Abstract
Objectives
The main challenges facing composite restorations are secondary caries and bulk fracture. The objectives of this study were to synthesize novel nanoparticles of amorphous calcium phosphate (NACP), develop NACP nanocomposite with calcium (Ca) and phosphate (PO4) ion release to combat caries, and investigate the effects of NACP filler level and glass co-filler reinforcement on composite properties.
Methods
NACP (diameter = 116 nm) were synthesized via a spray-drying technique for the first time. Since the local plaque pH in the oral cavity can decrease to 5 or 4, photo-activated composites were tested with immersion in solutions of pH 7, 5.5, and 4. Composite mechanical properties as well as Ca and PO4 ion release were measured vs. pH and filler level.
Results
Increasing the NACP filler level increased the ion release. At 28 d and pH 4, the Ca release was (4.66 ± 0.05) mmol/L at 20% NACP, much higher than (0.33 ± 0.08) at 10% NACP (p < 0.05). Decreasing the pH increased the ion release. At 20% NACP, the PO4 release at 28 d was (1.84 ± 0.12) mmol/L at pH 4, higher than (0.59 ± 0.08) at pH 5.5, and (0.12 ± 0.01) at pH 7 (p < 0.05). However, pH had little effect on composite mechanical properties. Flexural strength at 15% NACP was (96 ± 13) MPa at pH 4, similar to (89 ± 13) MPa at pH 5.5, and (89 ± 19) MPa at pH 7 (p > 0.1). The new NACP nanocomposites had strengths that were 2-fold those of previous calcium phosphate composites and resin-modified glass ionomer control.
Significance
NACP composites were developed for the first time. Their strengths matched or exceeded a commercial composite with little ion release, and were 2-fold those of previous Ca-PO4 composites. The nanocomposite was “smart” as it greatly increased the ion release at a cariogenic pH 4, when these ions would be most needed to inhibit caries. Hence, the new NACP composite may be promising for stress-bearing and caries-inhibiting restorations.
Keywords: dental nanocomposite, amorphous calcium phosphate, filler level, stress-bearing, caries inhibition, ion release
1. Introduction
Approximately 200 million dental restorations are placed annually in the United States [1]. Resin composites, consisting of fillers in a polymer matrix, are increasingly popular due to their esthetics, direct-filling ability, and enhanced performance [2–6]. Previous efforts have significantly improved the resin compositions, fillers types, and cure conditions [7–13]. However, recent reports showed that “The two main challenges are secondary caries and bulk fracture” [14,15]. Caries at the restoration margins is a frequent reason for replacement of existing restorations [16], which accounts for 50–70% of all restorations [17,18]. Replacement dentistry costs $5 billion/year in the U.S. [19]. Therefore, there is a need for mechanically-strong composites with caries-inhibition capabilities.
Calcium phosphate (CaP) particles have been filled into resins to develop composites with remineralizing capabilities [20–25]. One group of composites contained amorphous calcium phosphate (ACP) fillers, with ACP particles having a median diameter of 7.4 µm [26]. Another study reported an ACP median diameter of 55 µm [27]. These composites are promising because ACP is a precursor that forms initially and then transforms to apatite [28]. Hydroxyapatite [HA: Ca10(PO4)6(OH)2], the structural prototype of the major mineral component of teeth and bones, is the final stable product in the precipitation of calcium and phosphate ions in neutral or basic solutions [29]. Indeed, the ACP composites released supersaturating levels of calcium (Ca) and phosphate (PO4) ions in aqueous solutions, and effectively remineralized enamel lesions in vitro [27]. One drawback of the ACP composites is that they are mechanically weak, with flexural strength about half that of unfilled resin [20]. Such a low strength was “inadequate to make these composites acceptable as bulk restoratives” [21]. Therefore, there is a need to develop load-bearing ACP composites for a wide range of tooth cavity restorations.
Recently, CaP particles were combined with reinforcing whiskers or glass particles to yield composites with Ca and PO4 ion release and good mechanical properties [24,30,31]. These composites achieved Ca and PO4 release similar to previous CaP composites, while their mechanical properties nearly matched commercial load-bearing composites without ion release. Another study combined CaF2 nanoparticles with reinforcing fillers to develop composites with fluoride release and stress-bearing capability [32]. A literature search revealed no report on the synthesis of nanoparticles of ACP (NACP) or dental composites containing NACP.
Therefore, the objective of the present study was to develop the first NACP dental composite with load-bearing and caries-inhibiting capabilities. It was hypothesized that combining NACP with esthetic glass fillers would yield photo-cured composite with high levels of Ca and PO4 ion release and improved mechanical properties, and the composite would be “smart” to greatly increase the ion release at acidic, cariogenic pH, when these ions are most needed to inhibit caries.
2. Materials and methods
2.1 Synthesis of ACP nanoparticles
A spray-drying technique was used to make NACP. While nanoparticles of HA [33], dicalcium phosphate anhydrous (CaHPO4, or DCPA) [24] and CaF2 [32,34] were recently reported, ACP (Ca3[PO4]2) nanoparticles are reported here for the first time. ACP is important because it is a precursor that can convert to apatite, similar to the minerals in tooth enamel and dentin. A spraying solution was prepared by adding 1.5125 g of acetic acid glacial (J.T. Baker, Phillipsburg, NJ) into 500 mL of distilled water. Then, 0.8 g of calcium carbonate (CaCO3, Fisher, Fair Lawn, NJ) and 5.094 g of DCPA (Baker) were dissolved into the acetic acid solution. This solution was then added with distilled water to a total of 1 liter. The final Ca and PO4 ionic concentrations were 8 mmol/L and 5.333 mmol/L, respectively. This yielded a Ca/P molar ratio of 1.5, the same as that for ACP. The acetic acid concentration was 25 mmol/L. This solution was sprayed at a feed rate of 10 mL/min, through a nozzle (PNR, Poughkeepsie, NY) that was situated on a spray chamber with heated air flow. An electrostatic precipitator (AirQuality, Minneapolis, MN) was connected to the lower end of the column and drew air from the column to create a steady flow of air/mist. The water/volatile acid were evaporated into the dry, heated column and expelled from the precipitator into an exhaust-hood. The dried particles were collected by the electrostatic precipitator.
The collected powder was examined with X-ray diffractometry (XRD, DMAX2200, Rigaku, Woodlands, TX). The specific surface area of the powder was analyzed using a multipoint-BET (Brunauer, Emmet, and Teller) method (AUTOSORB-1, Quantachrome, Boynton Beach, FL) and transmission electron microscopy (TEM, 3010-HREM, JEOL, Peabody, MA).
2.2 Nanocomposite fabrication
Barium boroaluminosilicate glass particles of a median diameter of 1.4 µm (Caulk/Dentsply, Milford, DE) were selected as a co-filler because it is a typical dental glass filler similar to those in a hybrid composite (TPH, Cault/Dentsply). The glass particles were silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine (mass %) [35]. A resin of Bis-GMA (bisphenol glycidyl dimethacrylate) and TEGDMA (triethylene glycol dimethacrylate) at 1:1 mass ratio was rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoate.
Four composites were made with the following fillers: (1) 0% NACP + 75% glass (all mass fractions); (2) 10% NACP + 65% glass; (3) 15% NACP + 60% glass; and (4) 20% NACP + 50% glass. The total filler mass fraction was 75% except for (4) which had 70%. This was because with 20% NACP, the paste was relatively dry at 75% total filler level. NACP filler levels higher than 20% were not used in order for the NACP nanocomposite to have mechanical properties matching/exceeding a commercial control composite. The fillers and resin were mixed and the paste was placed into a stainless steel mold of 2×2×25 mm3. The specimen was photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each side. All the specimens were cured in air. The specimens were then incubated at 37 °C for 24 h prior to testing.
Two commercial materials were used as comparative controls. A composite with nano-fillers (40–200 nm) and a low level of fluoride release served as a control (Heliomolar, Ivoclar, Ontario, Canada). The fillers consisted of silica and ytterbium-trifluoride (total filler mass fraction = 66.7%). Heliomolar is indicated for Class I and Class II restorations in the posterior region, Class III and Class IV anterior restorations, Class V restorations, and pit and fissure sealing in molar and premolar teeth. A resin-modified glass ionomer (Vitremer, 3M, St. Paul, MN), another control, consisted of fluoroaluminosilicate glass, and a light-sensitive, aqueous polyalkenoic acid. Indications include Class III, V and root-caries restoration, Class I and II in primary teeth, and core-buildup. A powder/liquid ratio of 2.5/1 was used (filler mass fraction = 71.4%) according to the manufacturer. Both materials were photo-cured.
2.3 Flexural testing
Flexural strength and elastic modulus were measured using a three-point flexural test with a 20-mm span at a crosshead-speed of 1 mm/min on a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC). Flexural strength was calculated by: S = 3PmaxL/(2bh2), where Pmax is the fracture load, L is span, b is specimen width and h is thickness. Elastic modulus was calculated by: E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope of the load-displacement curve in the linear elastic region.
2.4 Ca and PO4 ion release
A sodium chloride (NaCl) solution (133 mmol/L) was buffered to three different pHs: pH 4 with 50 mmol/L lactic acid, pH 5.5 with 50 mmol/L acetic acid, and pH 7 with 50 mmol/L HEPES. Following previous studies [24,25], three specimens of approximately 2×2×12 mm3 were immersed in 50 mL of solution at each pH, yielding a specimen volume/solution of 2.9 mm3/mL. This compared to a specimen volume per solution of approximately 3.0 mm3/mL in a previous study [20]. For each solution, the concentrations of Ca and PO4 released from the specimens were measured at 1, 3, 7, 14, 21, and 28 days (d). At each time, aliquots of 0.5 mL were removed and replaced by fresh solution. The aliquots were analyzed for Ca and PO4 via a spectrophotometric method (DMS-80 UV-visible, Varian, Palo Alto, CA) using known standards and calibration curves [20,22]. The released ions were reported in cumulative concentrations.
One-way and two-way ANOVA were performed to detect the significant effects of the variables. Tukey’s multiple comparison test was used to compare the data at a p value of 0.05.
3. Results
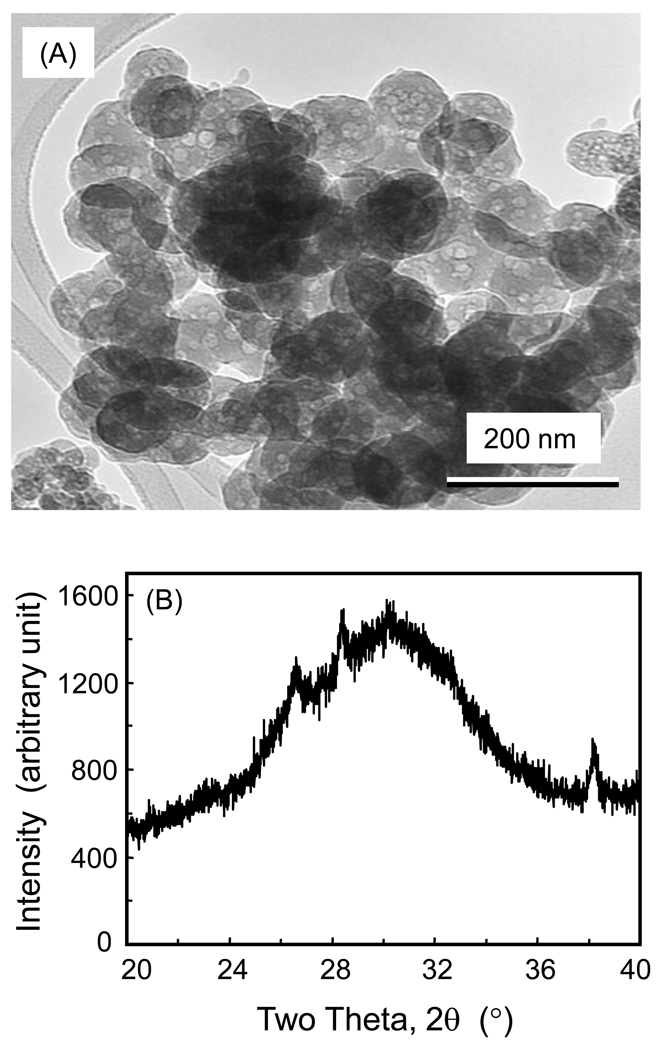
Fig. 1A shows the spray-dried NACP powder. The NACP particles appeared to have sizes of about 100 nm. The particles appeared to contain many smaller particles and had numerous spherical protuberances on the surfaces, suggesting that they were formed during the spry-drying process through the fusion of much smaller particles. The XRD pattern in (B) showed that the powder was amorphous. The BET method yielded a NACP surface area A = 17.76 m2/g. With ACP density ρ = 2.9 g/cm3, the particle diameter d = 6/(A ρ) = 116 nm, which is consistent with TEM observations.
Figure 1.
Nanoparticles of amorphous calcium phosphate (NACP) synthesized via a spray-drying technique. (A) A typical TEM micrograph of the NACP particles. (B) The XRD pattern shows that the spray-dried powder was amorphous.
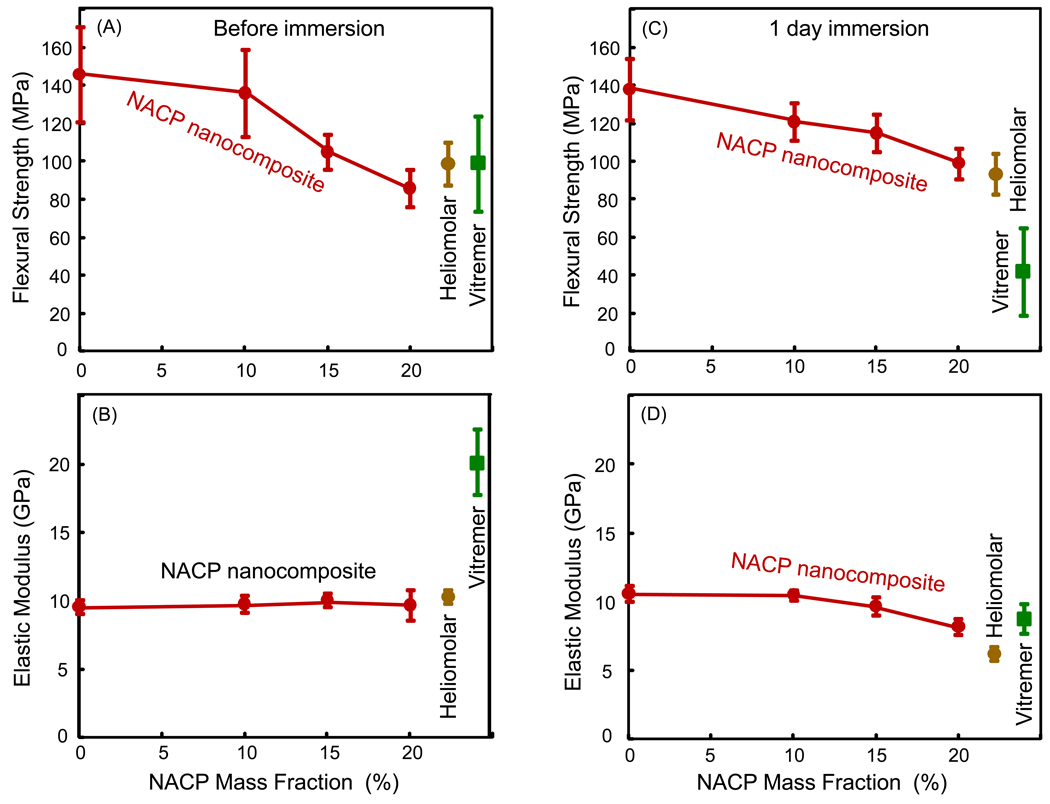
The composite mechanical properties are plotted in Fig. 2: (A and B) Before immersion, and (C and D) after 1 d immersion at pH 7. In (A), the strength (mean ± sd; n = 6) at 0% and 10% NACP were significantly higher than that at 20% NACP (p < 0.05). The strengths of the two commercial materials were not significantly different from any of the NACP composites (p > 0.1). Vitremer had a significantly (p < 0.05) higher elastic modulus than the other materials (B). After 1 d immersion (C), the strength of each material was not significantly lower than that before immersion, except for Vitremer which showed a significant decrease (p < 0.05).
Figure 2.
Mechanical properties of NACP nanocomposites. (A) Flexural strength before immersion. (B) Elastic modulus before immersion. (C) Flexural strength after 1 d immersion at pH 7. (B) Elastic modulus after 1 d immersion at pH 7. The properties for the NACP composites are plotted vs. NACP filler level. The two commercial materials are near the right axis as controls. Each value is the mean of six measurement, with the error bar showing one standard deviation (mean ± sd; n = 6).
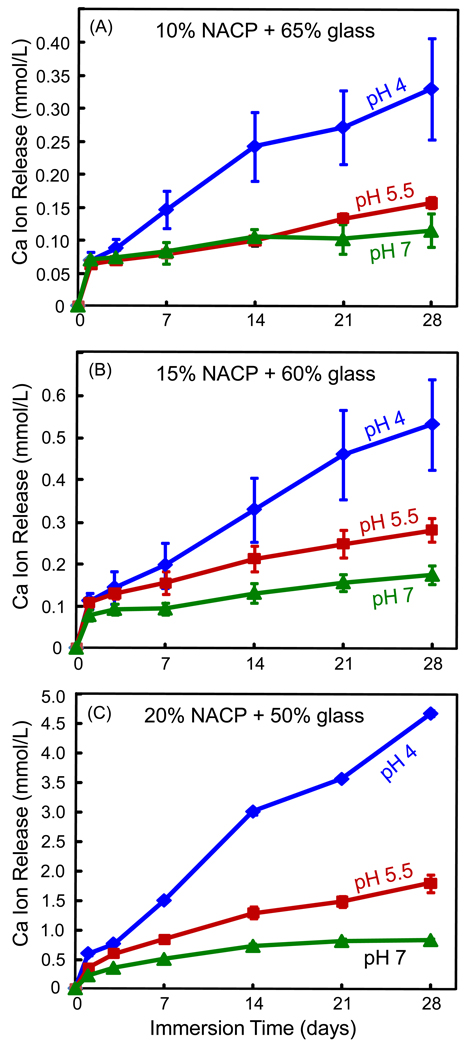
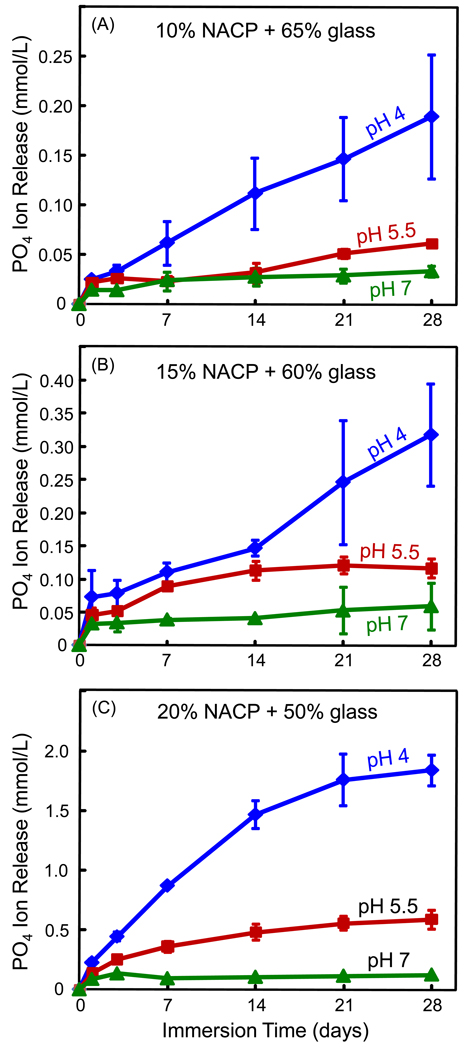
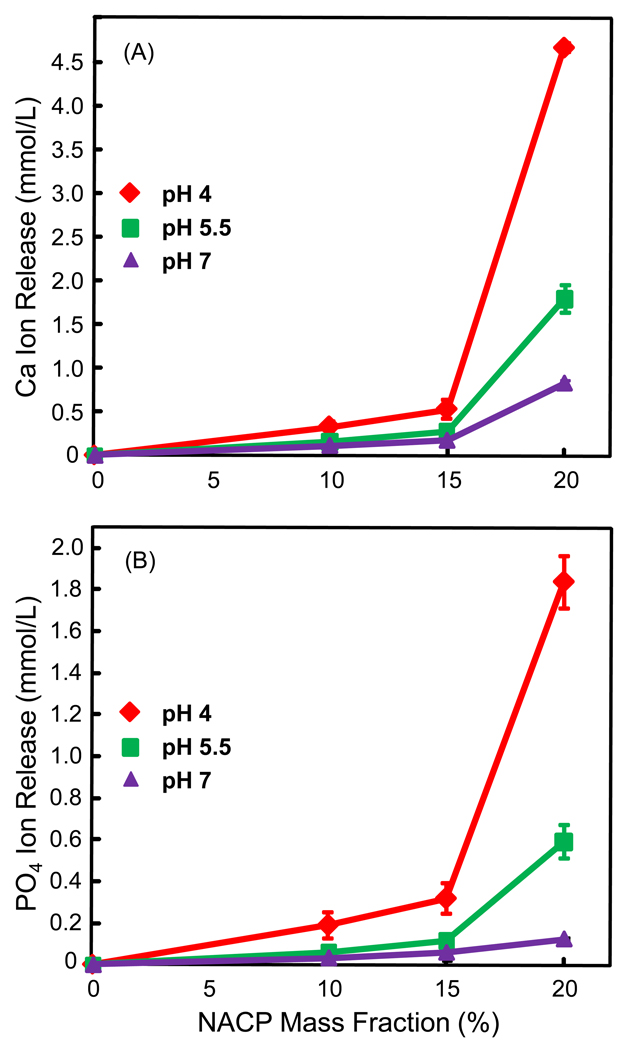
Ca and PO4 ion release are plotted in Figs. 3 and 4, respectively, at 10%, 15% and 20% NACP. Note the y-scale difference in order to show the data points with clarity. Increasing the NACP filler level increased the ion release. For example, at 28 d and pH 4, the Ca release was (4.66 ± 0.05) mmol/L at 20% NACP, much higher than (0.53 ± 0.11) at 15% NACP, and (0.33 ± 0.08) at 10% NACP (p < 0.05). Decreasing the solution pH significantly increased the ion release. At 20% NACP, for example, the PO4 release at 28 d was (1.84 ± 0.12) mmol/L at pH 4, significantly higher than (0.59 ± 0.08) at pH 5.5, and (0.12 ± 0.01) at pH 7 (p < 0.05).
Figure 3.
Ca ion release from the nanocomposite filled with: (A) 10% NACP and 65% glass, (B) 15% NACP and 60% glass, and (C) 20% NACP and 50% glass. Ca ion release increased with increasing the immersion time and the NACP filler level. Ca release increased with decreasing the solution pH. Each value is the mean of three measurement, with the error bar showing one standard deviation (mean ± sd; n = 3).
Figure 4.
PO4 ion release (mean ± sd; n = 3) from the nanocomposite filled with: (A) 10% NACP and 65% glass, (B) 15% NACP and 60% glass, and (C) 20% NACP and 50% glass. The release of PO4 significantly increased with longer immersion time and higher NACP filler level, or with decreasing the solution pH.
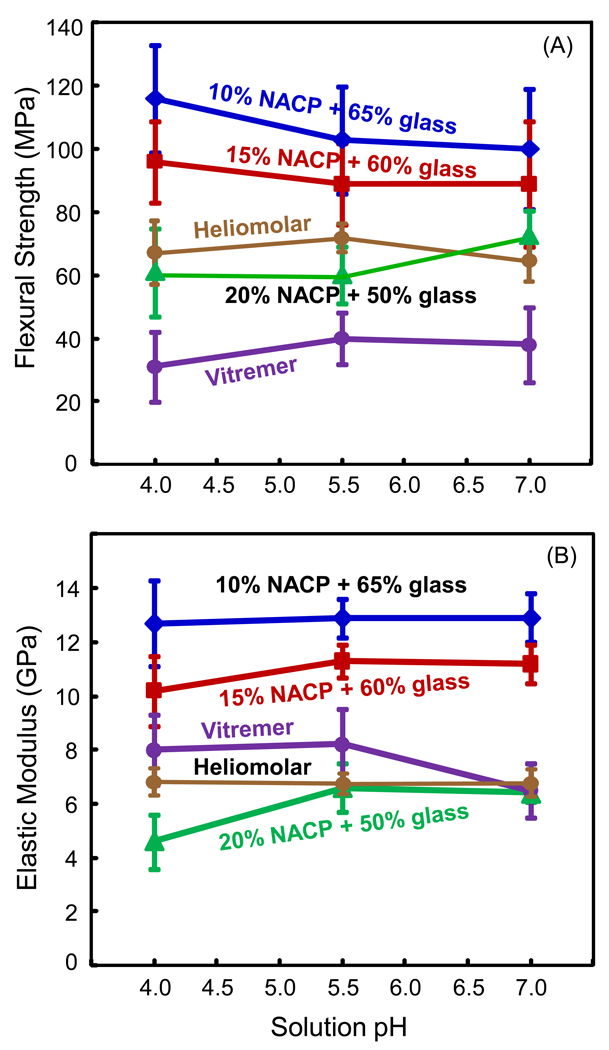
The composite mechanical properties after immersion for 28 d at different pH are shown in Fig. 5. In (A), for each material, varying the pH from 4 to 7 did not significantly change the strength (p > 0.1). Flexural strength at 15% NACP was (96 ± 13) MPa at pH 4, similar to (89 ± 13) MPa at pH 5.5, and (89 ± 19) MPa at pH 7 (p > 0.1). Among the materials, nanocomposites with 10% and 15% NACP had the highest strengths. Heliomolar and nanocomposite with 20% NACP had similar strengths, and Vitremer had slightly lower strengths.
Figure 5.
Mechanical properties of NACP nanocomposites after immersion for 28 d in solutions at different pH. (A) Flexural strength. (B) Elastic modulus. For every material, varying the solution pH from 4 to 7 did not significantly change the strength or modulus (p > 0.1).
4. Discussion
Extensive efforts have been undertaken to improve the performance of tooth cavity restorations [36–43]. Secondary caries currently limits the lifetime of composite restorations [14–19], hence it is highly desirable clinically to stabilize or regress tooth decay. Demineralization refers to the dissolution of calcium and phosphate ions from the tooth structure into the saliva, while remineralization refers to the mineral precipitation into the tooth structure [44,45]. Although saliva contains calcium and phosphate ions, the remineralization of tooth lesions can be significantly promoted by increasing the solution calcium-phosphate concentrations to levels higher than those in natural oral fluids. Therefore, an important approach to the inhibition of demineralization and the promotion of remineralization was to develop calcium-phosphate composites [20,22,24]. These composites released Ca and PO4 ions to supersaturating levels with respect to tooth mineral, and was shown to protect the teeth from demineralization, or even regenerate lost tooth mineral in vitro [22,27]. To achieve such ion release, previous studies have used micron-sized ACP [20], micron-sized tetracalcium phosphate [TTCP: Ca4(PO4)2O]) and DCPA [22], and DCPA nanoparticles [24,25] as fillers in the resins. The present study is the first report on the development of nano-sized ACP composite.
A major advantage of the NACP composite was that high levels of Ca and PO4 release was achieved at relatively low NACP filler levels because of the high surface area of the nanoparticles. The NACP particles of the present study had a specific surface area of 17.76 m2/g. In comparison, the specific surface area for previous ACP particles was 0.5 m2/g [26]. The new NACP particle size of 116 nm was 64-fold smaller than the reported 7.4 µm of traditional ACP particles [26], and 474-fold smaller than the reported 55 µm ACP particles [27]. The new nanocomposite with a low filler level of 20% NACP released Ca and PO4 to concentrations comparable with those of previous CaP composites [20,22]. This left room in the resin for significant amount of reinforcing, non-releasing, glass fillers. This method enabled the nanocomposite to rely on the stable glass fillers, not the releasing CaP fillers, for mechanical strength. As a result, the NACP composite with high levels of ion release possessed mechanical properties matching or exceeding those of a commercial composite with little ion release.
For comparison, the flexural strength of a previous ACP composite was approximately 30 MPa after 2 weeks of immersion [26]. This was consistent with a reported flexural strength of about 30 MPa for another resin containing calcium phosphate fillers after 90 d immersion [23]. These strengths were similar to the strength of Vitremer measured in the present study after 28 d immersion, suggesting that these previous CaP-filled resins might be useful in restorations where Vitremer is placed. The new NACP nanocomposite of the present study had flexural strengths of 60–120 MPa after immersion in solutions of pH 4, 5.5 and 7 for 28 d. Therefore, the new NACP nanocomposite had strengths of at least 2-fold that of the previous ACP composite.
The new NACP nanocomposite had mechanical properties that matched and exceeded those of a commercial composite with little ion release (Heliomolar). As shown in Fig. 5, after 28 d of immersion, the nanocomposites with 10% and 15% NACP had higher strengths and higher elastic moduli than Heliomolar. Therefore, they are promising to survive in restorations where Heliomolar is being used. According to the manufacturer, Heliomolar is indicated for Class I and II posterior restorations, Class III and IV anterior restorations and Class V restorations. The new nanocomposites may also be suitable for these applications, with an important additional benefit of Ca and PO4 ion release to inhibit caries. Hence, combining NACP with glass fillers yielded nanocomposites with a combination of Ca and PO4 release and stress-bearing properties, which may help reduce secondary-caries and restoration fracture. However, while the flexural strength and elastic modulus of the NACP nanocomposites are promising, further studies on their wear properties, color stability, fatigue resistance and long-term durability need to be performed, before these new nanocomposites can be proposed as load-bearing cavity filling materials.
To aid in the discussion on the effect of NACP filler level on ion release, the Ca and PO4 concentrations at 28 d are plotted in Fig. 6. It shows that when the NACP content was increased from 10% to 15%, there was a moderate increase in ion release. However, further increase to 20% NACP resulted in dramatically higher ion release. Hence, the Ca and PO4 release increased with NACP filler level at a rate faster than being linear. This is consistent with a previous study on the relationship between the amount of ion release and the dicalcium phosphate filler level in nanocomposite [25]. The first factor that contributed to the ion release was the ion source, i.e., the NACP fillers in the resin. It is expected that increasing the filler level would increase the ion source and hence the ion release. The second factor was that when the NACP content in the resin was increased, it not only increased the source of release, it also increased the amount of interfaces in the resin. The interface between the NACP particles and the resin served as relatively easier passageways for water to diffuse in, and ions to diffuse out. The third factor was that increasing the filler surface area due to the nanoparticles would moderately decrease the polymerization conversion, likely due to a higher concentration of air on the filler surfaces which decreased the conversion [35]. For example, a previous study showed that a nanocomposite with nanoparticles having a diameter of 112 nm at a nanoparticle:whisker ratio of 1:1 had a degree of polymerization conversion (DC) of 69.6%, measured using a Fourier Transform Infrared Spectrometer [35]. However, when these nanoparticles were replaced by traditional particles of a diameter of 0.88 µm, the DC was increased significantly to 75.4% (Table 2 in Ref. 35). This was attributed to the nanoparticles having a higher surface area than the traditional particles, hence likely having a higher concentration of air on the filler surfaces resulting in a slightly lower DC. In the present study, when the NACP filler level was increased from 10% to 20%, there was also an increase in the filler particle surface area. Based on these three factors, when the NACP filler level was increased, there was not only more source for ion release, there was also enhanced diffusion of water and ions through the resin due to a higher filler-resin interfacial area and lower polymerization conversion. These factors likely contributed to the observation that the Ca and PO4 release increased with NACP filler level at a rate faster than being linear.
Figure 6.
Effect of NACP filler level on ion release from nanocomposite. Ca and PO4 concentrations (mean ± sd; n = 3) at 28 d are plotted vs. NACP filler level. The ion release increased with NACP filler level at a rate faster than being linear, suggesting that the higher ion release resulted from not only an increase in the source of release, but also a higher interfacial area in the composite facilitating the water diffusion and ion release.
Another key factor on ion release is the pH. In the oral cavity, a local plaque pH of above 6 is the safe zone, pH of 6.0 - 5.5 is potentially cariogenic, and pH of 5.5 - 4 is the cariogenic or danger zone [46]. The present study showed that the new NACP composites were “smart” and greatly increased the Ca and PO4 release when the pH was reduced from neutral to a cariogenic pH of 4. There was a 5–10 fold increase in ion release as the pH was lowered from 7 to 4, when these ions are most needed for caries inhibition. The immersion for 28 d in solutions of pH 7, 5.5, and 4 reduced the mechanical properties of the composites, compared to those before immersion. However, as long as the specimens were immersed, pH variation from 4 to 7 did not have a significant effect on the mechanical properties. For every material tested, the flexural strength and elastic modulus were not significantly different among the three different pH values.
The NACP nanocomposites have potential for a range of tooth cavity restorations. For example, they could be used in load-bearing restorations for which the current CaP composites and resin-modified glass ionomer restoratives are inadequate. In addition, as the release of Ca and PO4 ions could remineralize existing lesions, the NACP composites could also be used in treatments where complete removal of caries tissue is contra-indicated, in teeth where carious lesions are beginning to occur, and for patients at high risk for dental caries including those receiving radiation treatments or with dry mouth. Furthermore, the NACP composites could be used in combination with fluoride-releasing dentifrices and mouthrinses, which could enhance the caries-inhibition and remineralization efficacy via the formation of fluoroapatite with increased resistance to acid attacks. Further research is needed to investigate these applications.
5. Conclusion
The present study developed NACP composites for the first time, and determined the effects of NACP filler level, glass reinforcement, and solution pH on Ca and PO4 release and mechanical properties. The nanoparticles with a high surface area enabled the composite to have high levels of ion release at low filler levels, thereby making room in the resin for significant amounts of reinforcement fillers. This resulted in the NACP composite to possess flexural strength and elastic modulus that matched or exceeded those of a commercial load-bearing composite with little ion release. The Ca and PO4 release greatly increased with NACP filler level. The nanocomposites were “smart” to dramatically increase the ion release at a cariogenic pH 4, when these ions would be most needed to inhibit caries. Decreasing the solution pH to 4 did not decrease the composite mechanical properties compared to those immersed at pH 7. The Ca and PO4 release from the NACP composite matched those of previous CaP composites known to remineralize tooth lesions, while the mechanical properties of the NACP composite were 2-fold higher. Therefore, the new NACP nanocomposite may have potential for stress-bearing and caries-inhibiting restorations.
Acknowledgments
We gratefully thank Dr. J.M. Antonucci, Dr. S. Dickens and Dr. D. Skrtic for discussions, and A.A. Giuseppetti for experimental assistance. This study was supported by NIH R01 grants DE17974 (HX) and DE16416 (LC), the University of Maryland Dental School, NIST, and ADAF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
Certain commercial materials and equipment are identified to specify experimental procedures. This does not imply recommendation by NIST/ADA.
REFERENCES
- 1.American Dental Association (ADA) The 1999 survey of dental services rendered. Chicago, IL: ADA Survey Center; 2002. [Google Scholar]
- 2.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 3.Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 4.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater. 2002;18:436–444. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 5.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruddell DE, M. M. Maloney MM, Thompson JY. Effect of novel filler particles on the mechanical and wear properties of dental composites. Dent Mater. 2002;18:72–80. doi: 10.1016/s0109-5641(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 8.Imazato S. Review: Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 9.Drummond JL, Bapna MS. Static and cyclic loading of fiber-reinforced dental resin. Dent Mater. 2003;19:226–231. doi: 10.1016/s0109-5641(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 10.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu H, Stansbury JW, C. N. Bowman CN. Impact of curing protocol on conversion and shrinkage stress. J Dent Res. 2005;84:822–826. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Ling L, Wang R, Burgess JO. Formation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006;22:1014–1023. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Krämer N, García-Godoy F, Reinelt C, Frankenberger R. Clinical performance of posterior compomer restorations over 4 years. Am J Dent. 2006;19:61–66. [PubMed] [Google Scholar]
- 14.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. International Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 17.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 18.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Prim Dent Care. 2002;9:31–36. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 19.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commision Projects 2-95. International Dent J. 2001;51:117–158. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 20.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 21.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53B:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 23.Dickens SH, Flaim GM, Floyd CJE. Effect of resin composition on mechanical and physical properties of calcium phosphate filled bonding systems. Polymer Preprints. 2004;45:329–330. [Google Scholar]
- 24.Xu HHK, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC. Nano dicalcium phosphate anhydrous-whisker composites with high strength and Ca and PO4 release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu HHK, Weir MD, Sun L, Takagi S, Chow LC. Effect of calcium phosphate nanoparticles on Ca-PO4 composites. J Dent Res. 2007;86:378–383. doi: 10.1177/154405910708600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regnault WF, Icenogle TB, Antonucci JM, Skrtic D. Amorphous calcium phosphate/urethane methacrylate resin composites. I. Physicochemical characterization. J Mater Sci: Mater Med. 2008;19:507–515. doi: 10.1007/s10856-007-3178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langhorst SE, O’Donnell JNR, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative microradiographic study. Dent Mater. 2009;25:884–891. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeGeros RZ. In: Calcium phosphates in oral biology and medicine. Chapters 3–4. Myers HM, editor. Basel, Switzerland: S. Karger; 1991. [PubMed] [Google Scholar]
- 29.Chow LC, Markovic M, Takagi S. Calcium phosphate cements. In: Struble LJ, editor. Cements research progress. Westerville, OH: American Ceramic Society; 1999. pp. 2152–2380. [Google Scholar]
- 30.Xu HHK, Weir MD, Sun L. Calcium and phosphate ion releasing composite: Effect of pH on release and mechanical properties. Dent Mater. 2009;25:535–542. doi: 10.1016/j.dental.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu HHK, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, Antonucci JM. Strong nanocomposites with Ca, PO4 and F release for caries inhibition. J Dent Res. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu HHK, Moreau JL, Sun L, Chow LC. Strength and fluoride release characteristics of a calcium fluoride based dental nanocomposite. Biomaterials. 2008;29:4261–4267. doi: 10.1016/j.biomaterials.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow LC, Sun L, Hockey B. Properties of nanostructured hydroxyapatite prepared by a spray drying technique. J Res NIST. 2004;109:543–551. doi: 10.6028/jres.109.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L, Chow LC. Preparation and properties of nano-sized calcium fluoride for dental applications. Dent Mater. 2008;24:111–116. doi: 10.1016/j.dental.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu HHK, Weir MD, Sun L. Dental nanocomposites with Ca-PO4 release: Effects of reinforcement, dicalcium phosphate particle size and silanization. Dent Mater. 2007;23:1482–1491. doi: 10.1016/j.dental.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Condon JR, Ferracane JL. Reduced polymerization stress through non-bonded nanofiller particles. Biomaterials. 2002;23:3807–3815. doi: 10.1016/s0142-9612(02)00099-6. [DOI] [PubMed] [Google Scholar]
- 37.Ellakuria J, Triana R, Minguez N, Soler I, Ibaseta G, García-Godoy F. Effect of one-year water storage on the surface microhardness of resin-modified versus conventional glass-ionomer cements. Dent Mater. 2003;19:286–290. doi: 10.1016/s0109-5641(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003;24:2451–2461. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi RL, Wiltbank BD, Murchison CF. Prediction of composite elastic modulus and polymerization shrinkage by computational micromechanics. Dent Mater. 2004;20:397–401. doi: 10.1016/j.dental.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent Mater. 2005;21:962–970. doi: 10.1016/j.dental.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Griggs JA. Recent advances in materials for all-ceramic restorations. Dent Clin N Am. 2007;51:713–727. doi: 10.1016/j.cden.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Della Bona A, Mecholsky JJ, Barrett AA, Griggs JA. Characterization of glass-infiltrated alumina-based ceramics. Dent Mater. 2008;24:1568–1574. doi: 10.1016/j.dental.2008.06.005. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denry I, Kelly JR. State of the art of zirconia for dental applications. Dental Mater. 2008;24:299–307. doi: 10.1016/j.dental.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Deng DM, ten Cate JM. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Res. 2004;38:54–61. doi: 10.1159/000073921. [DOI] [PubMed] [Google Scholar]
- 45.Featherstone JDB. The continuum of dental caries - Evidence for a dynamic disease process. J Dent Res. 2004;83:C39–C42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 46.Hefferren JJ, Koehler HM. Foods, nutrition and dental health. Park Forest South, IL: Pathotox Publishers; 1981. p. 138. [Google Scholar]