Abstract
The relationship between top-down enhancement and suppression of sensory cortical activity and large-scale, neural-networks remains unclear. Functional connectivity analysis of human fMRI data revealed visual cortical areas that selectively process relevant information are functionally connected with the frontal-parietal network, while those processing irrelevant information are simultaneously coupled with the default-network. This provides the first evidence that sensory cortical regions are differentially and dynamically coupled with distinct networks based on task goals.
Keywords: suppression, enhancement, top-down modulation, default network, frontal-parietal network
Goal-directed decisions influence our perception and result in corresponding modulation of sensory cortical activity. This phenomenon, known as top-down modulation, is characterized by increased cortical responses when stimuli are task-relevant (i.e., enhancement) and decreased responses when stimuli are task-irrelevant (i.e., suppression)1, 2. It is this modulatory ability that allows us to successfully navigate multiple streams of sensory information in a flexible manner. Top-down modulation is not thought to be an intrinsic property of visual cortices, but rather achieved via distributed connections between brain regions, or neural-networks, notably involving the prefrontal cortex, parietal cortex and the visual association cortex3-5. Recent evidence suggests that enhancement and suppression are distinct neural processes; e.g., they are differentially impacted by aging6 and cognitive load manipulations7.
Similar to top-down modulation of sensory cortices, distinct sets of parietal and frontal regions exhibit enhanced and suppressed activity depending upon an individual’s goals. Specifically, the frontal-parietal network (FPN)8, 9 is co-activated during a wide-array of externally-directed tasks (e.g., selective attention3, 10), while the default network9, 11 is de-activated during these tasks11 and actived by introspective cognitive processes (e.g. prospective/retrospective memory, internal monitoring12). To our knowledge, the relationship between these large-scale networks and top-down enhancement and suppression of sensory cortical activity has not been reported.
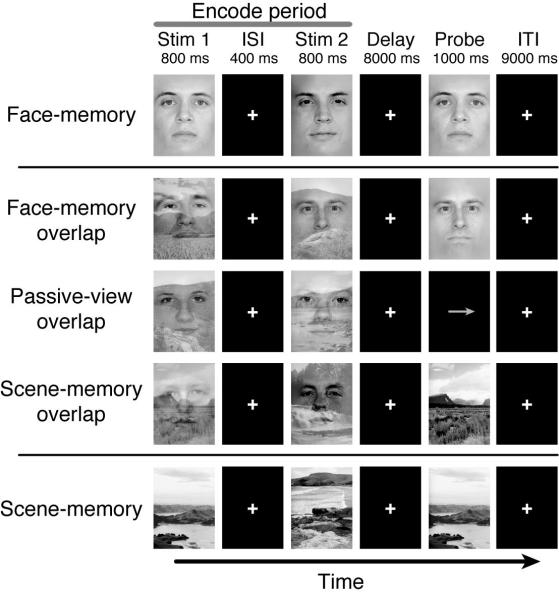
The present study evaluated whole-brain networks associated with top-down modulation in the setting of simultaneous, competing visual processing demands. We utilized fMRI during a selective, delayed-recognition task in which participants were required to remember specific stimuli, while simultaneously ignoring irrelevant stimuli (i.e., superimposed faces and natural scenes) over a brief delay period13 (Fig. 1). Both working memory accuracy and response time were negatively impacted by the presence of irrelevant stimuli, compared to trials where relevant stimuli were presented in isolation (Supplementary Fig. 1). To derive measures of neural enhancement and suppression, encode-period activity was assessed in scene-selective, visual association cortex (parahippocampal place area, (PPA)) and face-selective regions (fusiform face area (FFA))1, 3, 7. Significant top-down modulation was observed in the PPA, such that there was enhancement of activity when scenes were relevant compared to passive-view baseline (Scene-memory overlap (SM-O) > Passive-view overlap (PV-O); p<0.001), and suppression when scenes were irrelevant (PV-O > Face-memory overlap (FM-O); p<0.005) (Supplementary Fig. 2a), consistent with previous findings using sequentially presented images1. This activity modulation represents a pure top-down effect because bottom-up (stimulus-driven) information was constant across all three conditions; only task goals were manipulated. A similar pattern of activation was observed for the FFA (Supplementary Fig. 2b), except suppression was not significant, consistent with previous findings1 (Supplementary Discussion).
Figure 1.
Experimental Paradigm. Participants were instructed to remember Stim1 and Stim2 and respond yes/no if the probe image matched either of the previous two relevant stimuli as indicated by the task instructions: Face-memory (FM), Face-memory overlap (FM-O), Passive-view overlap (PV-O), Scene-memory overlap (SM-O), and Scene-memory (SM). Participants maintained fixation on the white crosshairs throughout experiment. ISI – inter-stimulus interval, ITI – inter-trial interval.
Functional connectivity maps were generated by correlating trial-by-trial variation in activity from the PPA and FFA with every other voxel in the brain3,14. Enhancement and suppression networks were derived by differentially pairing these visual regions (i.e., the seeds) with a condition and then contrasting the maps with those obtained by pairing seeds with the passive viewing condition. For example, an enhancement network was generated by contrasting PPA connectivity for SM-O > PV-O and a suppression network by contrasting PPA connectivity for FM-O > PV-O.
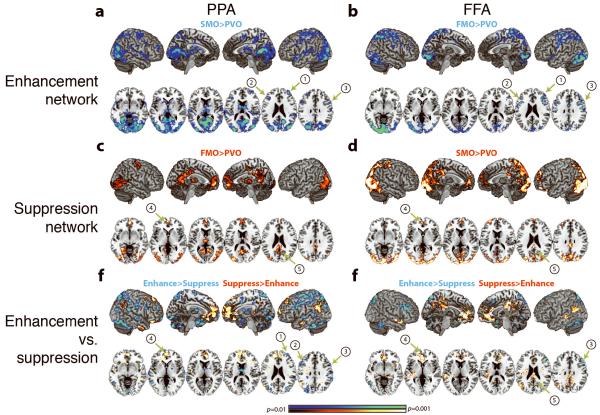
Analysis revealed that the enhancement network, independent of seed, was associated with brain regions of the FPN8, 9, notably the right middle frontal gyrus (MFG) and bilateral inferior frontal junction (IFJ) (Fig. 2a, b; Supplementary Table 1). In contrast, the suppression network was associated with regions of the default network9, 11, notably the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) (Fig. 2c, d; Supplementary Table 2). This was also independent of seed, and these regions were distinct from those identified in the enhancement network, as confirmed by a direct contrast between the networks (Fig. 2e, f; Supplementary Table 3). It was verified that these regions were nodes of the canonical default network by a whole-brain conjunction analysis between the suppression networks and the default network derived using an independent localizer task (Supplementary Methods, Supplementary Fig. 3). Further analysis of default network regions of interest derived from the localizer task (notably, the mPFC and PCC) supported that these regions were differentially coupled with visual cortical regions based on task goals, i.e., greater functional connectivity when activity in visual regions were being suppressed (Supplementary Fig. 4). Moreover, analysis revealed that the differential connectivity of stimulus-selective visual regions with the FPN and default network occurred simultaneously and switched dynamically with task goals. Importantly, visual cortical coupling with default network required the presence of irrelevant information, i.e. in conditions without task-irrelevant information (Face-memory (FM) and Scene-memory (SM)), but the same goals of remembering the face or scene, neither PPA nor FFA were functionally connected with default network regions (Supplementary Fig. 5).
Figure 2.
Network Connectivity. Connectivity Maps associated with enhancement (A and B, SM-O>PV-O and FM-O>PV-O, respectively), suppression (C and D, FM-O>PV-O and SM-O>PV-O, respectively), and contrast maps between suppression and enhancement networks (E and F) for both PPA (A, C, and E) and FFA (B, D, and F). Whole-brain maps were cluster corrected for multiple comparisons at p=0.05 and displayed at p<0.01. Labeled regions are as follows: 1) right MFG, 2) left IFJ, 3) right IFJ, 4) mPFC, and 5) PCC.
Regression analyses revealed two important aspects of the relationship between functional coupling, activity modulation and performance: 1) Participants who demonstrated the strongest connectivity between the PPA and mPFC region of the default network during FM-O (relative to PV-O), were those who also showed the greatest PPA activity suppression (Supplementary Fig. 6). This neural-behavioral relationship was limited to the PPA, consistent with the finding that FFA was not significantly suppressed. 2) Trial-by-trial fluctuations within participants in default network activity (particularly the mPFC and PCC) negatively correlated with response time on the delayed-recognition task (p<0.05, Supplementary Methods), such that trials with the most reduced (suppressed) activity in default network regions were those trials with the fastest RT. This is similar to previous findings that neural signatures of ignoring task-irrelevant stimuli are predictive of working memory performance15. Of note, this neural-behavioral relationship occurred only for tasks when irrelevant stimuli were present (Supplementary Fig. 7), consistent with functional connectivity being dependent on the presence of irrelevant information. A converging result was obtained using an across-participant regression analysis of RT and functional connectivity (overlap vs. non-overlap conditions), which revealed greater PPA-default network coupling (specifically, the mPFC and PCC) was associated with resistance to distraction on behavioral performance (Supplementary Fig. 8).
This study provides novel evidence that sensory cortical regions are functionally connected with distinct large-scale neural networks based on attentional goals. Consistent with previous studies, the FPN was associated with top-down enhancement of task-relevant stimuli3. The current results demonstrate for the first time that the default network is functionally connected with stimulus-selective visual cortex (i.e. PPA) only in the presence of irrelevant information, and that this functional coupling is predictive of both the neural suppression of task-irrelevant information and the resistance to the negative impact of distraction on working memory performance. This pattern of convergent results suggests that the connectivity finding was of functional significance. One interpretation is that suppression of externally generated distracting information (i.e. suppression of visual cortex) is intimately coupled with the suppression of internally generated distracting information (i.e. suppression of default network) (Supplementary Discussion). Although connectivity was also observed between the FFA and default network, it was not associated with significant suppression; the reason for this dissociation, as well as the generalization of this finding to other sensory areas remains to be determined. Of note, fMRI data is correlational and mechanistic interpretations of causality will require the support of a direct perturbation approach, such as transcranial magnetic stimulation (Supplementary Discussion). In summary, these results reveal a novel form of flexible, dissociable network dynamics between visual cortices and frontal and parietal regions based on task goals and the presence of irrelevant information.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health Grants and NIH Grant 5R01AG030395 (AG) and Larry L. Hillblom Center for the Biology of Aging Graduate Fellowship (JZC). We would like to thank Aaron Rutman, Michael Ruben, Joaquin A. Anguera, Ezequiel Morsella, and Theodore Zanto for their assistance.
REFERENCES
- 1.Gazzaley A, Cooney J, McEvoy K, Knight RT. D’Esposito, M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- 2.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 3.Gazzaley A, et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuster J. Network memory. Trends Neurosci. 1997;20:451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- 5.Fuster J, Bauer R, Jervey J. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 1985;330:299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- 6.Gazzaley A, Cooney J, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 7.Rissman J, Gazzaley A, D’Esposito M. The effect of non-visual working memory load on top-down modulation of visual processing. Neuropsychologia. 2009;47:1637–1646. doi: 10.1016/j.neuropsychologia.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Nat Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Nat Acad Sci USA. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 12.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Rutman AM, Clapp WC, Chadick J, Gazzaley A. Early Top-Down Control of Visual Processing Predicts Working Memory Performance. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 15.Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. J Neurosci. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.