examination. Tissue sections were stained with hematoxylin and eosin, and slides scanned using the Aperio Scanscope T2 Scanner (Aperio Technologies, Inc., Vista, CA). Images were viewed using the Aperio Scanscope v. 6.25.0.1117 for photomicrograph construction, combined with a measurement scale internally calibrated for each image.
Growth kinetics of Tbx1-expressing carcinoma cells
Tbx1-expressing cell lines and control cells were seeded at passage 9 into either T25-vented cap culture flasks (experiment 1) or 6–well culture plates (experiment 2) at 1 × 104 cells per well or flask. Media as described above was supplemented with 400 μg/ml G418. Cells were harvested in triplicate daily for 6 days and viable cells counted using 0.4% Trypan Blue (Invitrogen) exclusion and a hemacytometer.
Flow cytometric analysis of DNA content analysis
DNA content of Tbx1-expressing and control cells from passage 9 was analyzed by flow cytometry as described elsewhere [24]. Cell cycle was analyzed using ModFit software (Verity Software House, Topsham, ME) to determine the percent of cells in each phase of the cell cycle.
Colony morphology
To visualize colony morphology, three wells per clone (from experiment 2) were fixed in 10% neutral buffered formalin, then stained for 1 hr with 1% Rhodamine B (Sigma-Aldrich, St. Louis, MO). Images of stained cells were collected with an Olympus IX70 inverted microscope equipped with a digital camera (Sony DXC-S500).
Colony thickness measurements
Confocal images of Rhodamine B-stained cells in 6-well culture plates were taken on a Zeiss LSM 510 meta using an Achroplan 40X/0.8 Water Immersion long working distance objective connected to an objective inverter manufactured by LSM Technologies. (LSM Tech, Etters, PA), allowing the inverted microscope to become an upright microscope, permitting confocal imaging of the colonies. A 543 nm laser line from a Helium-Neon laser was used for excitation, and a 565-615 nm band pass emission filter was used to collect the images with a pinhole setting of 56 μm, yielding a confocal optical section with a thickness of 1.6 μm. A single Z-stack image was acquired for each of the 6 locations in a well, with three replicate wells assessed per clone (n=18 colonies per clone).
Anchorage-independent growth in soft agar
Colony formation in soft agar was assayed as described elsewhere [27]. 1×104 cells were suspended in 60-mm tissue culture dishes (three dishes per clone) in 0.33% Noble agar (Difco, Kansas City, MI) containing RPMI-1640 supplemented with 10% FBS and 400 μg/ml geneticin (G418). The cell mixture was layered over 5 ml 0.5% agar in RPMI-1640 with 10% FBS and G418 and grown at 37°C/ 5% CO2. Colonies with more than 8 cells were counted 9 days after seeding.
Statistics
Prior to statistical analysis, the distribution of each endpoint was checked for normality. Endpoints that were normally distributed were analyzed using parametric methods such as analysis of variance (ANOVA) with Tukey's multiple comparisons procedure, t-tests and regression. Endpoints that were not normally distributed were analyzed using nonparametric methods such as Kruskal-Wallis ANOVA and Mann-Whitney tests. P-values for the nonparametric tests were determined using exact calculations rather than asymptotic approximations, because of the relatively small sample sizes. All p-values are two-sided and are considered significant if less than 0.05.
Results
Tbx1 is not expressed in mouse skin tumors
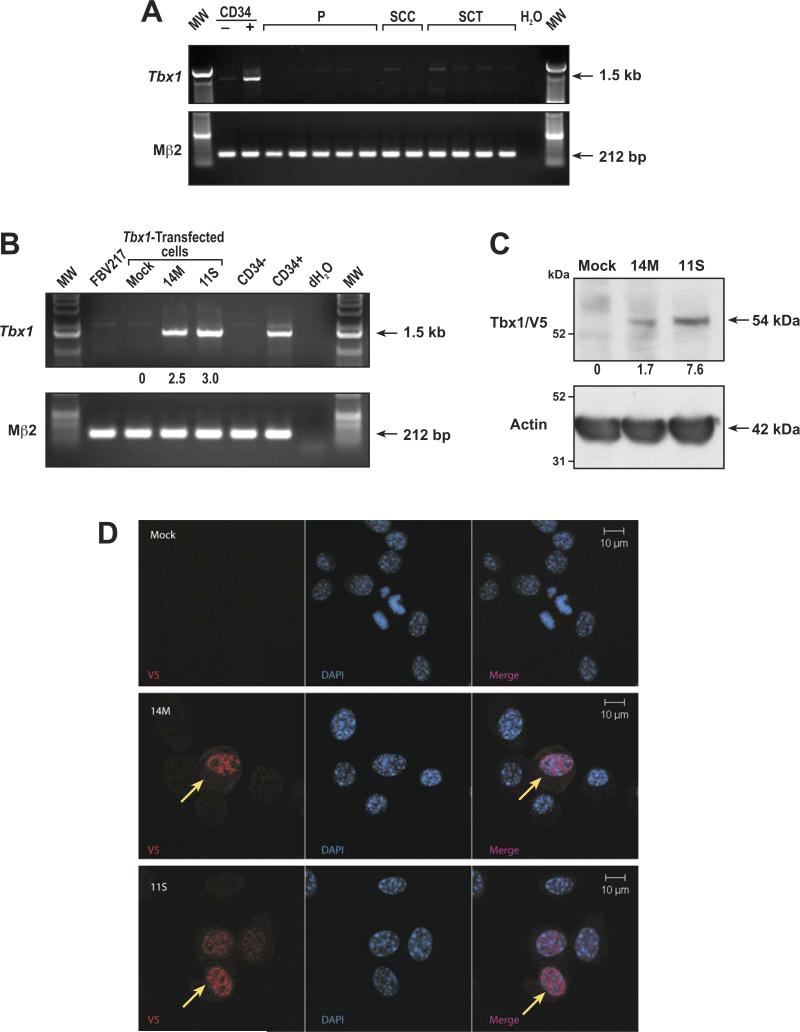
We and others have previously observed that the T-box transcription factor, Tbx1, is highly expressed in mouse hair follicle (HF) CD34-positive bulge stem and progenitor cells [9,11,12], as shown in Figure 1A. Because HF stem cells are thought to be a major target for chemical carcinogens and because skin tumors can arise from initiated stem cells [6], we first examined a series of benign lesions (papillomas) and malignacies (squamous cell carcinoma (SCC) and spindle cell tumors (SCT)) obtained from mice for Tbx1 expression. As shown in Figure 1A, Tbx1 message was below the detection limit in all skin lesions analyzed by RT-PCR. Therefore, Tbx1 expression appears to be lost or downregulated in cutaneous tumors in mice.
Figure 1.
Generation of mouse skin tumor cell lines expressing Tbx1. (A) RT-PCR detection of Tbx1 expression in mouse cells and tumors. CD34- keratinocytes; CD34+ hair follicle stem cells; P = papilloma; SCC = squamous cell carcinoma; SCT = spindle cell tumor. (B) RT-PCR detection of Tbx1 expression in transfectants. FVB217 = parental cell line; Mock = control; 14M and 11S = Tbx1-expressing cell lines; CD34-keratinocytes; CD34+ hair follicle stem cells. (C) Tbx1/V5 fusion protein expression in cell lines. Mock = control line; 14M and 11S = Tbx1-expressing cell lines. (D) Cellular localization of Tbx1 expression in cell lines. V5 antibody (red); DAPI = blue. Scale bar =10 μm.
Transfection of mouse skin tumor cells with Tbx1
While it is interesting to speculate that Tbx1 may play a functional role in the evolution of skin tumors from stem cells harboring carcinogenic mutations, there is no substantive data on how Tbx1 functions post-developmentally. Therefore, we examined the effect of ectopic expression of Tbx1 on growth and tumorigenic properties of cancer cells, using a mouse skin tumor cell line model.
Full-length V5-tagged mouse Tbx1 was transfected into a mouse skin tumor cell line (FVB217) generated from a tumor obtained from a DMBA-initiated, TPA-promoted FVB/N mouse [22]. These cells were selected because they do not express mouse Tbx1 (Figure 1B), are highly tumorigenic, forming spindle cell tumors (SCTs) following intradermal injection into FVB/N mice (Figure S1A). In addition, FVB217 cells harbor the expected DMBA-specific A-to-T transversion in the c-Ha-ras proto-oncogene [28] (Figure S1B; Table S1), which is retained in tumors that develop from these cells (Table S1). In culture, the cells show spindle-like, mesenchymal cell morphology (Figure S1C) [29].
Following transfection, cells were maintained under G418 selection before expansion of single colonies to establish stable transfectants. Twenty-four clonal cell lines were screened for Tbx1 expression using TaqMan (data not shown), and from these, two Tbx1-expressing cell lines were selected for further experiments, as well as one empty vector transfected line (hereafter referred to as 14M, 11S, and Mock, respectively). The cell lines were assessed for expression of Tbx1 by semi-quantitative RT-PCR (Figure 1B), and by Western blot analysis, using a V5 antibody to detect the Tbx1/V5 fusion protein (Figure 1C). Cell line 11S was found to express slightly more Tbx1 message than 14M by RT-PCR (Figure 1B), but substantially more protein (Figure 1C). Finally, because Tbx1 is a transcription factor, localization to the nucleus in the transfected cell lines was confirmed using immunofluorescent staining with the V5 antibody (Figure 1D). Taken together, these data confirm expression and correct nuclear localization of Tbx1 in two independent stably transfected cell lines.
Ectopic expression of Tbx1 delays SCT tumor growth in vivo
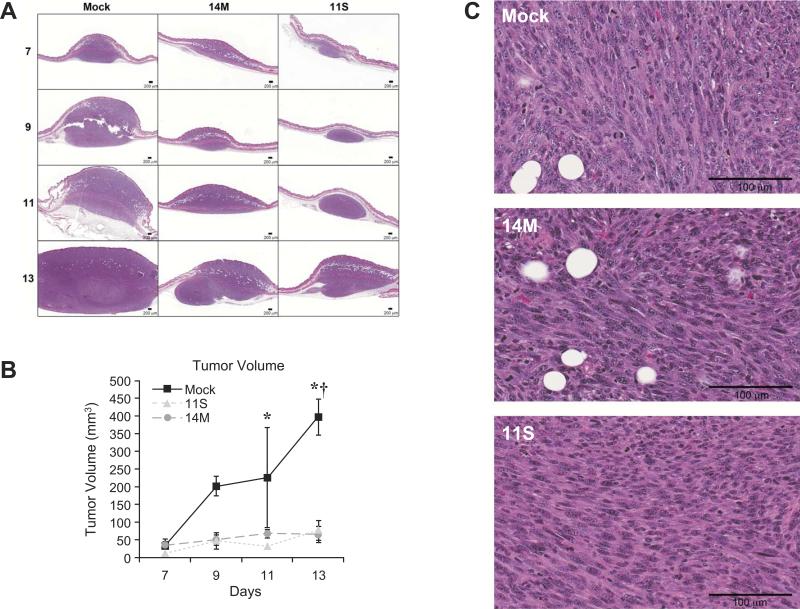
To test the effect of ectopic expression of Tbx1 on tumor growth in vivo, we injected the Tbx1-expressing and Mock control cell lines (2×105 cells per injection) intradermally into 7-week old female FVB/N mice. Tissues were collected 3, 5, 7, 9, 11, and 13 days post-injection (3 mice per cell line per time point) for tumor volume measurements and histological examination. As shown in Figure 2A and B, tumors derived from both Tbx1-expressing cell lines developed at a slower rate than tumors from the Mock cell line. At days 3 and 5, lesions were evident only upon microscopic examination of the injection sites for all three cell lines (Figure S2A). By day 13, however, tumors from the Mock cell line reached a volume of 396.60 +/- 51.05 mm3 (mean +/- SE), while tumors from the two Tbx1-transfected cell lines only reached volumes of 64.97 +/- 22.82 mm3 and 76.29 +/- 27.89 mm3 (14M and 11S, respectively; p<0.05, both 14M and 11S compared to Mock at day 13). An additional study was conducted using 1×105 cells per injection to determine if fewer cells at injection would result in a more profound reduction in tumor growth. As shown in Figure S2B, by day 7, tumors from Mock cells were similar in size to control tumors shown in Figure 2. Although there were no measureable tumors at this time point from 14M cells, there was histological evidence of aggregation into tumor masses (Figure S2B). However, for 11S, tumor cells were only diffusely localized to the injection site, again with no palpable tumors, (Figure S2B), indicating that tumor growth from the Tbx1-expressing clones was effected by cell number at injection, while control tumors grew at similar rates regardless of initial cell number. Taken together, ectopic expression of Tbx1 in mouse SCT cells resulted in decreased tumor growth rate in vivo, with even low expression levels having a potent suppressive effect.
Figure 2.
Growth of Tbx1-expressing clones in vivo. Cells (2 × 105) were injected intradermally into FVB/N mice. (A) H&E stained sections of tumors collected on days 7, 9, 11, and 13. Scale bar = 200 μm. (B) Tumor volume was measured (width × length × depth) for each tumor. *, Mock is significantly greater than 11S at p < 0.05, using the Mann-Whitney test. †,Mock is significantly greater than 14M at p < 0.05, using the Mann-Whitney test. (C) High magnification view of tumors from Mock, 14M and 11S. Scale bar = 100 μm.
Histological examination of the tumors did not reveal any differences in tumor morphology between Tbx1- and mock-transfected cells (Figure 2C), with all tumors displaying the herringbone pattern consistent with SCTs [1]. Furthermore, all tumors were found to be diffusely vimentin positive and keratin negative (data not shown). In addition, the DMBA-specific codon 61 A-to-T transversion in c-Ha-ras was detected in the parental FVB217 cell line, the Mock control and both Tbx1-expressing cell lines, as well as in the tumors derived from each cell line (Table SI). Finally, analysis of activated ras demonstrated that the constitutively activated GTP-bound form of ras was present in all cell lines (Figure S3). Therefore, while ectopic expression of Tbx1 in tumor cells delayed tumor growth, the morphological characteristics of the tumor and mutational status of the ras oncogene remained unchanged.
Tbx1 reduces tumor cell growth kinetics and affects colony morphology in culture
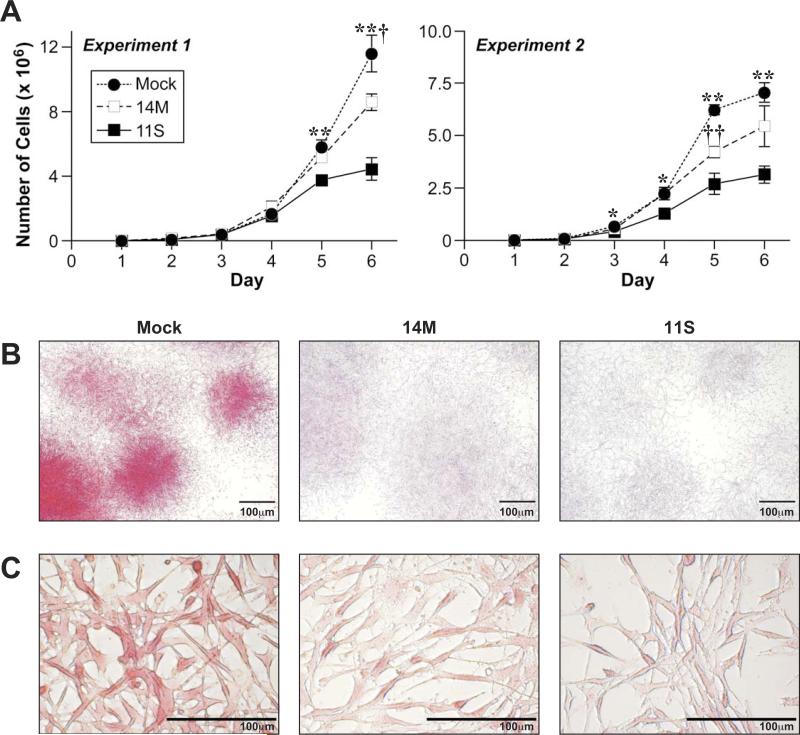
Because of the marked reduction in the rate of in vivo tumor growth from the Tbx1-transfected cells, we investigated the functional consequences of ectopic expression of Tbx1 by examination of the growth and morphological characteristics of the transfectants in culture. To test the effect of Tbx1 on cell growth of the tumor cell line, culture flasks (experiment 1) or plates (experiment 2) were seeded with 1 × 104 cells, then cells counted every 24 h for 6 days (Figure 3A). Cell counts revealed that both Tbx1-transfectants had decreased growth rates in culture relative to control cells (Figure 3A; Table SII).
Figure 3.
Growth kinetics and colony morphology of Tbx1-expressing tumor cells. (A) Cell lines were seeded (1×104 cells) into either T25 culture flasks (experiment 1) or 6 well culture dishes (experiment 2) and counted daily for 6 days. *, Mock is significantly greater than 11S at p < 0.01. **, Mock is significantly greater than 11S at p < 0.001. †, Mock is significantly greater than 14M at p < 0.01; ††, Mock is significantly greater than 14M at p < 0.001. (B) Cells were stained with Rhodamine B to visualize colony morphology. Scale bar = 100 μm. (C) Cell morphology of Rhodamine B-stained Mock, 14M, and 11S cells. Scale bar = 100 μm.
Replicate wells were stained with Rhodamine B to study colony morphology (Figure 3B, day 6 shown). As cell numbers increased and colony growth expanded, the Tbx1-expressing cells demonstrated distinct differences in colony morphology from the Mock control cells. By the end of the study (day 6), Mock transfected cells formed dense, compact colonies that stained intensely with Rhodamine B, while both of the Tbx1-expressing cell lines formed diffuse colonies, with increasingly lighter staining intensity from 14M to 11S (Figure 3B). Higher magnification images, shown in Figure 3C, reveal similar cell morphology between the three clones, regardless of Tbx1 expression.
Tbx1-expressing tumor cells exhibit reduced proliferation and colony thickness
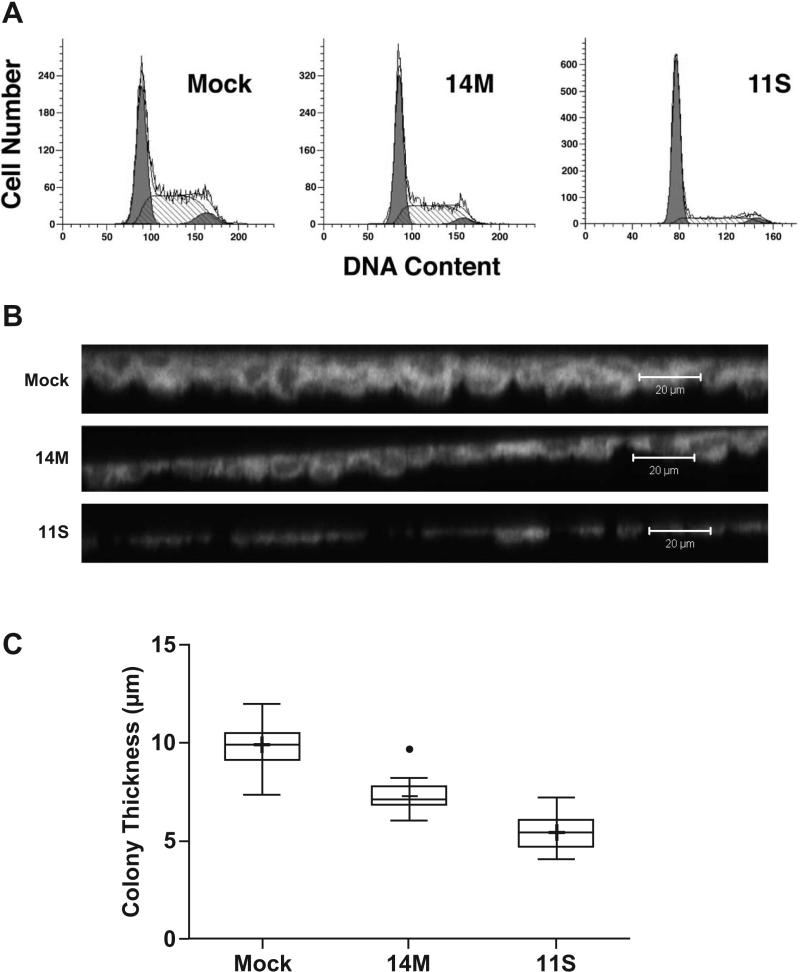
Tumor cells in culture, at high densities, tend to continue to proliferate in spite of close cell-to-cell contact [27]. Because of the decreased growth rate found in the Tbx1-expressing cell lines, we determined the effect of Tbx1 expression on the cell cycle kinetics, measuring DNA content by flow cytomtery. As shown in Figure 4A, there was an increased accumulation of cells in G1 and reduction in S and G2/M in both Tbx1-expressing clones, representing a trend toward a more quiescent cell cycle profile. As with cell growth, there was a Tbx1-expression dosage effect on the cell cycle kinetics, with 11S having a higher percentage of cells in G1/G0 than 14M, along with reduced S and G2/M phases. These data suggest that as Tbx1-expressing cells reach high densities in culture, proliferation is reduced, and this occurs in a Tbx1 dose-dependent manner.
Figure 4.
Cell proliferation and colony thickness of Tbx1-expressing cell lines. (A) Cell lines were analyzed for DNA content by flow cytometry following staining with propidium iodide and using Modfit software. (B) Confocal images were taken of Rhodamine B-stained cells (6 days in culture) to collect thickness measurements. Scale bar = 20 μm. (C) Measurements were collected from 6 colonies in each of 3 wells per cell line. Mock is significantly greater than 11S (*) and 14M (†) at p < 0.0001 by Tukey's multiple comparisons procedure.
It appeared from the Rhodamine B-staining intensity that colonies from the Tbx1-transfectants were not multilayered compared to the Mock-transfected cells. To assess colony thickness, fixed and stained wells from day 6 in culture were examined with confocal microscopy to generate 3-dimensional images of the culture surface, from which measurements were taken to determine relative colony thickness (Figure 4B and C). As shown in Figure 4C, colony thickness was found to be as follows, in descending order of thickness: Mock > 14M > 11S (mean ± SEs of 9.85 ± 0.30 μm, 7.29 ± 0.20 μm, and 5.44 ± 0.22 μm, respectively). Cell lines 14M and 11S had significantly fewer cell layers per colony than Mock (p<0.0001), which can be seen in the side view images in Figure 4B. These data demonstrate that Mock-transfected cells tended to “pile” up and become more multilayered at high density, while both Tbx1-expressing cell lines spread out over the culture surface with comparatively less 3-dimensional development.
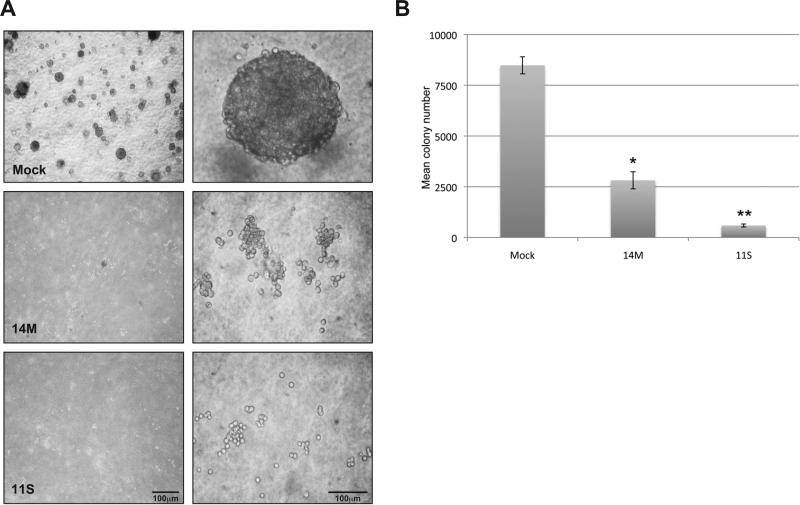
Tbx1 abrogates anchorage independent growth of mouse SCT cells
The cumulative evidence obtained thus far (decreased growth kinetics in culture and the changes in colony morphology) suggested that ectopic expression of Tbx1 had restored contact inhibition [30,31] to the tumor cells. A hallmark of tumor cells in culture is the ability to form colonies in the absence of attachment to a solid substrate, termed anchorage independent growth [27]. Conversely, cells that are contact inhibited fail to develop colonies when suspended in soft agar. As shown in Figure 5A and B, Mock cells generated many multicellular colonies (8485 ± 243 colonies, mean ± SE), as expected of a transformed cell line. However, Tbx1 expression significantly reduced colony formation in soft agar (p<0.05 for both 14M and 11S compared to Mock), with the highest expressing cell line, 11S, greatly reduced in multicellular colony growth (2816 ± 243 colonies and 592 ± 42 colonies, 14M and 11S, respectively). These data support the hypothesis that Tbx1 restored contact inhibition to the tumor cells, impairing the ability of these cells to grow in absence of substrate attachment.
Figure 5.
Tbx1 expression abrogates anchorage independent growth in skin tumor cells. (A) Cell lines were suspended in soft agar to study anchorage independent growth. Photographs were taken after 8 days in culture. Scale bar = 100 μm. (B) Colonies with more than 8 cells were counted on day 9 (in triplicate). †, Mock is significantly greater than 14M at p < 0.005. *, Mock is significantly greater than 11S at p < 0.0006.
Discussion
We report here that ectopic expression of the T-box transcription factor, Tbx1, in mouse skin tumor cells suppressed tumor growth in vivo through a mechanism involving restoration of contact inhibition. These data provide evidence of a novel role for Tbx1 as a negative regulator of tumor growth, in contrast to its pro-proliferative function during development.
T-box transcription factors are a family of important developmental regulators, with 17 members identified to date [13]. In addition to critical roles in development, emerging evidence supports a role for some members of the T-box family in carcinogenesis, with effects on cell proliferation, invasion and metastasis. For example, two closely related T-box genes, Tbx2 and Tbx3, have been shown to be overexpressed in several human cancers, including breast, pancreatic, ovarian, cervical cancers, as well as melanomas [32]. Unlike other T-box genes, Tbx2 and Tbx3 are transcriptional repressors [33] and both facilitate tumorigenesis through suppression of p14ARF (p19ARF in mice) [34,35], bypassing senescence and, thereby, facilitating malignant transformation. While there is overlapping function in suppression of senescence, Tbx2 and Tbx3 also affect different aspects of transformation, growth, and migration. It was recently demonstrated that Tbx2 regulates transformation and proliferation but not migration of melanoma and breast cancer cells, while Tbx3 promotes tumor formation and metastasis [36]. In contrast, ectopic expression of Tbx1 in skin tumor cells resulted in negative regulation of transformation and tumor cell proliferation, reverting the cells to a phenotype less permissive for unregulated, anchorage independent growth.
Another member of the T-box family, brachyury, is expressed in various human tumors, including esophageal, bladder, and ovarian [37]. A recent study demonstrated that ectopic expression of brachyury in carcinoma cells resulted in epithelial-mesenchymal transition (EMT), with evidence of increased migration and invasion, but with limited effect on primary tumor growth [38]. In addition, brachury was shown to suppress cell cycle progression by downregulating cyclin D1 and inhibiting cyclin/CDK complex formation, demonstrating a role in cell cycle regulation at the G1/S checkpoint [38]. Finally, while Tbx2, Tbx3, and brachyury appear to have permissive effects on tumorigenesis, it is interesting to note that another T-box gene, Tbx5, similar to Tbx1, shows evidence of growth suppression of cancer cells [39,40], with decreased growth and reduced colony formation in vitro [40]. Therefore, the T-box family of transcription factors displays mechanistic differences in regulating cancer cell proliferation, invasion, and metastasis.
All of the effects of Tbx1 described herein occur in the presence of an irreversible genetic alternation, constitutively activated oncogenic ras. Mutant ras is a key mediator of dysregulated tumor cell growth and is a primary effector of malignant transformation and anchorage independent growth [41-43]. It is therefore interesting that cells expressing Tbx1 were able to mitigate the effects of oncogenic ras under the in vitro culture conditions used here. While tumor growth in vivo was suppressed, the fact that tumors were able to develop at all was likely facilitated at least in part by mutant ras expression, which was retained in the tumors that grew out from the cell lines. In addition, Tbx1/V5 expression was undetectable in tumors analyzed at days 11 and 13 (data not shown), indicating that Tbx1 expression was lost as tumors developed. Indeed, all experiments were conducted with cells at passage 8 or 9 because Tbx1/V5 expression was undetectable after passage 11 or 12, even in the presence of G418 selection (data not shown). However, the decrease in the rate of tumor development in Tbx1-expressing clones was likely mediated by the diminished proliferative state and contact inhibited phenotype of the cells at the time of injection. In addition, these effects were not completely overcome in spite of loss of Tbx1 expression in the tumors, as evidenced by the continued slow rate of growth over time compared to control tumor growth.
In summary, the data shown here demonstrates that the developmentally important transcription factor, Tbx1, serves as a negative regulator of growth in mouse skin tumor cells, via restoration of contact inhibition. In addition, tumorigenicity is reduced in these cells, as reflected by loss of anchorage independent growth and delayed tumor growth in vivo. Taken together, these data provide evidence for a novel role for Tbx1 in carcinogenesis as a potential negative regulator of tumor development.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of the NIEHS Histology and Special Techniques Core, (especially Norris Flagler), NIEHS Arts and Photography (especially Lois Wyrick), and the NIEHS animal husbandry staff. We thank Drs. Rick Paules and Stavros Garantziotis for review of this manuscript. This work was conducted in the Intramural Research Division of the NIH at the National Institute of Environmental Health Sciences.
References
- 1.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4(9):1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54(1):63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 3.Yuspa SH, Dlugosz AA, Denning MF, Glick AB. Multistage carcinogenesis in the skin. J Investig Dermatol Symp Proc. 1996;1(2):147–150. [PubMed] [Google Scholar]
- 4.Kangsamaksin T, Park HJ, Trempus CS, Morris RJ. A perspective on murine keratinocyte stem cells as targets of chemically induced skin cancer. Mol Carcinog. 2007;46(8):579–584. doi: 10.1002/mc.20355. [DOI] [PubMed] [Google Scholar]
- 5.Kim DJ, Kataoka K, Rao D, Kiguchi K, Cotsarelis G, Digiovanni J. Targeted disruption of stat3 reveals a major role for follicular stem cells in skin tumor initiation. Cancer Res. 2009;69(19):7587–7594. doi: 10.1158/0008-5472.CAN-09-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris RJ. Keratinocyte stem cells: targets for cutaneous carcinogens. J Clin Invest. 2000;106(1):3–8. doi: 10.1172/JCI10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22(4):411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 9.Trempus CS, Dang H, Humble MM, et al. Comprehensive microarray transcriptome profiling of CD34-enriched mouse keratinocyte stem cells. J Invest Dermatol. 2007;127(12):2904–2907. doi: 10.1038/sj.jid.5700917. [DOI] [PubMed] [Google Scholar]
- 10.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3(11):e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoupa M, Seppala M, Mitsiadis T, Cobourne MT. Tbx1 is expressed at multiple sites of epithelial-mesenchymal interaction during early development of the facial complex. Int J Dev Biol. 2006;50(5):504–510. doi: 10.1387/ijdb.052116mz. [DOI] [PubMed] [Google Scholar]
- 13.Scambler PJ. 22q11 deletion syndrome: a role for TBX1 in pharyngeal and cardiovascular development. Pediatr Cardiol. 2010;31(3):378–390. doi: 10.1007/s00246-009-9613-0. [DOI] [PubMed] [Google Scholar]
- 14.Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27(3):286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Fulcoli FG, Tang S, Baldini A. Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ Res. 2009;105(9):842–851. doi: 10.1161/CIRCRESAHA.109.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Viola A, Zhang Z, Gerken CP, Lindsay-Illingworth EA, Baldini A. Tbx1 regulates population, proliferation and cell fate determination of otic epithelial cells. Dev Biol. 2007;302(2):670–682. doi: 10.1016/j.ydbio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esibizione D, Cui CY, Schlessinger D. Candidate EDA targets revealed by expression profiling of primary keratinocytes from Tabby mutant mice. Gene. 2008;427(1-2):42–46. doi: 10.1016/j.gene.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botchkarev VA, Fessing MY. Edar signaling in the control of hair follicle development. J Investig Dermatol Symp Proc. 2005;10(3):247–251. doi: 10.1111/j.1087-0024.2005.10129.x. [DOI] [PubMed] [Google Scholar]
- 19.Fulcoli FG, Huynh T, Scambler PJ, Baldini A. Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner. PLoS One. 2009;4(6):e6049. doi: 10.1371/journal.pone.0006049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Freyer L, Morrow BE. Canonical Wnt signaling modulates Tbx1, Eya1, and Six1 expression, restricting neurogenesis in the otic vesicle. Dev Dyn. 2010;239(6):1708–1722. doi: 10.1002/dvdy.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huh SH, Ornitz DM. Beta-catenin deficiency causes DiGeorge syndrome-like phenotypes through regulation of Tbx1. Development. 2010;137(7):1137–1147. doi: 10.1242/dev.045534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French JE, Libbus BL, Hansen L, et al. Cytogenetic analysis of malignant skin tumors induced in chemically treated TG-AC transgenic mice. Mol Carcinog. 1994;11(4):215–226. doi: 10.1002/mc.2940110407. [DOI] [PubMed] [Google Scholar]
- 23.Humble MC, Trempus CS, Spalding JW, Cannon RE, Tennant RW. Biological, cellular, and molecular characteristics of an inducible transgenic skin tumor model: a review. Oncogene. 2005;24(56):8217–8228. doi: 10.1038/sj.onc.1209000. [DOI] [PubMed] [Google Scholar]
- 24.Trempus CS, Morris RJ, Bortner CD, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120(4):501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 25.Cannon RE, Spalding JW, Trempus CS, et al. Kinetics of wound-induced v-Haras transgene expression and papilloma development in transgenic Tg.AC mice. Mol Carcinog. 1997;20(1):108–114. doi: 10.1002/(sici)1098-2744(199709)20:1<108::aid-mc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Wei SJ, Trempus CS, Cannon RE, Bortner CD, Tennant RW. Identification of Dss1 as a 12-O-tetradecanoylphorbol-13-acetate-responsive gene expressed in keratinocyte progenitor cells, with possible involvement in early skin tumorigenesis. J Biol Chem. 2003;278(3):1758–1768. doi: 10.1074/jbc.M206328200. [DOI] [PubMed] [Google Scholar]
- 27.Cox AD, Der CJ. Biological assays for cellular transformation. Methods Enzymol. 1994;238:277–294. doi: 10.1016/0076-6879(94)38026-0. [DOI] [PubMed] [Google Scholar]
- 28.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 29.Stoler AB, Stenback F, Balmain A. The conversion of mouse skin squamous cell carcinomas to spindle cell carcinomas is a recessive event. J Cell Biol. 1993;122(5):1103–1117. doi: 10.1083/jcb.122.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruse PF, Jr., Miedema E. Production and characterization of multiple-layered populations of animal cells. J Cell Biol. 1965;27(2):273–279. doi: 10.1083/jcb.27.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoker MG, Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez M, Aladowicz E, Lanfrancone L, Goding CR. Tbx3 represses E-cadherin expression and enhances melanoma invasiveness. Cancer Res. 2008;68(19):7872–7881. doi: 10.1158/0008-5472.CAN-08-0301. [DOI] [PubMed] [Google Scholar]
- 33.Rowley M, Grothey E, Couch FJ. The role of Tbx2 and Tbx3 in mammary development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2004;9(2):109–118. doi: 10.1023/B:JOMG.0000037156.64331.3f. [DOI] [PubMed] [Google Scholar]
- 34.Brummelkamp TR, Kortlever RM, Lingbeek M, et al. TBX-3, the gene mutated in Ulnar-Mammary Syndrome, is a negative regulator of p19ARF and inhibits senescence. J Biol Chem. 2002;277(8):6567–6572. doi: 10.1074/jbc.M110492200. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs JJ, Keblusek P, Robanus-Maandag E, et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nat Genet. 2000;26(3):291–299. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- 36.Peres J, Davis E, Mowla S, et al. The highly homologous T-box transcription factors, TBX2 and TBX3, have distinct roles in the oncogenic process. Genes & Cancer. 2010;1(3):272–282. doi: 10.1177/1947601910365160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13(8):2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 38.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120(2):533–544. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatcher CJ, Kim MS, Mah CS, et al. TBX5 transcription factor regulates cell proliferation during cardiogenesis. Dev Biol. 2001;230(2):177–188. doi: 10.1006/dbio.2000.0134. [DOI] [PubMed] [Google Scholar]
- 40.He ML, Chen Y, Peng Y, et al. Induction of apoptosis and inhibition of cell growth by developmental regulator hTBX5. Biochem Biophys Res Commun. 2002;297(2):185–192. doi: 10.1016/s0006-291x(02)02142-3. [DOI] [PubMed] [Google Scholar]
- 41.Colburn NH, Bruegge WF, Bates JR, et al. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978;38(3):624–634. [PubMed] [Google Scholar]
- 42.Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 43.Shin SI, Freedman VH, Risser R, Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.